Toxic Effect of Methyl-Thiophanate on Bombyx mori Based on Physiological and Transcriptomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silkworm Strains and Rearing
2.2. Methyl-Thiophanate Content Determination
2.3. Methyl-Thiophanate Treatment
2.4. Transmission Electron Microscopy (TEM)
2.5. Reactive Oxygen Species (ROS) Assay
2.6. Measurement of Superoxide Dismutase (SOD) Activity, Catalase (CAT) Activity, and Peroxidase (POD) Activity
2.7. RNA Preparation and Sequencing
2.8. Analysis of Transcriptome Data
2.9. Reverse Transcription Quantitative Transcriptase–Polymerase Chain Reaction (RT-qPCR)
2.10. Sequence Alignment
2.11. Motif Analysis
2.12. Statistical Analysis
3. Results
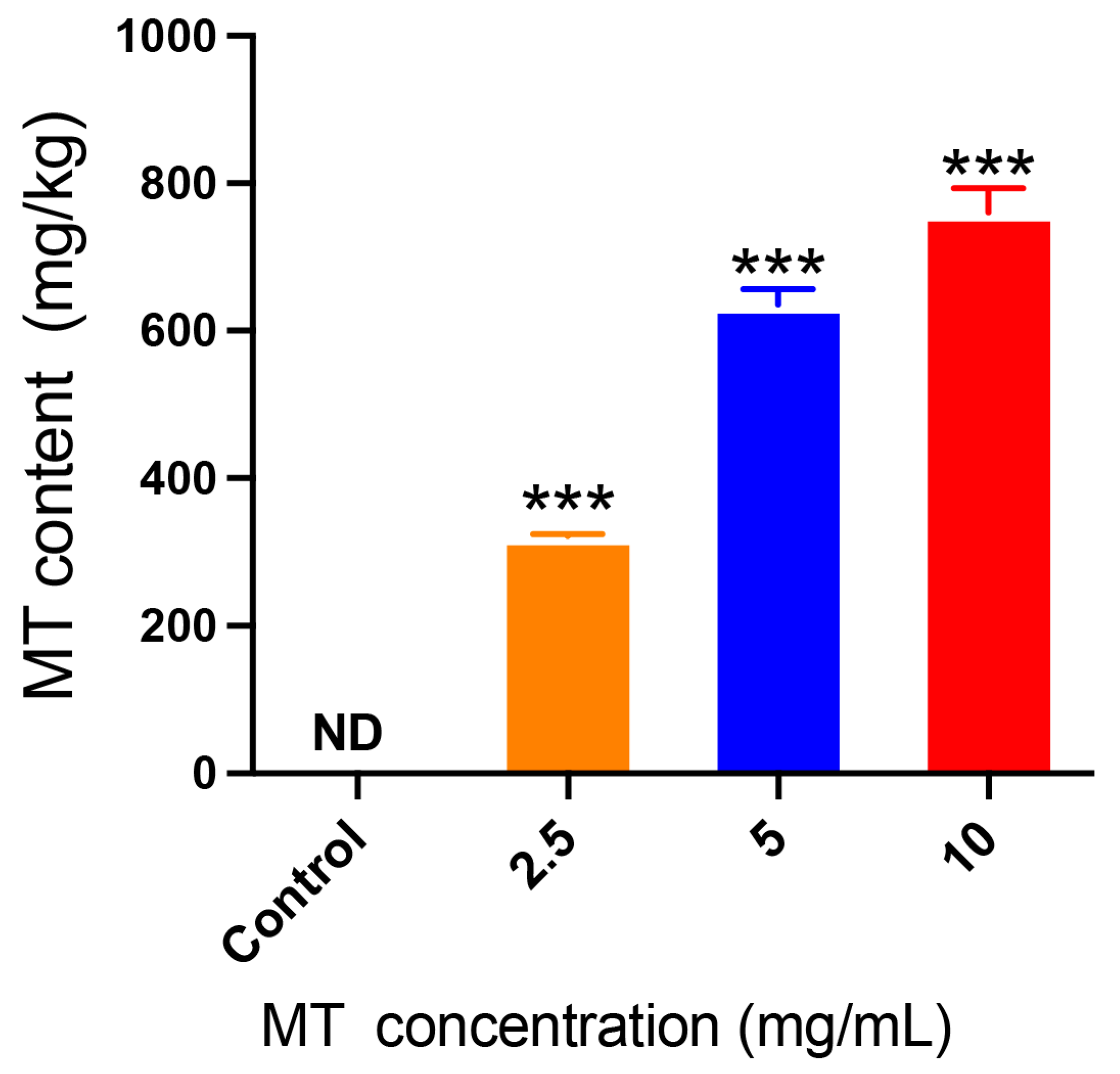
3.1. Content of Methyl-Thiophanate in Mulberry Leaves
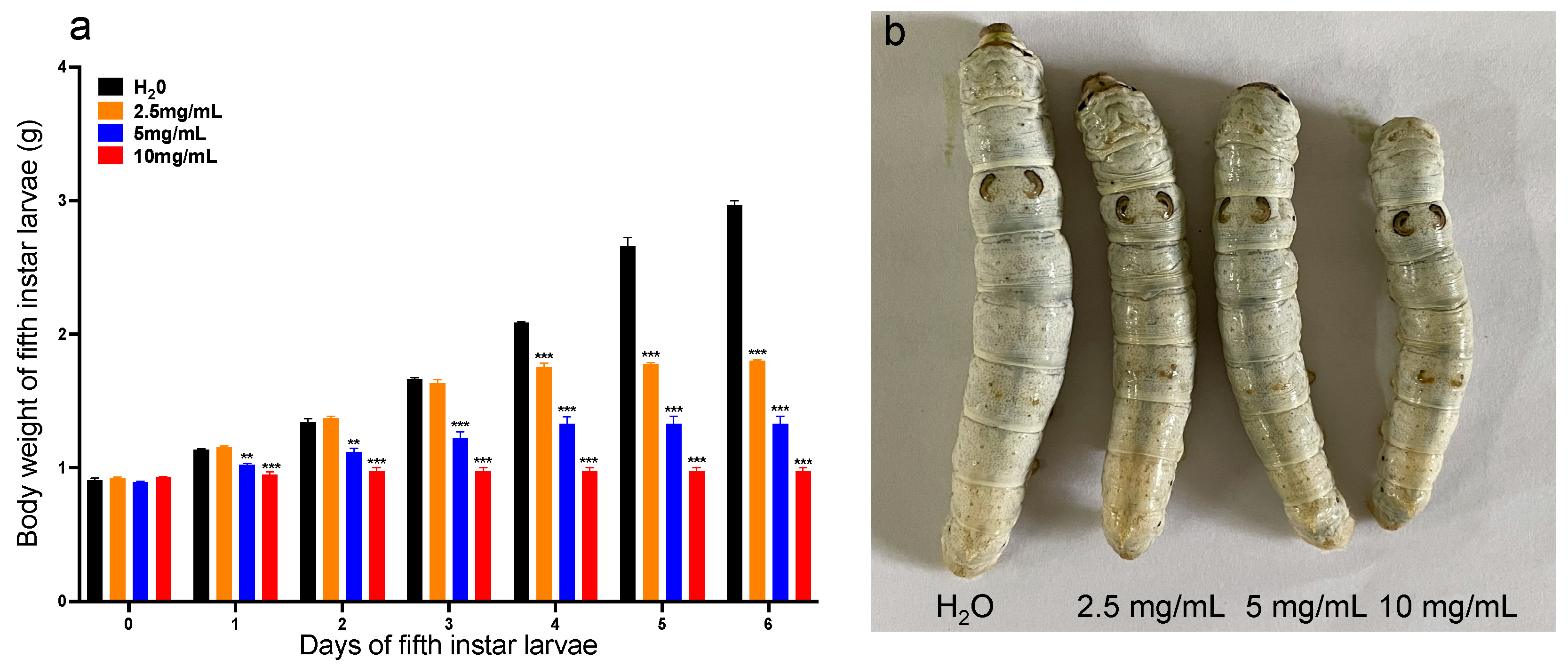
3.2. Effects of Methyl-Thiophanate on Larval Development
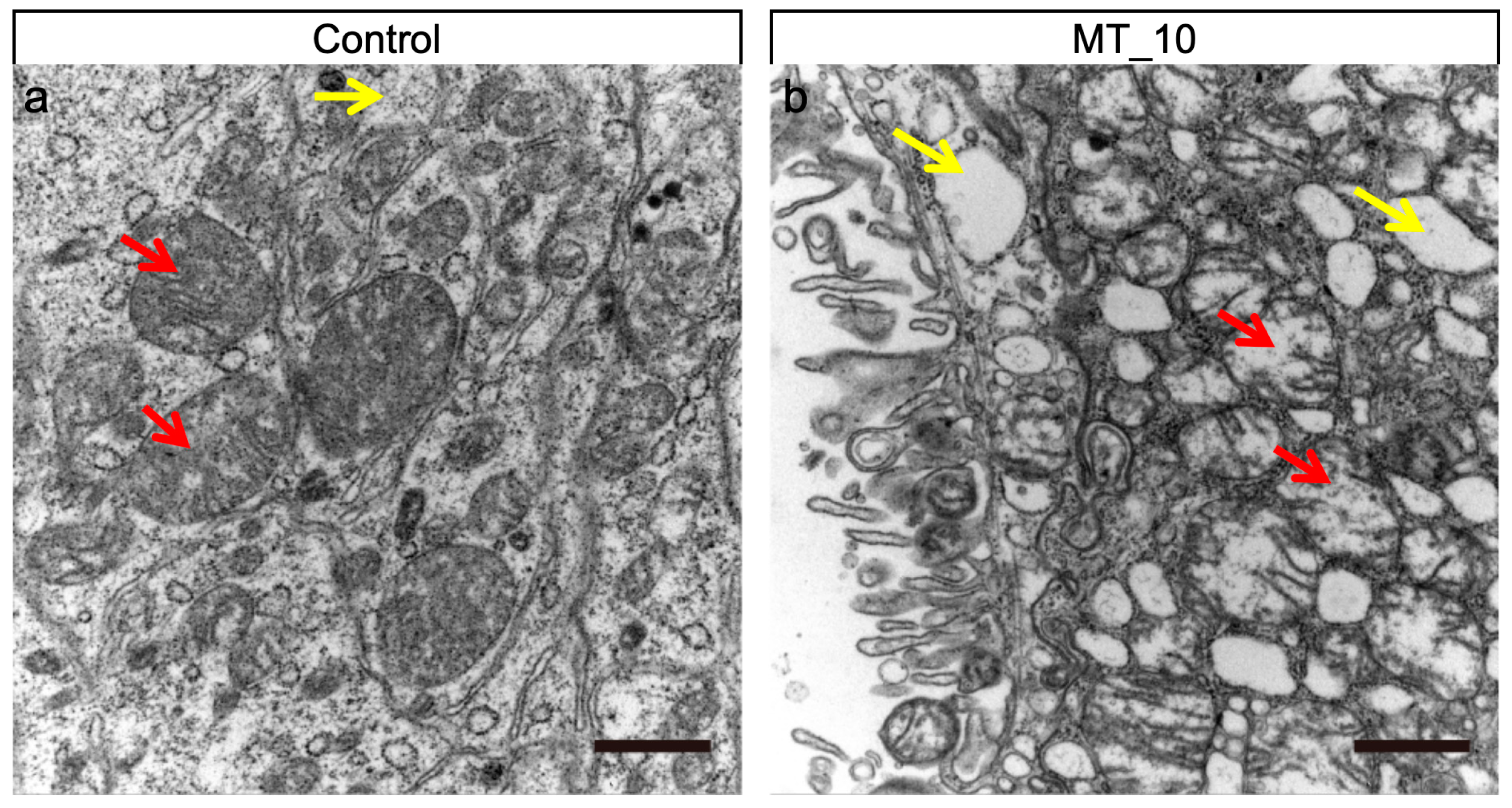
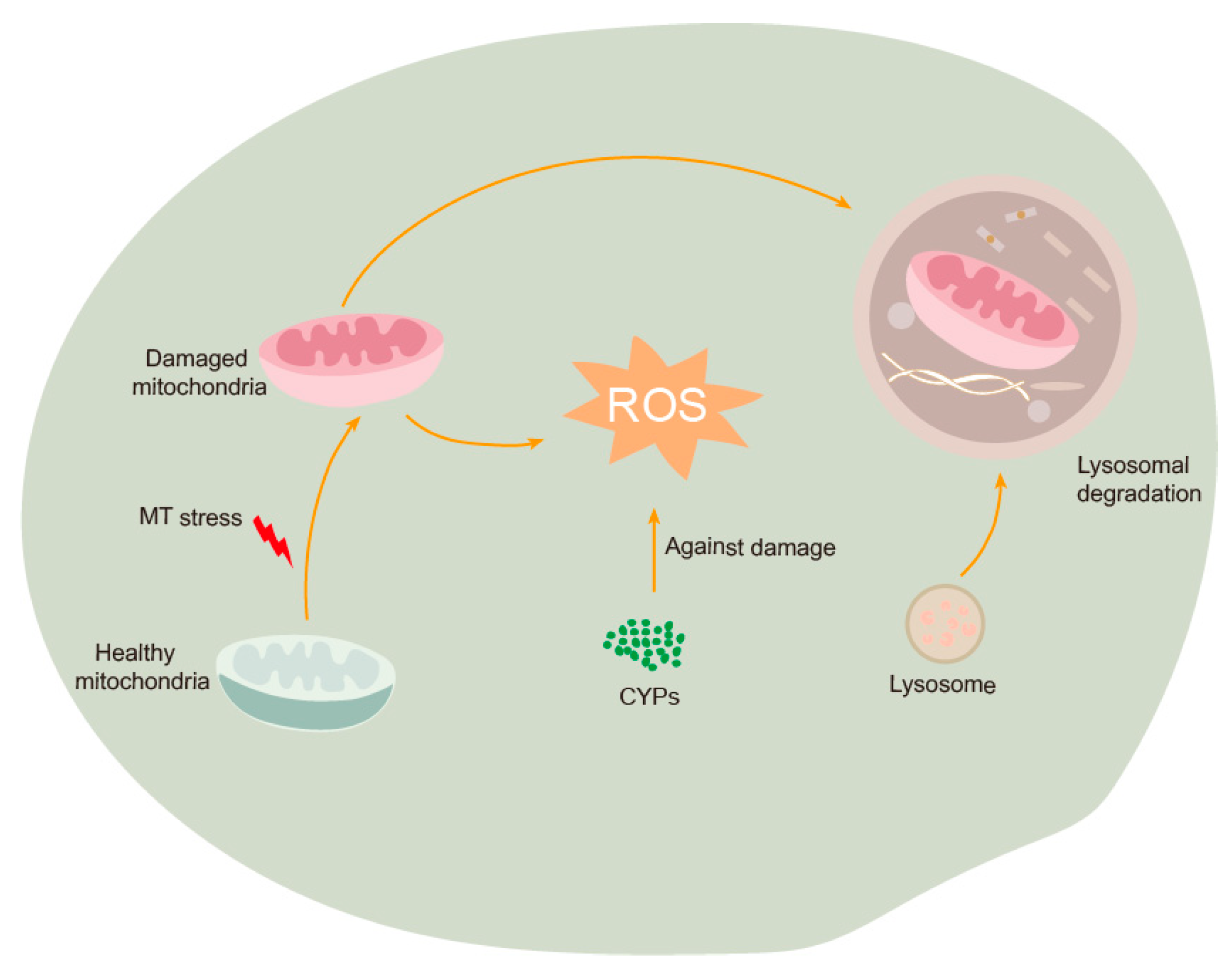
3.3. Methyl-Thiophanate Supplementation Induced Autophagy
3.4. Methyl-Thiophanate Supplementation Activated Larval Oxidative Stress
3.5. Transcriptome Sequence and Assembly
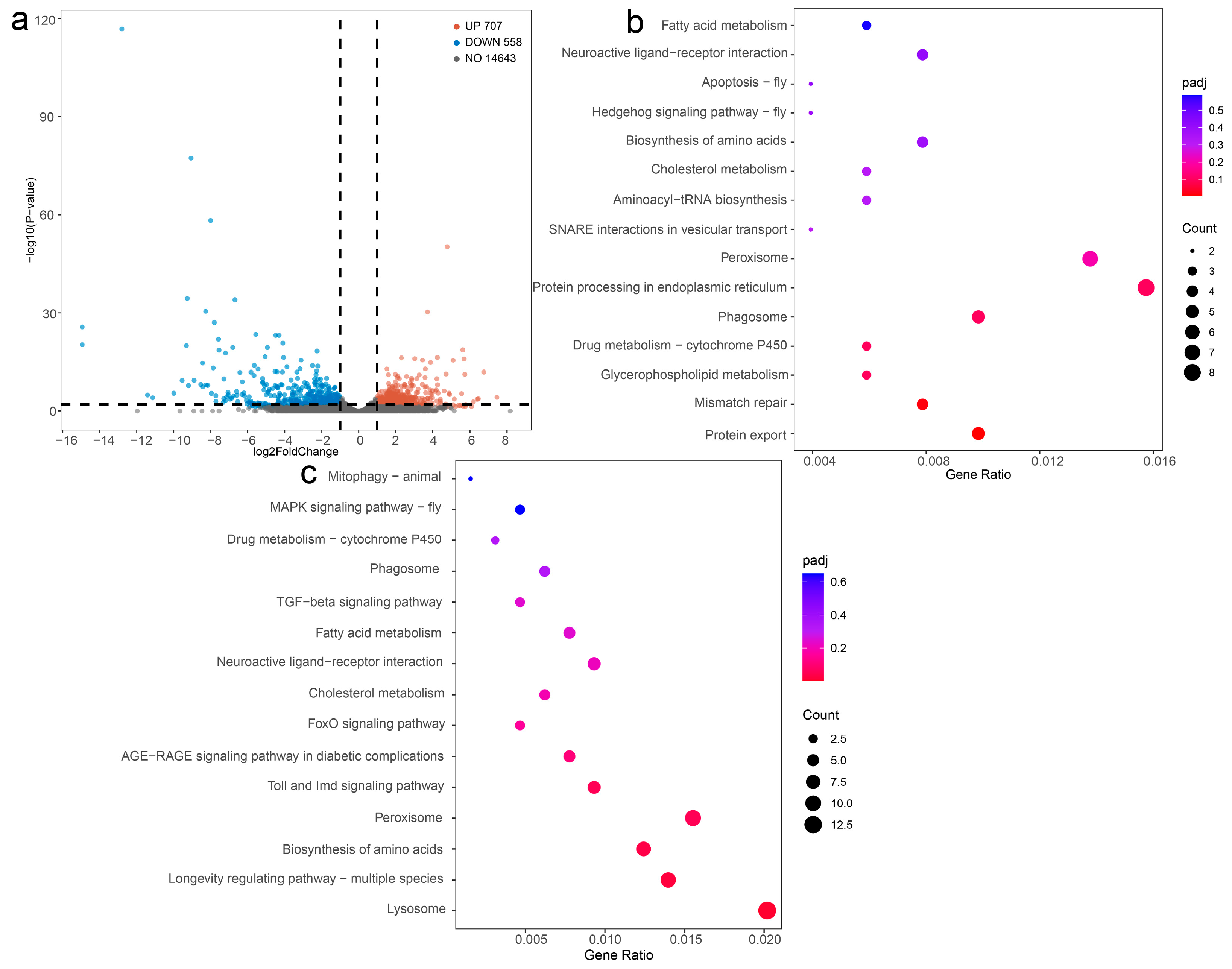
3.6. Identification and Analysis of DEGs
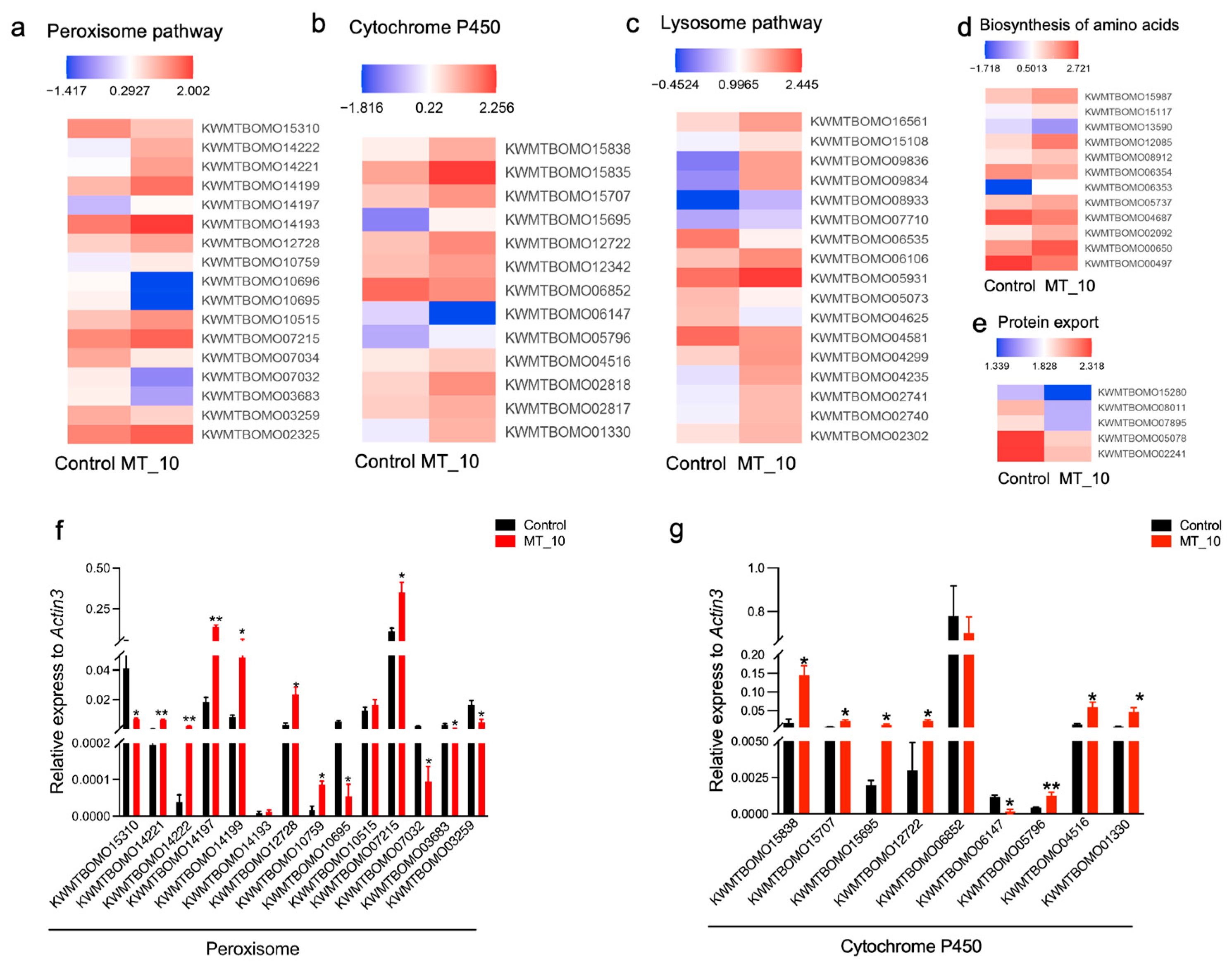
3.7. DEGs Involved in Antioxidation, Detoxification, Metabolism, and Autophagy
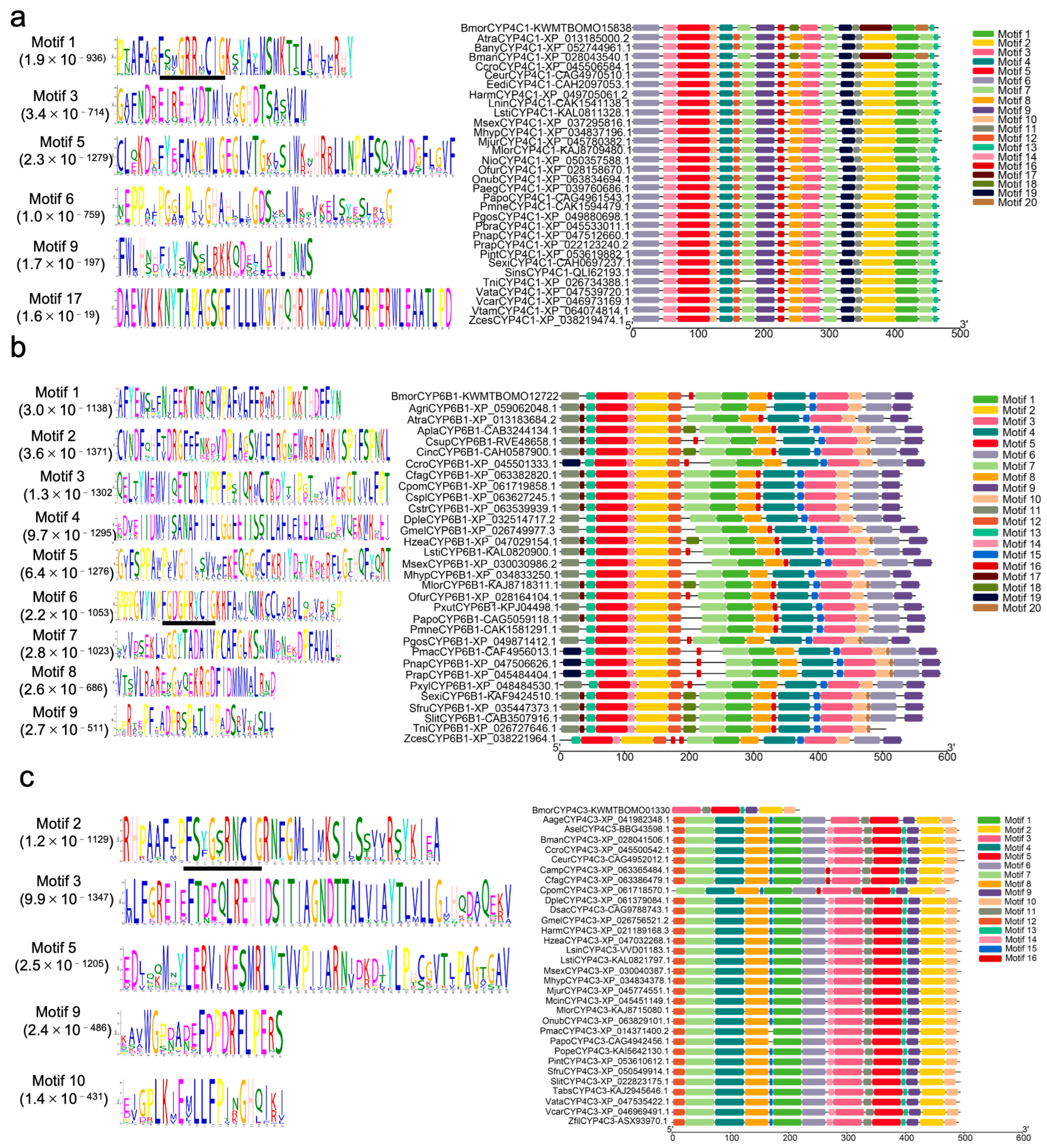
3.8. Motif Analysis of the Cytochrome P450 Proteins and Their Homologous Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.; Fang, Y.; Li, D.C.; Chen, D.S.; Wu, F. Toxicity of procymidone to Bombyx mori based on physiological and transcriptomic analysis. Arch. Insect Biochem. Physiol. 