Direct-to-Consumer Genetic Tests and Canadian Genetic Counselors: A Pilot Exploration of Professional Roles in Response to Novel Biotechnologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation and Data Analysis
2.2. Participants
2.3. Survey Limitations
3. Results and Discussion
3.1. Survey Results
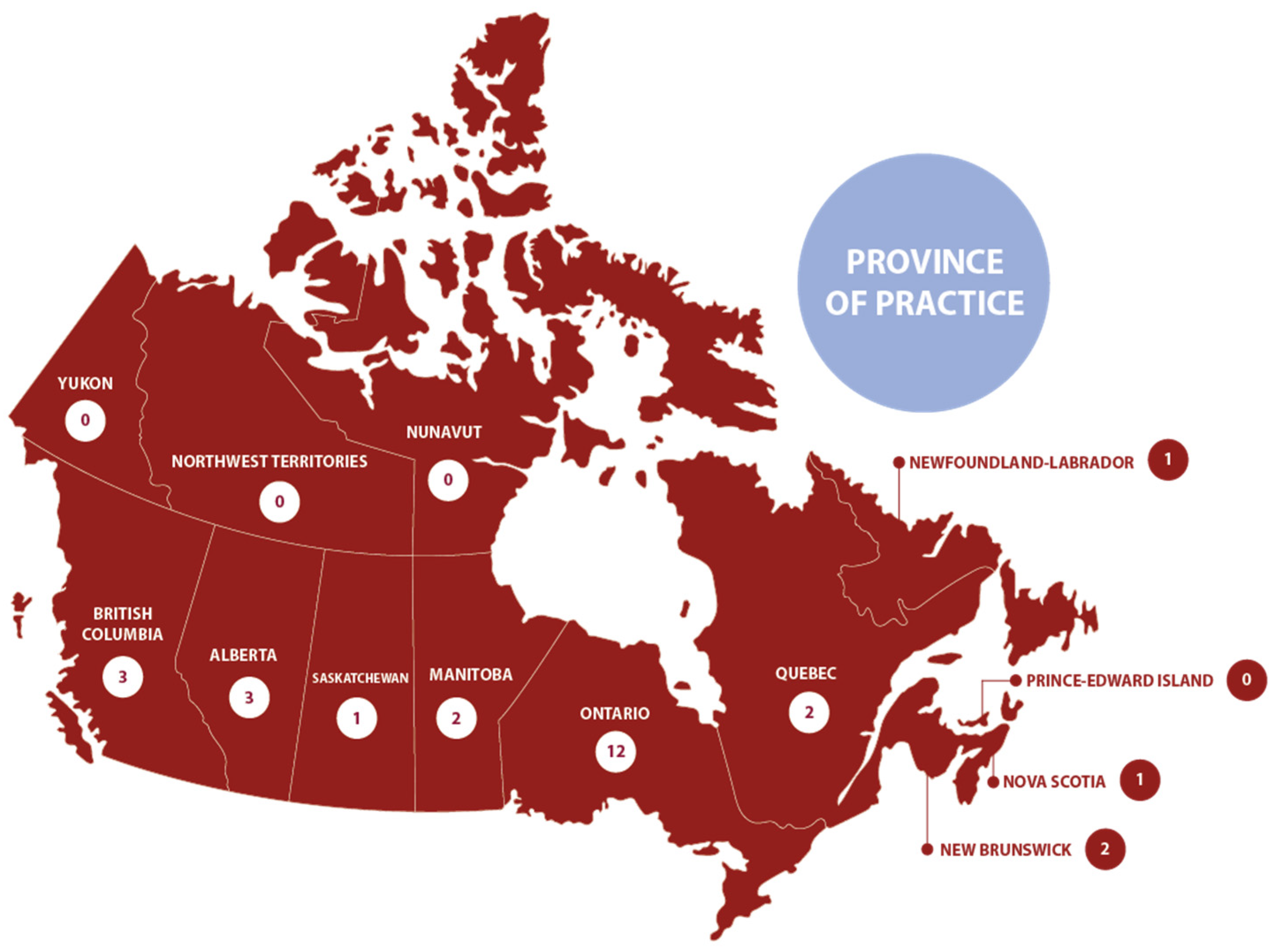
3.2. Survey Demographics
3.3. Practice Implications
3.4. Research Recommendations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stewart, K.F.J.; Wesselius, A.; Schreurs, M.A.C.; Schols, A.M.W.J.; Zeegers, M.P. Behavioural Changes, Sharing Behaviour and Psychological Responses after Receiving Direct-to-Consumer Genetic Test Results: A Systematic Review and Meta-Analysis. J. Community Genet. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Niemiec, E.; Borry, P.; Pinxten, W.; Howard, H.C. Content Analysis of Informed Consent for Whole Genome Sequencing Offered by Direct-to-Consumer Genetic Testing Companies. Hum. Mutat. 2016, 37, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Oh, B. Direct-to-Consumer Genetic Testing: Advantages and Pitfalls. Genom. Inf. 2019, 17, e33. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, T.; McGuire, A.L. Direct-to-Consumer Genetic Testing: Perceptions, Problems, and Policy Responses. Annu. Rev. Med. 2012, 63, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Mathews, R.; Hall, W.; Carter, A. Direct-to-Consumer Genetic Testing for Addiction Susceptibility: A Premature Commercialisation of Doubtful Validity and Value. Addiction 2012, 107, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Helmore, E. Genetic Testing Firm 23 and Me Admits Hackers Accessed DNA Data of 7 m Users. Available online: https://www.theguardian.com/technology/2023/dec/05/23andme-hack-data-breach (accessed on 10 December 2023).
