The Microphthalmia-Associated Transcription Factor (MITF) and Its Role in the Structure and Function of the Eye
Abstract
1. Introduction
2. The Role of Mitf in the RPE
Mitf and Ion Transport across the RPE
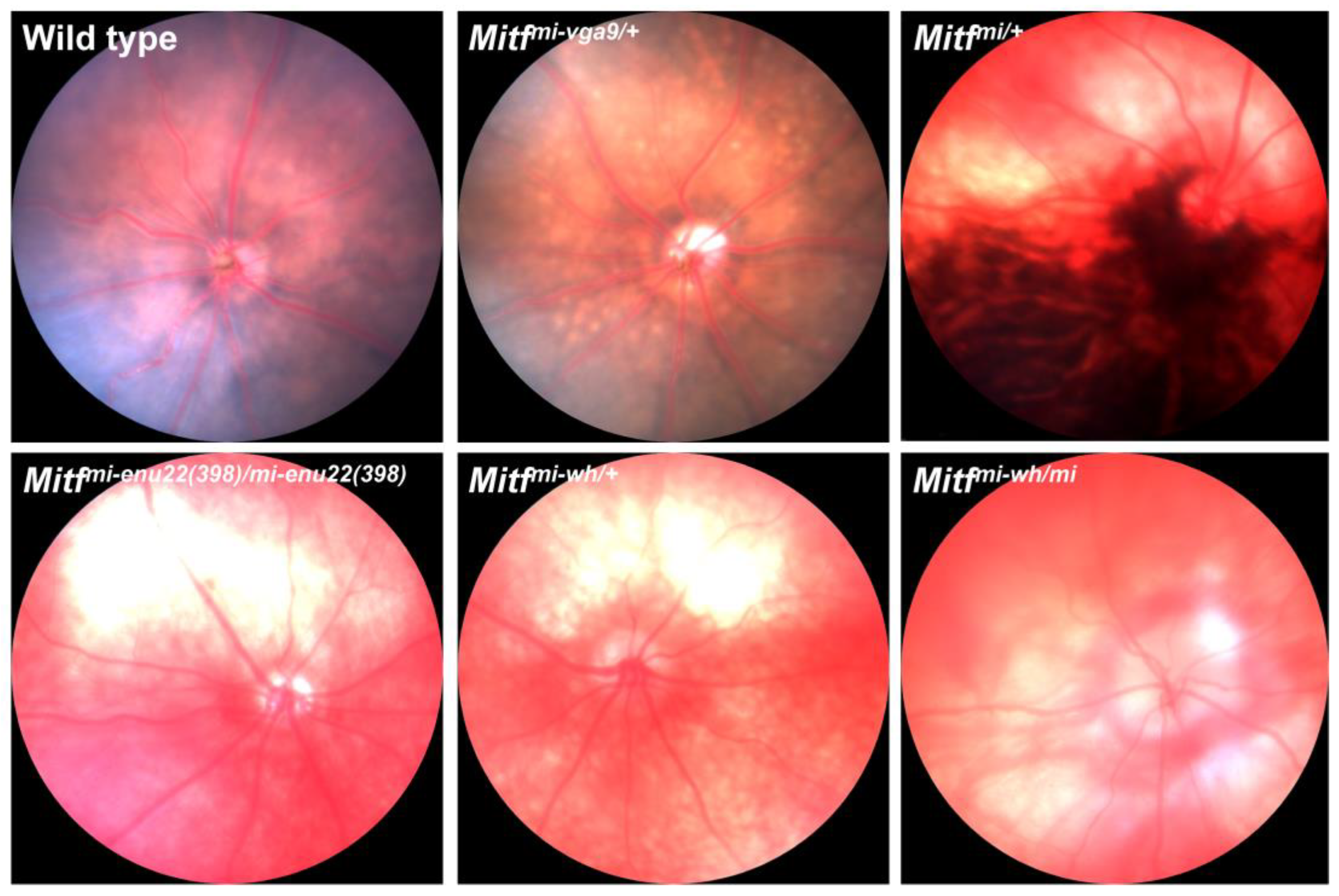
3. Mitf and the Vasculature of the Eye
| Genotype | Phenotype | Source |
|---|---|---|
| Mitfmi | White coat; eyes small and red; osteopetrosis; inner defects; incisors fail to erupt; deficiency of mast cells. | [7] |
| Mitfmi-rw | Colored marks around the neck; eyes small and red. | [75] |
| Mitfmi-bw | White coat with colored spots on rump and head; eyes small and red. | [76] |
| MitfMi-wh | White coat; eyes slightly pigmented and small. | [19] |
| Mitfmi-vga9 | White coat; eyes small and red. | [7] |
| Mitfmi-vit | Initial markings on the thorax and abdomen; gradual loss of pigmentation in coat and eye; defective RPE–photoreceptor interactions. | [77,78,79] |
| MitfMi-or | White coat; eyes small and red; osteopetrosis; incisors fail to erupt. | [80,81] |
| MitfMi-H | White coat; eyelids are closed at birth. | [82] |
| MitfMi-b | White coat; reduced eye pigmentation. | [83] |
4. Mitf Mutations and Microphthalmia
5. Mitf and Postnatal Retinal Degeneration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hertwig, P. Neue mutationen und koppelungsgruppen bei der hausmaus. Z. Indukt. Abstamm. Vererbungslehre 1942, 80, 220–246. [Google Scholar] [CrossRef]
- Gruneberg, H. Some observations on the microphthalmia gene in the mouse. J. Genet. 1948, 49, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Hara, Y.; Vyas, D.; Hodgkinson, C.; Fex, J.; Grundfast, K.; Arnheiter, H. Cochlear disorder associated with melanocyte anomaly in mice with a transgenic insertional mutation. Mol. Cell Neurosci. 1992, 3, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Hemesath, T.J.; Steingrimsson, E.; McGill, G.; Hansen, M.J.; Vaught, J.; Hodgkinson, C.A.; Arnheiter, H.; Copeland, N.G.; Jenkins, N.A.; Fisher, D.E. Microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes. Dev. 1994, 8, 2770–2780. [Google Scholar] [CrossRef]
- Steingrimsson, E.; Tessarollo, L.; Pathak, B.; Hou, L.; Arnheiter, H.; Copeland, N.G.; Jenkins, N.A. Mitf and Tfe3, two members of the Mitf-Tfe family of bHLH-Zip transcription factors, have important but functionally redundant roles in osteoclast development. Proc. Natl. Acad. Sci. USA 2002, 99, 4477–4482. [Google Scholar] [CrossRef]
- Goding, C.R. Mitf from neural crest to melanoma: Signal transduction and transcription in the melanocyte lineage. Genes. Dev. 2000, 14, 1712–1728. [Google Scholar] [CrossRef]
- Hodgkinson, C.A.; Moore, K.J.; Nakayama, A.; Steingrímsson, E.; Copeland, N.G.; Jenkins, N.A.; Arnheiter, H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 1993, 74, 395–404. [Google Scholar] [CrossRef]
- Nakayama, A.; Nguyen, M.T.T.; Chen, C.C.; Opdecamp, K.; Hodgkinson, C.A.; Amheiter, H. Mutations in microphthalmia, the mouse homolog of the human deafness gene MITF, affect neuroepithelial and neural crest-derived melanocytes differently. Mech. Dev. 1998, 70, 155–166. [Google Scholar] [CrossRef]