2022, 110, e21906. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ji, L.S.Z.; Jing, X.Y.; Zhao, P.; Xia, Q.Y. MicroRNA let-7 targets BmCDK1 to regulate cell proliferation and endomitosis of silk gland in the silkworm, Bombyx mori. Insect Sci. 2024, 31, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, Z.; Guo, Y.; Xiao, W.; Zhang, Y.; Zhou, A.; Chen, P. The role of BmTMED6 in female reproduction in silkworm, Bombyx mori. Insects 2024, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhu, F.; Chen, K. Silkworm: A promising model organism in life science. J. Insect Sci. 2017, 17, 97. [Google Scholar]
- Lu, N.; Zhang, L.; Tian, Y.; Yang, J.; Zheng, S.; Wang, L.; Guo, W. Biosynthetic pathways and related genes regulation of bioactive ingredients in mulberry leaves. Plant Signal Behav. 2023, 18, 2287881. [Google Scholar] [CrossRef]
- Kim, I.; Lee, J. Variations in Anthocyanin Profiles and Antioxidant Activity of 12 Genotypes of mulberry (Morus spp.) fruits and their changes during processing. Antioxidants 2020, 9, 242. [Google Scholar] [CrossRef]
- Deng, L.; Liu, D.; Zhang, Q.; Luo, J.; Zhong, G. Effect of the mixture of mulberry leaf powder and KGM flour on promoting calcium absorption and bone mineral density in vivo. J. Sci. Food Agric. 2020, 100, 3587–3597. [Google Scholar] [CrossRef]
- Walkowiak-Bródka, A.; Piekuś-Słomka, N.; Wnuk, K.; Kupcewicz, B. Analysis of white mulberry leaves and dietary supplements, ATR-FTIR combined with chemometrics for the rapid determination of 1-Deoxynojirimycin. Nutrients 2022, 14, 5276. [Google Scholar] [CrossRef]