- Tandy-Connor, S.; Guiltinan, J.; Krempely, K.; LaDuca, H.; Reineke, P.; Gutierrez, S.; Gray, P.; Tippin Davis, B. False-Positive Results Released by Direct-to-Consumer Genetic Tests Highlight the Importance of Clinical Confirmation Testing for Appropriate Patient Care. Genet. Med. 2018, 20, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Middleton, A.; Mendes, Á.; Benjamin, C.M.; Howard, H.C. Direct-to-Consumer Genetic Testing: Where and How Does Genetic Counseling Fit? Per. Med. 2017, 14, 249–257. [Google Scholar] [CrossRef]
- Rafiq, M.; Ianuale, C.; Ricciardi, W.; Boccia, S. Direct-to-Consumer Genetic Testing: A Systematic Review of European Guidelines, Recommendations, and Position Statements. Genet. Test. Mol. Biomarkers 2015, 19, 535–547. [Google Scholar] [CrossRef]
- Hurlbut, J.B.; Metzler, I.; Marelli, L.; Jasanoff, S. Bioconstitutional Imaginaries and the Comparative Politics of Genetic Self-Knowledge. Sci. Technol. Hum. Values 2020, 45, 1087–1118. [Google Scholar] [CrossRef]
- Allyse, M.A.; Robinson, D.H.; Ferber, M.J.; Sharp, R.R. Direct-to-Consumer Testing 2.0: Emerging Models of Direct-to-Consumer Genetic Testing. Mayo Clin. Proc. 2018, 93, 113–120. [Google Scholar] [CrossRef]
- Samuel, G.N.; Jordens, C.F.C.; Kerridge, I. Direct-to-Consumer Personal Genome Testing: Ethical and Regulatory Issues That Arise from Wanting to “know” Your DNA. Intern. Med. J. 2010, 40, 220–224. [Google Scholar] [CrossRef]
- Pomerantz, D. 23 and Me Had Devastating News about My Health. I Wish a Person Had Delivered It. Available online: https://www.statnews.com/2019/08/08/23andme-genetic-test-revealed-high-cancer-risk/ (accessed on 1 December 2021).