- Arnheiter, H. The discovery of the microphthalmia locus and its gene, Mitf. Pigment. Cell Melanoma Res. 2010, 23, 729–735. [Google Scholar] [CrossRef]
- Lu, S.Y.; Li, M.; Lin, Y.L. Mitf regulates osteoclastogenesis by modulating NFATc1 activity. Exp. Cell Res. 2014, 328, 32–43. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Chen, Y.; Yang, J.; Chen, H.; Arnheiter, H.; Hou, L. The transcription factor MITF in RPE function and dysfunction. Prog. Retin. Eye Res. 2019, 73, 100766. [Google Scholar] [CrossRef] [PubMed]
- Tshori, S.; Gilon, D.; Beeri, R.; Nechushtan, H.; Kaluzhny, D.; Pikarsky, E.; Razin, E. Transcription factor MITF regulates cardiac growth and hypertrophy. J. Clin. Invest. 2006, 116, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Bharti, K.; Nguyen, M.T.; Skuntz, S.; Bertuzzi, S.; Arnheiter, H. The other pigment cell: Specification and development of the pigmented epithelium of the vertebrate eye. Pigment. Cell Res. 2006, 19, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Bumsted, K.M.; Barnstable, C.J. Dorsal retinal pigment epithelium differentiates as neural retina in the microphthalmia (mi/mi) mouse. Invest. Ophthalmol. Vis. Sci. 2000, 41, 903–908. [Google Scholar]
- Nguyen, M.; Arnheiter, H. Signaling and transcriptional regulation in early mammalian eye development: A link between FGF and MITF. Development 2000, 127, 3581–3591. [Google Scholar] [CrossRef]
- García-Llorca, A.; Aspelund, S.G.; Ogmundsdottir, M.H.; Steingrimsson, E.; Eysteinsson, T. The microphthalmia-associated transcription factor (Mitf) gene and its role in regulating eye function. Sci. Rep. 2019, 9, 15386. [Google Scholar] [CrossRef]
- García-Llorca, A.; Ólafsson, K.H.; Sigurdsson, A.T.; Eysteinsson, T. Progressive Cone-Rod Dystrophy and RPE Dysfunction in Mitf(mi/+) Mice. Genes 2023, 14, 1458. [Google Scholar] [CrossRef]
- Steingrímsson, E.; Copeland, N.G.; Jenkins, N.A. Melanocytes and the microphthalmia transcription factor network. Annu. Rev. Genet. 2004, 38, 365–411. [Google Scholar] [CrossRef]
- Steingrímsson, E.; Moore, K.J.; Lamoreux, M.L.; Ferré-D’Amaré, A.R.; Burley, S.K.; Zimring, D.C.; Skow, L.C.; Hodgkinson, C.A.; Arnheiter, H.; Copeland, N.G.; et al. Molecular basis of mouse microphthalmia (mi) mutations helps explain their developmental and phenotypic consequences. Nat. Genet. 1994, 8, 256–263. [Google Scholar] [CrossRef]
- Yasumoto, K.; Amae, S.; Udono, T.; Fuse, N.; Takeda, K.; Shibahara, S. A big gene linked to small eyes encodes multiple Mitf isoforms: Many promoters make light work. Pigment. Cell Res. 1998, 11, 329–336. [Google Scholar] [CrossRef]
- Read, A.P.; Newton, V.E. Waardenburg syndrome. J. Med. Genet. 1997, 34, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Amiel, J.; Watkin, P.M.; Tassabehji, M.; Read, A.P.; Winter, R.M. Mutation of the MITF gene in albinism-deafness syndrome (Tietz syndrome). Clin. Dysmorphol. 1998, 7, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.; Kelley, P.M.; Kenyon, J.B.; Hoover, D. Tietz syndrome (hypopigmentation/deafness) caused by mutation of MITF. J. Med. Genet. 2000, 37, 446–448. [Google Scholar] [CrossRef]