- Sandoval-Insausti, H.; Chiu, Y.H.; Wang, Y.X.; Hart, J.E.; Bhupathiraju, S.N.; Mínguez-Alarcón, L.; Ding, M.; Willett, W.C.; Laden, F.; Chavarro, J.E. Intake of fruits and vegetables according to pesticide residue status in relation to all-cause and disease-specific mortality: Results from three prospective cohort studies. Environ. Int. 2022, 159, 107024. [Google Scholar] [CrossRef] [PubMed]
- Jallow, M.F.A.; Awadh, D.G.; Albaho, M.S.; Devi, V.Y.; Ahmad, N. Monitoring of pesticide residues in commonly used fruits and vegetables in Kuwait. Int. J. Environ. Res. Public Health 2017, 14, 833. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Lee, R.H.; Jeng, J.Y.; Lee, C.W.; Shiea, J. Fast screening of trace multiresidue pesticides on fruit and vegetable surfaces using ambient ionization tandem mass spectrometry. Anal. Chim. Acta 2020, 1102, 63–71. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Ren, C.; Li, J.; Sheng, F.; Hu, Z. In vitro study on the interaction between thiophanate methyl and human serum albumin. J. Photochem. Photobiol. B 2009, 94, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Schoeneberg, A.; Hu, M. Resistance to azoxystrobin and thiophanate-methyl is widespread in Colletotrichum spp. isolates from the mid-Atlantic strawberry fields. Plant Dis. 2021, 105, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wu, J.; Lin, X.; Wang, Y.; Zhu, J.; Chen, C.; Wang, Y.; Jia, H.; Shen, J. Effect of ozonated water, mancozeb, and thiophanate-methyl on the phyllosphere microbial diversity of strawberry. Front. Plant Sci. 2022, 13, 967797. [Google Scholar] [CrossRef]