- Garde, D. ‘What’s My Real Identity?’: As DNA Ancestry Sites Gather More Data, the Answer for Consumers Often Changes. Available online: https://www.statnews.com/2019/05/22/dna-ancestry-sites-gather-data-shifting-answers-consumers/ (accessed on 1 December 2021).
- Giovanni, M.A.; Fickie, M.R.; Lehmann, L.S.; Green, R.C.; Meckley, L.M.; Veenstra, D.; Murray, M.F. Health-Care Referrals from Direct-to-Consumer Genetic Testing. Genet. Test. Mol. Biomark. 2010, 14, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.A.; Guerrini, C.J.; Mcguire, A.L. Direct-to-Consumer Genetic Testing: Value and Risk. Annu. Rev. Med. 2021, 72, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H. The Problem with Direct-to-Consumer Genetic Tests. Available online: https://blogs.scientificamerican.com/observations/the-problem-with-direct-to-consumer-genetic-tests/ (accessed on 1 December 2021).
- Udesky, L. The Ethics of Direct-to-Consumer Genetic Testing. Lancet 2010, 376, 1377–1378. [Google Scholar] [CrossRef]
- Horton, R.; Crawford, G.; Freeman, L.; Fenwick, A.; Wright, C.F.; Lucassen, A. Direct-to-Consumer Genetic Testing. Br. Med. J. 2019, 367, 15688–15694. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, A.; Schwartz, M.; Colas, C.; Golmard, L.; Stoppa-Lyonnet, D. Direct-to-Consumer Misleading Information on Cancer Risks Calls for an Urgent Clarification of Health Genetic Testing Performed by Commercial Companies. Eur. J. Cancer 2020, 132, 100–103. [Google Scholar] [CrossRef]
- National Society of Genetic Counselors NSGC At-Home Genetic Testing Position Statement. Available online: https://www.nsgc.org/Policy-Research-and-Publications/Position-Statements/Position-Statements/Post/at-home-genetic-testing-position-statement (accessed on 10 January 2021).
- Harris, A.; Kelly, S.E.; Wyatt, S. Counseling Customers: Emerging Roles for Genetic Counselors in the Direct-to-Consumer Genetic Testing Market. J. Genet. Couns. 2013, 22, 277–288. [Google Scholar] [CrossRef]
- Hsieh, V.; Braid, T.; Gordon, E.; Hercher, L. Direct-to-Consumer Genetic Testing Companies Tell Their Customers to ‘see a Genetic Counselor’’. How Do Genetic Counselors Feel about Direct-to-Consumer Genetic Testing?’. J. Genet. Couns. 2021, 30, 191–197. [Google Scholar] [CrossRef]
- Unim, B.; De Vito, C.; Hagan, J.; Villari, P.; Knoppers, B.M.; Zawati, M. The Provision of Genetic Testing and Related Services in Quebec, Canada. Front. Genet. 2020, 11, 127. [Google Scholar] [CrossRef]
- American Medical Association Direct-to-Consumer Genetic Testing. Available online: https://www.ama-assn.org/delivering-care/precision-medicine/direct-consumer-genetic-testing (accessed on 5 February 2021).
- Canadian Association of Genetic Counsellors CAGC Statement on Direct-to-Consumer Genetic Testing. Available online: https://www.cagc-accg.ca/doc/BOD/Statements/CAGC%20DTC%20Statement%20-%20FINAL%202018-04-11(1).pdf (accessed on 10 January 2021).
- Canadian Medical Association Direct-to-Consumer Genetic Testing. Available online: https://www.cma.ca/sites/default/files/2018-11/cma-policy-direct-to-consumer-genetic-testing-pd17-05-e.pdf (accessed on 10 January 2021).
- Canadian Association of Genetic Counsellors Advertise to CAGC Members. Available online: https://www.cagc-accg.ca/index.php?page=339 (accessed on 1 September 2021).