- George, A.; Zand, D.J.; Hufnagel, R.B.; Sharma, R.; Sergeev, Y.V.; Legare, J.M.; Rice, G.M.; Scott Schwoerer, J.A.; Rius, M.; Tetri, L.; et al. Biallelic Mutations in MITF Cause Coloboma, Osteopetrosis, Microphthalmia, Macrocephaly, Albinism, and Deafness. Am. J. Hum. Genet. 2016, 99, 1388–1394. [Google Scholar] [CrossRef]
- Bertolotto, C.; Lesueur, F.; Giuliano, S.; Strub, T.; de Lichy, M.; Bille, K.; Dessen, P.; d’Hayer, B.; Mohamdi, H.; Remenieras, A.; et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 2011, 480, 94–98. [Google Scholar] [CrossRef]
- Murakami, H.; Arnheiter, H. Sumoylation modulates transcriptional activity of MITF in a promoter-specific manner. Pigment. Cell Res. 2005, 18, 265–277. [Google Scholar] [CrossRef]
- Yokoyama, S.; Woods, S.L.; Boyle, G.M.; Aoude, L.G.; MacGregor, S.; Zismann, V.; Gartside, M.; Cust, A.E.; Haq, R.; Harland, M.; et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature 2011, 480, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, S.; Karl, M.O.; Strauss, O. Ion channels in the RPE. Prog. Retin. Eye Res. 2007, 26, 263–301. [Google Scholar] [CrossRef]
- Weiter, J.J.; Delori, F.C.; Wing, G.L.; Fitch, K.A. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest. Ophthalmol. Vis. Sci. 1986, 27, 145–152. [Google Scholar]
- Cheli, Y.; Ohanna, M.; Ballotti, R.; Bertolotto, C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment. Cell Melanoma Res. 2010, 23, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Shao, J.; Smutko, J.S.; Dussault, B.J.; Nagle, D.L.; Woolf, E.A.; Holmgren, L.M.; Moore, K.J.; Shyjan, A.W. Chromosomal localization and genomic characterization of the mouse melastatin gene (Mlsn1). Genomics 1998, 54, 116–123. [Google Scholar] [CrossRef]
- Lu, S.; Slominski, A.; Yang, S.E.; Sheehan, C.; Ross, J.; Carlson, J.A. The correlation of TRPM1 (Melastatin) mRNA expression with microphthalmia-associated transcription factor (MITF) and other melanogenesis-related proteins in normal and pathological skin, hair follicles and melanocytic nevi. J. Cutan. Pathol. 2010, 37, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Krizaj, D.; Cordeiro, S.; Strauss, O. Retinal TRP channels: Cell-type-specific regulators of retinal homeostasis and multimodal integration. Prog. Retin. Eye Res. 2023, 92, 101114. [Google Scholar] [CrossRef] [PubMed]
- Gomez, N.M.; Lu, W.; Lim, J.C.; Kiselyov, K.; Campagno, K.E.; Grishchuk, Y.; Slaugenhaupt, S.A.; Pfeffer, B.A.; Fliesler, S.J.; Mitchell, C.H. Robust lysosomal calcium signaling through channel TRPML1 is impaired by lysosomal lipid accumulation. FASEB J. 2018, 32, 782–794. [Google Scholar] [CrossRef]
- Beckel, J.M.; Gomez, N.M.; Lu, W.; Campagno, K.E.; Nabet, B.; Albalawi, F.; Lim, J.C.; Boesze-Battaglia, K.; Mitchell, C.H. Stimulation of TLR3 triggers release of lysosomal ATP in astrocytes and epithelial cells that requires TRPML1 channels. Sci. Rep. 2018, 8, 5726. [Google Scholar] [CrossRef]