- Wu, D.; Meng, L.; Yang, L.; Wang, J.; Fu, X.; Du, X.; Li, S.; He, Y.; Huang, L. Feasibility of laser-induced breakdown spectroscopy and hyperspectral imaging for rapid detection of thiophanate-methyl residue on mulberry. Fruit. Int. J. Mol. Sci. 2019, 20, 2017. [Google Scholar] [CrossRef] [PubMed]
- Vieira, W.A.D.S.; Lima, W.G.; Nascimento, E.S.; Michereff, S.J.; Reis, A.; Doyle, V.P.; Câmara, M.P.S. Thiophanate-methyl resistance and fitness components of Colletotrichum musae Isolates from Banana in Brazil. Plant Dis. 2017, 101, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tsuji, S.S.; Li, Y.; Hu, M.; Bandeira, M.A.; Câmara, M.P.S.; Michereff, S.J.; Schnabel, G. Reduced sensitivity of azoxystrobin and thiophanate-methyl resistance in Lasiodiplodia theobromae from papaya. Pestic. Biochem. Physiol. 2020, 162, 60–68. [Google Scholar] [CrossRef]
- Ben Amara, I.; Ben Saad, H.; Cherif, B.; Elwej, A.; Lassoued, S.; Kallel, C.; Zeghal, N. Methyl-thiophanate increases reactive oxygen species production and induces genotoxicity in rat peripheral blood. Toxicol. Mech. Methods 2014, 24, 679–687. [Google Scholar] [CrossRef]
- Jia, K.; Cheng, B.; Huang, L.; Xiao, J.; Bai, Z.; Liao, X.; Cao, Z.; Shen, T.; Zhang, C.; Hu, C.; et al. Thiophanate-methyl induces severe hepatotoxicity in zebrafish. Chemosphere 2020, 248, 125941. [Google Scholar] [CrossRef]
- Che, X.; Huang, Y.; Zhong, K.; Jia, K.; Wei, Y.; Meng, Y.; Yuan, W.; Lu, H. Thiophanate-methyl induces notochord toxicity by activating the PI3K-mTOR pathway in zebrafish (Danio rerio) embryos. Environ. Pollut. 2023, 318, 120861. [Google Scholar] [CrossRef]