- National Society of Genetic Counselors Professional Status Survey 2020: Executive Summary. Available online: https://www.nsgc.org/Portals/0/Docs/Policy/PSS%20Executive%20Summary%202020%20FINAL%2005-03-20.pdf (accessed on 1 October 2021).
- Jones, T.L.; Baxter, M.; Khanduja, V. A Quick Guide to Survey Research. Ann. R. Coll. Surg. Engl. 2013, 95, 5–7. [Google Scholar] [CrossRef]
- Canadian Medical Association CMA National Physician Health Survey. Available online: https://www.cma.ca/sites/default/files/2018-11/nph-survey-e.pdf (accessed on 5 July 2022).
- Hock, K.T.; Christensen, K.D.; Yashar, B.M.; Roberts, J.S.; Gollust, S.E.; Uhlmann, W.R. Direct-to-Consumer Genetic Testing: An Assessment of Genetic Counselors’ Knowledge and Beliefs. Genet. Med. 2011, 13, 325–332. [Google Scholar] [CrossRef]
- Direct-to-Consumer Genetic Testing and Privacy. Available online: https://www.priv.gc.ca/en/privacy-topics/health-genetic-and-other-body-information/02_05_d_69_gen/ (accessed on 1 April 2021).
- ACMG Board of Directors Direct-to-Consumer Genetic Testing: A Revised Position Statement of the American College of Medical Genetics and Genomics. Genet. Med. 2016, 18, 207–208. [CrossRef]
- Sassano, M.; Hoxhaj, I.; Stojanovic, J.; Acampora, A.; Boccia, S. Survey of Public Health Professionals on Perspectives towards Direct-to-Consumer Genetic Tests. Eur. J. Public Health 2021, 31, 449–450. [Google Scholar] [CrossRef]
- European Society of Human Genetics. Statement of the ESHG on Direct-to-Consumer Genetic Testing for Health-Related Purposes. Eur. J. Hum. Genet. 2010, 18, 1271–1273. [Google Scholar] [CrossRef]
- The American Society of Human Genetics. Advancing Research and Privacy: Achievements, Challenges, and Core Principles. Am. J. Hum. Genet. 2019, 105, 445–447. [Google Scholar] [CrossRef]
- American Medical Association AMA Calls for Privacy Guidelines Governing Mail-Order DNA Tests. Available online: https://www.ama-assn.org/press-center/press-releases/ama-calls-privacy-guidelines-governing-mail-order-dna-tests (accessed on 1 February 2022).
- Hoxhaj, I.; Stojanovic, J.; Boccia, S. European Citizens’ Perspectives on Direct-to-Consumer Genetic Testing: An Updated Systematic Review. Eur. J. Public Health 2020, ckz246, 1–7. [Google Scholar] [CrossRef]
- Blell, M.; Hunter, M.A. Direct-to-Consumer Genetic Testing’s Red Herring: “Genetic Ancestry” and Personalized Medicine. Front. Med. 2019, 6, 48. [Google Scholar] [CrossRef]
- Padawer, R. Sigrid Johnson Was Black. A DNA Test Said She Wasn’t. Available online: https://www.nytimes.com/2018/11/19/magazine/dna-test-black-family.html (accessed on 1 October 2021).
- Powell, K.P.; Christianson, C.A.; Cogswell, W.A.; Dave, G.; Verma, A.; Eubanks, S.; Henrich, V.C. Educational Needs of Primary Care Physicians Regarding Direct-to-Consumer Genetic Testing. J. Genet. Couns. 2012, 21, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, A.K.; Hayden, M.R. A Grand Challenge: Providing Benefits of Clinical Genetics to Those in Need. Genet. Med. 2011, 13, 197–200. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.P.; Walton, N.; Williams, M.S.; Kim, K.K.; Bastola, K. Are Providers Prepared for Genomic Medicine: Interpretation of Direct-to-Consumer Genetic Testing (DTC-GT) Results and Genetic Self-Efficacy by Medical Professionals. BMC Health Serv. Res. 2019, 19, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Direct-to-Consumer Genetic Testing FAQ for Healthcare Professionals. Available online: https://www.genome.gov/For-Health-Professionals/Provider-Genomics-Education-Resources/Healthcare-Provider-Direct-to-Consumer-Genetic-Testing-FAQ (accessed on 1 October 2022).