- Adijanto, J.; Castorino, J.J.; Wang, Z.X.; Maminishkis, A.; Grunwald, G.B.; Philp, N.J. Microphthalmia-associated transcription factor (MITF) promotes differentiation of human retinal pigment epithelium (RPE) by regulating microRNAs-204/211 expression. J. Biol. Chem. 2012, 287, 20491–20503. [Google Scholar] [CrossRef]
- Du, S.W.; Komirisetty, R.; Lewandowski, D.; Choi, E.H.; Panas, D.; Suh, S.; Tabaka, M.; Radu, R.A.; Palczewski, K. Conditional deletion of miR-204 and miR-211 in murine retinal pigment epithelium results in retinal degeneration. J. Biol. Chem. 2024, 300, 107344. [Google Scholar] [CrossRef]
- Gilliam, J.C.; Wensel, T.G. TRP channel gene expression in the mouse retina. Vis. Vision. Res. 2011, 51, 2440–2452. [Google Scholar] [CrossRef]
- Morgans, C.W.; Zhang, J.; Jeffrey, B.G.; Nelson, S.M.; Burke, N.S.; Duvoisin, R.M.; Brown, R.L. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc. Natl. Acad. Sci. USA 2009, 106, 19174–19178. [Google Scholar] [CrossRef]
- Shen, Y.; Heimel, J.A.; Kamermans, M.; Peachey, N.S.; Gregg, R.G.; Nawy, S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J. Neurosci. 2009, 29, 6088–6093. [Google Scholar] [CrossRef]
- Xu, Y.; Dhingra, A.; Fina, M.E.; Koike, C.; Furukawa, T.; Vardi, N. MGluR6 deletion renders the TRPM1 channel in retina inactive. J. Neurophysiol. 2012, 107, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.M.; Mohr, F.; Behrendt, M.; Oberwinkler, J. Properties and functions of TRPM1 channels in the dendritic tips of retinal ON-bipolar cells. Eur. J. Cell Biol. 2015, 94, 420–427. [Google Scholar] [CrossRef]
- Peachey, N.S.; Pearring, J.N.; Bojang, P., Jr.; Hirschtritt, M.E.; Sturgill-Short, G.; Ray, T.A.; Furukawa, T.; Koike, C.; Goldberg, A.F.; Shen, Y.; et al. Depolarizing bipolar cell dysfunction due to a Trpm1 point mutation. J. Neurophysiol. 2012, 108, 2442–2451. [Google Scholar] [CrossRef][Green Version]
- Audo, I.; Kohl, S.; Leroy, B.P.; Munier, F.L.; Guillonneau, X.; Mohand-Said, S.; Bujakowska, K.; Nandrot, E.F.; Lorenz, B.; Preising, M.; et al. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am. J. Hum. Genet. 2009, 85, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Esumi, N.; Kachi, S.; Campochiaro, P.A.; Zack, D.J. VMD2 promoter requires two proximal E-box sites for its activity in vivo and is regulated by the MITF-TFE family. J. Biol. Chem. 2007, 282, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Esumi, N.; Kachi, S.; Hackler, L., Jr.; Masuda, T.; Yang, Z.; Campochiaro, P.A.; Zack, D.J. BEST1 expression in the retinal pigment epithelium is modulated by OTX family members. Hum. Mol. Genet. 2009, 18, 128–141. [Google Scholar] [CrossRef]
- Masuda, T.; Esumi, N. SOX9, through interaction with microphthalmia-associated transcription factor (MITF) and OTX2, regulates BEST1 expression in the retinal pigment epithelium. J. Biol. Chem. 2010, 285, 26933–26944. [Google Scholar] [CrossRef] [PubMed]
- Marmorstein, A.D.; Marmorstein, L.Y.; Rayborn, M.; Wang, X.; Hollyfield, J.G.; Petrukhin, K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2000, 97, 12758–12763. [Google Scholar] [CrossRef]