- Feki, A.; Kammoun, I.; Eleroui, M.; Kallel, R.; Megdiche, F.; Hariz, L.; Boudawara, T.; Kallel, C.; Kallel, H.; Pujo, J.M.; et al. Bioactivity of Falkenbergia rufolanosa methanolic extract: Assessment of its effect on methyl-thiophanate induced bone and blood disorders. Pharmaceuticals 2023, 16, 529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, L.; Xu, C.; Wang, D.; Yang, G.; Wang, X.; Weng, H.; Wang, Q. Mixture toxicity of thiophanate-methyl and fenvalerate to embryonic zebrafish (Danio rerio) and its underlying mechanism. Sci. Total Environ. 2021, 56, 143754. [Google Scholar] [CrossRef]
- He, Z.; Fang, Y.; Li, D.C.; Chen, D.S.; Wu, F. Effect of lactic acid supplementation on the growth and reproduction of Bombyx mori (Lepidopteria: Bombycidae). J. Insect Sci. 2021, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Fang, Y.; Zhang, F.; Liu, Y.; Cheng, X.; Wang, J.; Li, D.; Chen, D.; Wu, F. Adenine nucleotide translocase 2 (Ant2) is required for individualization of spermatogenesis of Drosophila melanogaster. Insect Sci. 2024, 31, 1055–1072. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, F.; Zhan, Y.; Lu, M.; Xu, D.; Wang, J.; Li, Q.; Zhao, L.; Su, Y. RpS3 Is Required for Spermatogenesis of Drosophila melanogaster. Cells 2023, 12, 573. [Google Scholar] [CrossRef]
- Cao, Z.; Zou, L.; Wang, H.; Zhang, H.; Liao, X.; Xiao, J.; Zhang, S.; Lu, H. Exposure to diclofop-methyl induces immunotoxicity and behavioral abnormalities in zebrafish embryos. Aquat. Toxicol. 2019, 214, 105253. [Google Scholar] [CrossRef]
- Karthi, S.; Sankari, R.; Shivakumar, M.S. Ultraviolet-B light induced oxidative stress: Effects on antioxidant response of Spodoptera litura. J. Photochem. Photobiol. B 2014, 135, 1–6. [Google Scholar] [CrossRef]