- Right to Know. Available online: https://righttoknow.us/ (accessed on 1 August 2022).
- DNA Painter. Available online: https://dnapainter.com/ (accessed on 1 August 2022).
- Killbride, M.K.; Bradbury, A.R. The Need to Improve the Clinical Utility of Direct-to-Consumer Genetic Tests: Either Too Narrow or Too Broad. J. Am. Med. Assoc. 2020, 323, 1443–1444. [Google Scholar] [CrossRef] [PubMed]
- Popejoy, A.B.; Fullerton, S.M. Genomics Is Failing on Diversity. Nature 2016, 538, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.; Patrick-Miller, L.; Bradbury, A.R. Controversies in Communication of Genetic Risk for Hereditary Breast Cancer. Breast J. 2009, 15, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Direct-to-Consumer Tests. Available online: https://www.fda.gov/medical-devices/vitro-diagnostics/direct-consumer-tests (accessed on 1 June 2021).
- Wade, C.H.; Wilfond, B.S. Ethical and Clinical Practice Considerations for Genetic Counselors Related to Direct-to-Consumer Marketing of Genetic Tests. Am. J. Med. Genet. 2006, 142C, 284–292. [Google Scholar] [CrossRef]
- Faucett, W.A. Invited Commentary—Genetic Counselor’s Ethical and Professional Duty to Discuss DTC Tests. Am. J. Med. Genet. Part C Semin. Med. Genet. 2006, 142C, 293. [Google Scholar] [CrossRef]
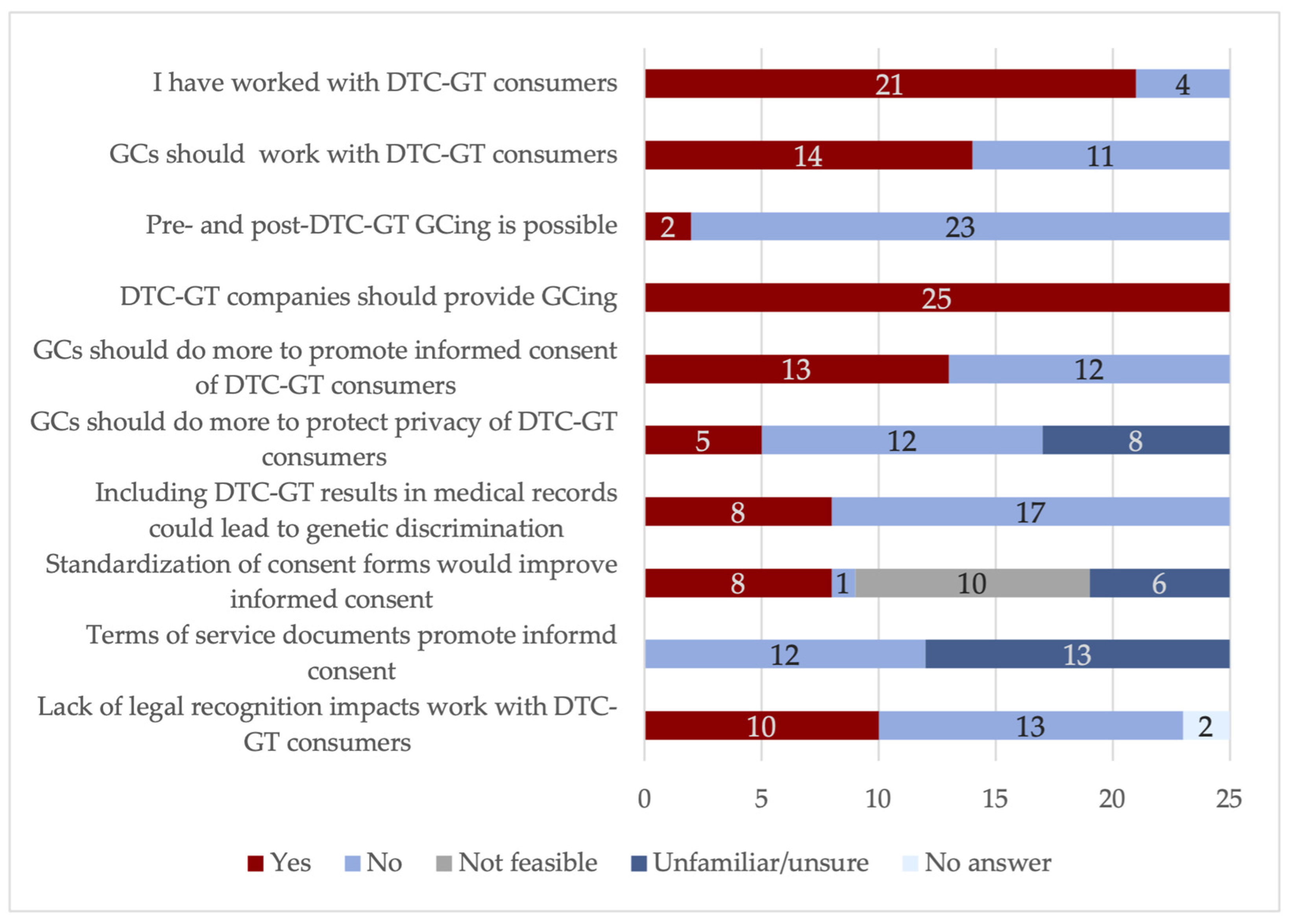
| Question | Response | |
|---|---|---|
| Do DTC-GT terms of service documents sufficiently describe the limitations of DTC-GTs? | 20% (n = 5): Too varied | |
| 0% (n = 0): Clearly describe | ||
| 32% (n = 8): Poorly describe | ||
| 0% (n = 0): Do not describe | ||
| 48% (n = 12): Unfamiliar | ||
| Does the language in DTC-GT terms of service documents sufficiently promote informed consent? | 0% (n = 0): Yes | |
| 48% (n = 12) No | ||
| 52% (n = 13): Unfamiliar | ||
| How would you rate the following statement: DTC-GTs remove GCs from discussions of genetic health data, which negatively impacts consumer informed consent. | 8% (n = 2): Strongly agree | |
| 52% (n = 13): Agree | ||
| 24% (n = 6): Disagree | ||
| 16% (n = 4) Disagree | ||
| 0% (n = 0): Strongly disagree | ||
| Do you interpret the Canadian Association of Genetic Counsellors (CAGC) Code of Ethics tenant to “promote awareness of the roles of medical genetics professionals” to include collaboration with the DTC-GT industry? | 76% (n = 19): Yes | |
| 24% (n = 6): No | ||
| Why no? | 8% (n = 2): Beyond the scope of practice | |
| 12% (n = 3): Conflict of interest | ||
| Why yes? | 4% (n = 1): No response | |
| 64% (n = 16): Would allow for evaluation of DTC-GTs | ||