- Martínez-Morales, J.R.; Dolez, V.; Rodrigo, I.; Zaccarini, R.; Leconte, L.; Bovolenta, P.; Saule, S. OTX2 activates the molecular network underlying retina pigment epithelium differentiation. J. Biol. Chem. 2003, 278, 21721–21731. [Google Scholar] [CrossRef]
- Poche, R.A.; Furuta, Y.; Chaboissier, M.C.; Schedl, A.; Behringer, R.R. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Muller glial cell development. J. Comp. Neurol. 2008, 510, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tsunenari, T.; Yau, K.W.; Nathans, J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc. Natl. Acad. Sci. USA 2002, 99, 4008–4013. [Google Scholar] [CrossRef]
- Rosenthal, R.; Bakall, B.; Kinnick, T.; Peachey, N.; Wimmers, S.; Wadelius, C.; Marmorstein, A.; Strauss, O. Expression of bestrophin-1, the product of the VMD2 gene, modulates voltage-dependent Ca2+ channels in retinal pigment epithelial cells. FASEB J. 2006, 20, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.A.; Guziewicz, K.E.; Lee, C.J.; Kalathur, R.C.; Pulido, J.S.; Marmorstein, L.Y.; Marmorstein, A.D. Bestrophin 1 and retinal disease. Prog. Retin. Eye Res. 2017, 58, 45–69. [Google Scholar] [CrossRef]
- Marmorstein, A.D.; Kinnick, T.R.; Stanton, J.B.; Johnson, A.A.; Lynch, R.M.; Marmorstein, L.Y. Bestrophin-1 influences transepithelial electrical properties and Ca2+ signaling in human retinal pigment epithelium. Mol. Vis. 2015, 21, 347–359. [Google Scholar]
- Marmorstein, L.Y.; Wu, J.; McLaughlin, P.; Yocom, J.; Karl, M.O.; Neussert, R.; Wimmers, S.; Stanton, J.B.; Gregg, R.G.; Strauss, O.; et al. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (best-1). J. Gen. Physiol. 2006, 127, 577–589. [Google Scholar] [CrossRef]
- Chowers, I.; Tiosano, L.; Audo, I.; Grunin, M.; Boon, C.J. Adult-onset foveomacular vitelliform dystrophy: A fresh perspective. Prog. Retin. Eye Res. 2015, 47, 64–85. [Google Scholar] [CrossRef] [PubMed]
- Moller, A.; Eysteinsson, T.; Steingrimsson, E. Electroretinographic assessment of retinal function in microphthalmia mutant mice. Exp. Eye Res. 2004, 78, 837–848. [Google Scholar] [CrossRef]
- Saint-Geniez, M.; Kurihara, T.; Sekiyama, E.; Maldonado, A.E.; D’Amore, P.A. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. USA 2009, 106, 18751–18756. [Google Scholar] [CrossRef]
- Sakagami, K.; Kodama, T.; Puro, D.G. PDGF-induced coupling of function with metabolism in microvascular pericytes of the retina. Invest. Ophthalmol. Vis. Sci. 2001, 42, 1939–1944. [Google Scholar]
- Rousseau, B.; Larrieu-Lahargue, F.; Bikfalvi, A.; Javerzat, S. Involvement of fibroblast growth factors in choroidal angiogenesis and retinal vascularization. Exp. Eye Res. 2003, 77, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Schlunck, G.; Hansen, L.L.; Agostini, H.T. Differential expression of angioregulatory factors in normal and CNV-derived human retinal pigment epithelium. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Murugeswari, P.; Subramani, M.; Jayadev, C.; Shetty, R.; Das, D. Retinal pigment epithelium-secretome: A diabetic retinopathy perspective. Cytokine 2017, 95, 126–135. [Google Scholar] [CrossRef]