- El-Bakry, H.A.; El-Sherif, G.; Rostom, R.M. Therapeutic dose of green tea extract provokes liver damage and exacerbates paracetamol-induced hepatotoxicity in rats through oxidative stress and caspase 3-dependent apoptosis. Biomed. Pharmacother. 2017, 96, 798–811. [Google Scholar] [CrossRef]
- Yan, S.; Wang, J.; Zhu, L.; Chen, A.; Wangm, J. Toxic effects of nitenpyram on antioxidant enzyme system and DNA in zebrafish (Danio rerio) livers. Ecotoxicol. Environ. Saf. 2015, 122, 54–60. [Google Scholar] [CrossRef]
- Dogan, D.; Deveci, H.A.; Nur, G. Manifestations of oxidative stress and liver injury in clothianidin exposed Oncorhynchus mykiss. Toxicol Res (Camb) 2021, 10, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, T.; Wang, J.; Wang, J.; Zhang, C.; Zhu, L. Genotoxicity and oxidative stress induced by the fungicide azoxystrobin in zebrafish (Danio rerio) livers. Pestic. Biochem. Physiol. 2016, 133, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gonis, E.; Fraichard, S.; Chertemps, T.; Hecker, A.; Schwartz, M.; Canon, F.; Neiers, F. Expression patterns of Drosophila melanogaster glutathione transferases. Insects 2022, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.Y.; Zhang, C.Y.; Zhu, F.; Wang, X.P.; Lei, C.L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 2009, 55, 588–592. [Google Scholar] [CrossRef]
- Guo, S.H.; Yu, L.; Liu, Y.M.; Wang, F.F.; Chen, Y.C.; Wang, Y.; Qiu, B.L.; Sang, W. Digital gene expression profiling in larvae of Tribolium castaneum at different periods post UV-B exposure. Ecotoxicol. Environ. Saf. 2019, 174, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Meng, J.Y.; Yao, M.S.; Zhang, C.Y. Transcriptome analysis of Myzus persicae to UV-B Stress. J. Insect Sci. 2021, 21, 7. [Google Scholar] [CrossRef]