| 56% (n = 14): Would allow for standardized informed consent | ||
| 20% (n = 5): DTC-GT company funding could facilitate research and development in GCing | ||
| The CAGC Code of Ethics states that GCs should: “... promote awareness of the roles of medical genetics professionals through activities such as participation in multi-disciplinary teams, providing public education, contributing to policy-making and provincial/national consultation”. Do you think that “providing public education” should include information on DTC-GTs? | 84% (n = 21): Yes | |
| 16% (n = 4): No | ||
| Do you believe the vocation of GCing needs to do more to protect the informed consent of DTC-GT customers compared to traditional patients seeking counseling? | 52% (n = 13): Yes | |
| 48% (n = 12): No | ||
| Why no? | 20% (n = 5): DTC-GT is not medically necessary; does not merit intervention from healthcare professionals | |
| 36% (n = 9): Inefficient use of clinical time and resources | ||
| Why yes? | 0% (n = 0): GCs are not adequately trained to counsel DTC-GT customers on informed consent | |
| 44% (n = 11): The tests contain significant limitations which may be unclear to consumers | ||
| 44% (n = 11): The absence of healthcare professionals creates additional challenges to informed consent that merit attention | ||
| 32% (n = 8): There is limited information about the accuracy of DTC-GT results | ||
| DTC-GTs continue to improve the quality and accuracy of results every year. How does the continual improvement of DTC-GT results impact GCing? | 24% (n = 6): Multi-disciplinary medical collaborations | |
| 40% (n = 10): Longer relationship with DTC-GT customers with changing results | ||
| 24% (n = 6): Diverting clinical resources with expanding knowledge demands | ||
| 56% (n = 14): Does not impact GCing; field evolving | ||
| Question | Response | |
|---|---|---|
| How concerned are you with the privacy policies of DTC-GT companies in Canada? Scale of 1 (unconcerned)–5 (concerned) | 4% (n = 1): 1 (unconcerned) | |
| 8% (n = 2): 2 | ||
| 28% (n = 7): 3 | ||
| 52% (n = 13): 4 | ||
| 8% (n = 2): 5 (concerned) | ||
| Average response: 3.52 (concerned, standard deviation = 0.92) | ||
| Where do you perceive privacy issues in DTC-GTs? | 84% (n = 21): Sale of aggregated data | |
| 72% (n = 18): Retention of samples | ||
| 40% (n = 10): Anonymization of genetic data | ||
| 4% (n = 1): Canadian Federal Law sufficiently protects consumer privacy | ||
| 12% (n = 3): DTC-GTs adequately safeguard personal genetic security | ||