- Ma, X.; Pan, L.; Jin, X.; Dai, X.; Li, H.; Wen, B.; Chen, Y.; Ma, A.; Qu, J.; Hou, L. Microphthalmia-associated transcription factor acts through PEDF to regulate RPE cell migration. Exp. Cell Res. 2012, 318, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Saint-Geniez, M.; Maldonado, A.E.; D’Amore, P.A. VEGF expression and receptor activation in the choroid during development and in the adult. Invest. Ophthalmol. Vis. Sci. 2006, 47, 3135–3142. [Google Scholar] [CrossRef]
- Ford, K.M.; D’Amore, P.A. Molecular regulation of vascular endothelial growth factor expression in the retinal pigment epithelium. Mol. Vis. 2012, 18, 519–527. [Google Scholar]
- Marneros, A.G.; Fan, J.; Yokoyama, Y.; Gerber, H.P.; Ferrara, N.; Crouch, R.K.; Olsen, B.R. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am. J. Pathol. 2005, 167, 1451–1459. [Google Scholar] [CrossRef]
- Shibuya, H.; Watanabe, R.; Maeno, A.; Ichimura, K.; Tamura, M.; Wakana, S.; Shiroishi, T.; Ohba, K.; Takeda, K.; Tomita, H.; et al. Melanocytes contribute to the vasculature of the choroid. Genes. Genet. Syst. 2018, 93, 51–58. [Google Scholar] [CrossRef]
- Becerra, S.P.; Fariss, R.N.; Wu, Y.Q.; Montuenga, L.M.; Wong, P.; Pfeffer, B.A. Pigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: Apical secretion and distribution. Exp. Eye Res. 2004, 78, 223–234. [Google Scholar] [CrossRef]
- Karakousis, P.C.; John, S.K.; Behling, K.C.; Surace, E.M.; Smith, J.E.; Hendrickson, A.; Tang, W.X.; Bennett, J.; Milam, A.H. Localization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissues. Mol. Vis. 2001, 7, 154–163. [Google Scholar]
- Huang, Q.; Wang, S.; Sorenson, C.M.; Sheibani, N. PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration. Exp. Eye Res. 2008, 87, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Geng, H.; Li, L.; Li, J.; Cheng, B.; Ma, X.; Li, H.; Hou, L. Photoreceptor degeneration in microphthalmia (Mitf) mice: Partial rescue by pigment epithelium-derived factor. Dis. Model. Mech. 2019, 12, dmm035642. [Google Scholar] [CrossRef] [PubMed]
- Au—García-Llorca, A.; Au—Reynisson, H.; Au—Eysteinsson, T. Measuring Retinal Vessel Diameter from Mouse Fluorescent Angiography Images. J. Vis. Exp. 2023, 195, e64964. [Google Scholar] [CrossRef]
- Danielsson, S.B.; Garcia-Llorca, A.; Reynisson, H.; Eysteinsson, T. Mouse microphthalmia-associated transcription factor (Mitf) mutations affect the structure of the retinal vasculature. Acta Ophthalmol. 2022, 100, 911–918. [Google Scholar] [CrossRef]
- Bharti, K.; Liu, W.; Csermely, T.; Bertuzzi, S.; Arnheiter, H. Alternative promoter use in eye development: The complex role and regulation of the transcription factor MITF. Development 2008, 135, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Steingrímsson, E.; Arnheiter, H.; Hallsson, J.H.; Lamoreux, M.L.; Copeland, N.G.; Jenkins, N.A. Interallelic complementation at the mouse Mitf locus. Genetics 2003, 163, 267–276. [Google Scholar] [CrossRef]