- Wan, L.; Zhou, A.; Xiao, W.; Zou, B.; Jiang, Y.; Xiao, J.; Deng, C.; Zhang, Y. Cytochrome P450 monooxygenase genes in the wild silkworm, Bombyx mandarina. PeerJ 2021, 9, e10818. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, F.; Zhu, J.; Fu, D.; Wang, Z.; Xiao, R. Combined PacBio Iso-Seq and Illumina RNA-Seq analysis of the Tuta absoluta (Meyrick) transcriptome and Cytochrome P450 genes. Insects 2023, 14, 363. [Google Scholar] [CrossRef]
- Lamb, D.C.; Follmer, A.H.; Goldstone, J.V.; Nelson, D.R.; Warrilow, A.G.; Price, C.L.; True, M.Y.; Kelly, S.L.; Poulos, T.L.; Stegeman, J.J. On the occurrence of cytochrome P450 in viruses. Proc. Natl. Acad. Sci. USA 2019, 116, 12343–12352. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, X. Lysosome biogenesis: Regulation and functions. J. Cell Biol. 2021, 220, e202102001. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mukherjee, R.; Kuncha, S.K.; Brunstein, M.E.; Rathore, R.; Junek, S.; Münch, C.; Dikic, I. A lysosome membrane regeneration pathway depends on TBC1D15 and autophagic lysosomal reformation proteins. Nat. Cell Biol. 2023, 25, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.X.; Finkel, T. A phosphoinositide signaling pathway mediates rapid lysosomal repair. Nature 2022, 609, 815–821. [Google Scholar] [CrossRef] [PubMed]

| Samples | Control_1 | Control_2 | Control_3 | MT_10 1 | MT_10 2 | MT_10 3 |
|---|---|---|---|---|---|---|