| Do you believe the vocation of GCing needs to do more to protect the privacy of DTC-GT customers compared to traditional patients seeking counseling? | 48% (n = 12): No | |
| 20% (n = 5): Yes | ||
| 32% (n = 8): Unsure | ||
| Why no? | 32% (n = 8): Intervention not feasible | |
| 24% (n = 6): Beyond the scope of GCing | ||
| 24% (n = 6): Intervention was a misuse of clinical time | ||
| 20% (n = 5): The lack of regulation of DTC-GT company privacy policies is concerning | ||
| Why yes? | 4% (n = 1): Since informed consent is difficult to achieve without the mediation of a healthcare professional, privacy policies must be closely monitored | |
| 20% (n = 5): The lack of regulation of DTC-GT company privacy policies is concerning | ||
| Question | Response | |
|---|---|---|
| Generally speaking, how heavily do you think the GCing vocation should be involved with DTC-GTs? 1–not at all involved, 5–heavily involved. | 0% (n = 0): 1 | |
| 24% (n = 6): 2 | ||
| 36% (n = 9): 3 | ||
| 32% (n = 8): 4 | ||
| 8% (n = 2): 5 | ||
| How would you rate the following statement: DTC-GTs are sufficiently regulated. | 0% (n = 0): Strongly agree | |
| 0% (n = 0): Agree | ||
| 24% (n = 6): Neutral | ||
| 76% (n = 19): Disagree | ||
| 0% (n = 0): Strongly disagree | ||
| What level of involvement should the CAGC hold with respect to DTC-GT evaluation? | 20% (n = 5): Grade tests (maintain a list on the website) | |
| 12% (n = 3): Recommend tests (through the website) | ||
| 16% (n = 4): Accredit tests (label products) | ||
| 48% (n = 12): Currently not feasible for the CAGC to be involved in this process | ||
| 56% (n = 14): The CAGC should not attempt to evaluate DTC-GTs | ||
| Do you believe it is feasible to offer pre- and post-clinical genetic counseling to DTC-GT customers? | 8% (n = 3): Yes | |
| 92% (n = 23): No | ||
| Why no? | 72% (n = 18): Not enough GCs | |
| 72% (n = 18): Should be the responsibility of DTC-GT companies | ||
| Does pre- or post-DTC-GT counseling have the potential for the most impact on consumers? | 28% (n = 7): Before | |
| 44% (n = 11): After | ||
| 28% (n = 7): Not feasible to offer any counseling | ||
| Which of the following methods could alleviate the burden of DTC-GT counseling? | 40% (n = 10): Virtual conferences within province | |
| 40% (n = 10): Virtual conferencing within the country | ||
| 56% (n = 14): Online resources managed by GCs | ||
| 40% (n = 10): Not feasible to offer any counseling | ||
| Given the high demand for counseling for DTC-GT customers, what is currently feasible for the CAGC to offer? | 48% (n = 12): Establish a special interest group (SIG) at the annual CAGC meeting to develop policy review of DTC-GTs | |