- Lerner, A.B.; Shiohara, T.; Boissy, R.E.; Jacobson, K.A.; Lamoreux, M.L.; Moellmann, G.E. A mouse model for vitiligo. J. Invest. Dermatol. 1986, 87, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B. C57BL/6J-vit/vit mouse model of retinal degeneration: Light microscopic analysis and evaluation of rhodopsin levels. Exp. Eye Res. 1992, 55, 903–910. [Google Scholar] [CrossRef]
- Smith, S.B.; Hamasaki, D.I. Electroretinographic study of the C57BL/6-mivit/mivit mouse model of retinal degeneration. Invest. Ophthalmol. Vis. Sci. 1994, 35, 3119–3123. [Google Scholar]
- Nii, A.; Steingrímsson, E.; Copeland, N.G.; Jenkins, N.A.; Ward, J.M. Mild osteopetrosis in the microphthalmia-oak ridge mouse. A model for intermediate autosomal recessive osteopetrosis in humans. Am. J. Pathol. 1995, 147, 1871–1882. [Google Scholar]
- Sharma, S.M.; Sif, S.; Ostrowski, M.C.; Sankar, U. Defective co-activator recruitment in osteoclasts from microphthalmia-oak ridge mutant mice. J. Cell Physiol. 2009, 220, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Thaung, C.; West, K.; Clark, B.J.; McKie, L.; Morgan, J.E.; Arnold, K.; Nolan, P.M.; Peters, J.; Hunter, A.J.; Brown, S.D.; et al. Novel ENU-induced eye mutations in the mouse: Models for human eye disease. Hum. Mol. Genet. 2002, 11, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Steingrímsson, E.; Nii, A.; Fisher, D.E.; Ferré-D’Amaré, A.R.; McCormick, R.J.; Russell, L.B.; Burley, S.K.; Ward, J.M.; Jenkins, N.A.; Copeland, N.G. The semidominant Mi(b) mutation identifies a role for the HLH domain in DNA binding in addition to its role in protein dimerization. Embo J. 1996, 15, 6280–6289. [Google Scholar] [CrossRef] [PubMed]
- Bumsted, K.M.; Rizzolo, L.J.; Barnstable, C.J. Defects in the MITF (mi/mi) apical surface are associated with a failure of outer segment elongation. Exp. Eye Res. 2001, 73, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Konyukhov, B.V.; Sazhina, M.V. Interaction of the genes of ocular retardation and microphthalmia in mice. Folia Biol. 1966, 12, 116–123. [Google Scholar]
- Konyukhov, B.V.; Osipov, V.V. Interallelic complementation of microphthalmia and white genes in mice. Genetika 1968, 4, 65–76. [Google Scholar]
- Steingrimsson, E. Interpretation of complex phenotypes: Lessons from the Mitf gene. Pigment. Cell Melanoma Res. 2010, 23, 736–740. [Google Scholar] [CrossRef]
- Justice, M.J.; Noveroske, J.K.; Weber, J.S.; Zheng, B.; Bradley, A. Mouse ENU mutagenesis. Hum. Mol. Genet. 1999, 8, 1955–1963. [Google Scholar] [CrossRef]
- Hansdottir, A.G.; Palsdottir, K.; Favor, J.; Neuhauser-Klaus, A.; Fuchs, H.; de Angelis, M.H.; Steingrimsson, E. The novel mouse microphthalmia mutations Mitfmi-enu5 and Mitfmi-bcc2 produce dominant negative Mitf proteins. Genomics 2004, 83, 932–935. [Google Scholar] [CrossRef]
- Hara, Y.; Battey, J.; Gainer, H. Structure of mouse vasopressin and oxytocin genes. Brain Res. Mol. Brain Res. 1990, 8, 319–324. [Google Scholar] [CrossRef]