| Clean reads | 41,628,434 | 38,167,832 | 40,405,632 | 38,790,212 | 39,364,340 | 43,447,812 |
| Clean bases | 6,244,265,100 | 5,725,174,800 | 6,060,844,800 | 5,818,531,800 | 5,904,651,000 | 6,517,171,800 |
| Q30 of clean reads | 93.35% | 93.18% | 93.88% | 93.36% | 95.70% | 93.66% |
| GC contents | 47.54% | 46.69% | 45.93% | 46.69% | 46.13% | 46.68% |
| Total mapped reads | 37,862,142 (90.95%) | 28,509,563 (74.70%) | 35,610,569 (88.13%) | 35,061,892 (90.39%) | 36,449,260 (92.59%) | 39,820,870 (91.65%) |
| Uniquely mapped reads | 33,216,284 (87.73%) | 25,060,252 (87.90%) | 31,468,498 (88.37%) | 32,053,845 (91.42%) | 33,896,516 (93.00%) | 36,639,623 (92.01%) |
| Multiple mapped reads | 4,645,858 (12.27%) | 3,449,311 (12.10%) | 4,142,071 (11.63%) | 3,008,347 (8.58%) | 2,552,744 (7.00%) | 3,118,347 (7.99%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Fang, Y.; Zhang, F.; Liu, Y.; Wen, X.; Yu, C.; Cheng, X.; Li, D.; Huang, L.; Ai, H.; et al. Toxic Effect of Methyl-Thiophanate on Bombyx mori Based on Physiological and Transcriptomic Analysis. Genes 2024, 15, 1279. https://doi.org/10.3390/genes15101279
He Z, Fang Y, Zhang F, Liu Y, Wen X, Yu C, Cheng X, Li D, Huang L, Ai H, et al. Toxic Effect of Methyl-Thiophanate on Bombyx mori Based on Physiological and Transcriptomic Analysis. Genes. 2024; 15(10):1279. https://doi.org/10.3390/genes15101279
Chicago/Turabian StyleHe, Zhen, Yang Fang, Fengchao Zhang, Yang Liu, Xiaoli Wen, Cui Yu, Xinkai Cheng, Dechen Li, Liang Huang, Hui Ai, and et al. 2024. "Toxic Effect of Methyl-Thiophanate on Bombyx mori Based on Physiological and Transcriptomic Analysis" Genes 15, no. 10: 1279. https://doi.org/10.3390/genes15101279