| 44% (n = 11): Organize seminars for primary care physicians or other healthcare professionals to prepare them to offer counseling of DTC-GT results | ||
| 60% (n = 15): Advocate for federal regulation of DTC-GTs | ||
| 68% (n = 17): Produce informative materials | ||
| How do you see the current role of genetic counselors changing with regards to the DTC-GT industry? | 60% (n = 15): Increased collaboration with primary care physicians | |
| 52% (n = 13): Increased collaboration with other healthcare professionals | ||
| 52% (n = 13): Increased work outside clinical roles | ||
| 52% (n = 13): Increased advocacy work | ||
| 24% (n = 6): GCs should focus on clinical patients rather than DTC-GT customers | ||
| How would you respond to the following statement: It is the responsibility of DTC-GT companies to provide counseling for consumers rather than clinical GCs | 100% (n = 25): Yes | |
| 0% (n = 0): No | ||
| Why yes? | 56% (n = 14): Too few accredited genetic counselors across Canada to provide this service | |
| 56% (n = 14): Uneven distribution of clinical GCs across Canada creates access issues | ||
| 60% (n = 15): Since tests are not medically necessary, customers do not merit access to a limited pool of GCs | ||
| 40% (n = 10): DTC-GT customers are not patients of the healthcare system | ||
| 84% (n = 21): The DTC-GT industry has sufficient resources to recruit GCs | ||
| 40% (n = 10): Offsetting the DTC-GT counseling responsibility helps the industry avoid accountability | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haley, C.E.; Zawati, M.H. Direct-to-Consumer Genetic Tests and Canadian Genetic Counselors: A Pilot Exploration of Professional Roles in Response to Novel Biotechnologies. Genes 2024, 15, 156. https://doi.org/10.3390/genes15020156
Haley CE, Zawati MH. Direct-to-Consumer Genetic Tests and Canadian Genetic Counselors: A Pilot Exploration of Professional Roles in Response to Novel Biotechnologies. Genes. 2024; 15(2):156. https://doi.org/10.3390/genes15020156
Chicago/Turabian StyleHaley, Cassandra E., and Ma’n H. Zawati. 2024. "Direct-to-Consumer Genetic Tests and Canadian Genetic Counselors: A Pilot Exploration of Professional Roles in Response to Novel Biotechnologies" Genes 15, no. 2: 156. https://doi.org/10.3390/genes15020156
APA StyleHaley, C. E., & Zawati, M. H. (2024). Direct-to-Consumer Genetic Tests and Canadian Genetic Counselors: A Pilot Exploration of Professional Roles in Response to Novel Biotechnologies. Genes, 15(2), 156. https://doi.org/10.3390/genes15020156