- Tang, M.; Pawlyk, B.S.; Kosaras, B.; Berson, E.L.; Sidman, R.L. ERG abnormalities in relation to histopathologic findings in vitiligo mutant mice. Exp. Eye Res. 1997, 65, 215–222. [Google Scholar] [CrossRef]
- Boissy, R.E.; Moellmann, G.E.; Lerner, A.B. Morphology of melanocytes in hair bulbs and eyes of vitiligo mice. Am. J. Pathol. 1987, 127, 380–388. [Google Scholar] [PubMed]
- Sidman, R.L.; Kosaras, B.; Tang, M. Pigment epithelial and retinal phenotypes in the vitiligo mivit, mutant mouse. Invest. Ophthalmol. Vis. Sci. 1996, 37, 1097–1115. [Google Scholar] [PubMed]
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal degeneration mutants in the mouse. Vis. Vision. Res. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Cope, B.K.; McCoy, J.R.; McCool, D.J.; Defoe, D.M. Reduction of phagosomes in the vitiligo (C57BL/6-mivit/mivit) mouse model of retinal degeneration. Invest. Ophthalmol. Vis. Sci. 1994, 35, 3625–3632. [Google Scholar]
- Nir, I.; Ransom, N.; Smith, S.B. Ultrastructural features of retinal dystrophy in mutant vitiligo mice. Exp. Eye Res. 1995, 61, 363–377. [Google Scholar] [CrossRef]
- Moore, K.J. Insight into the Microphthalmia Gene. Trends Genet. 1995, 11, 442–448. [Google Scholar] [CrossRef]
- Wolfe, H.G. New allele at the mi locus. Mouse News Let. 1962, 26, 35. [Google Scholar]
- Wolfe, H.G.; Coleman, D.l. Mi-spotted: A mutation in the mouse. Genet. Res. Camb. 1964, 5, 432–440. [Google Scholar] [CrossRef]
- Bertolotto, C.; Buscà, R.; Abbe, P.; Bille, K.; Aberdam, E.; Ortonne, J.P.; Ballotti, R. Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: Pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol. Cell Biol. 1998, 18, 694–702. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, H.; Chen, H.; Mei, L.; He, C.; Jiang, L.; Li, J.D.; Feng, Y. Functional analysis of MITF gene mutations associated with Waardenburg syndrome type 2. FEBS Lett. 2012, 586, 4126–4131. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hao, Z.; Luo, H.; He, C.; Mei, L.; Liu, Y.; Wang, X.; Niu, Z.; Chen, H.; Li, J.D.; et al. Functional analysis of a nonstop mutation in MITF gene identified in a patient with Waardenburg syndrome type 2. J. Hum. Genet. 2017, 62, 703–709. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Llorca, A.; Eysteinsson, T. The Microphthalmia-Associated Transcription Factor (MITF) and Its Role in the Structure and Function of the Eye. Genes 2024, 15, 1258. https://doi.org/10.3390/genes15101258
García-Llorca A, Eysteinsson T. The Microphthalmia-Associated Transcription Factor (MITF) and Its Role in the Structure and Function of the Eye. Genes. 2024; 15(10):1258. https://doi.org/10.3390/genes15101258
Chicago/Turabian StyleGarcía-Llorca, Andrea, and Thor Eysteinsson. 2024. "The Microphthalmia-Associated Transcription Factor (MITF) and Its Role in the Structure and Function of the Eye" Genes 15, no. 10: 1258. https://doi.org/10.3390/genes15101258
APA StyleGarcía-Llorca, A., & Eysteinsson, T. (2024). The Microphthalmia-Associated Transcription Factor (MITF) and Its Role in the Structure and Function of the Eye. Genes, 15(10), 1258. https://doi.org/10.3390/genes15101258





