Genetic Analysis of Egg Production Traits in Luhua Chickens: Insights from a Multi-Trait Animal Model and a Genome-Wide Association Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Chicken Population and Phenotypic Data
2.3. Phenotypic Data Analysis
2.4. Estimated Genetic Parameters
2.5. Genotypic Data and Quality Control
2.6. Principal Component Analysis
2.7. Association Analysis
2.8. Identification of Candidate Genes
3. Results
3.1. Descriptive Statistics
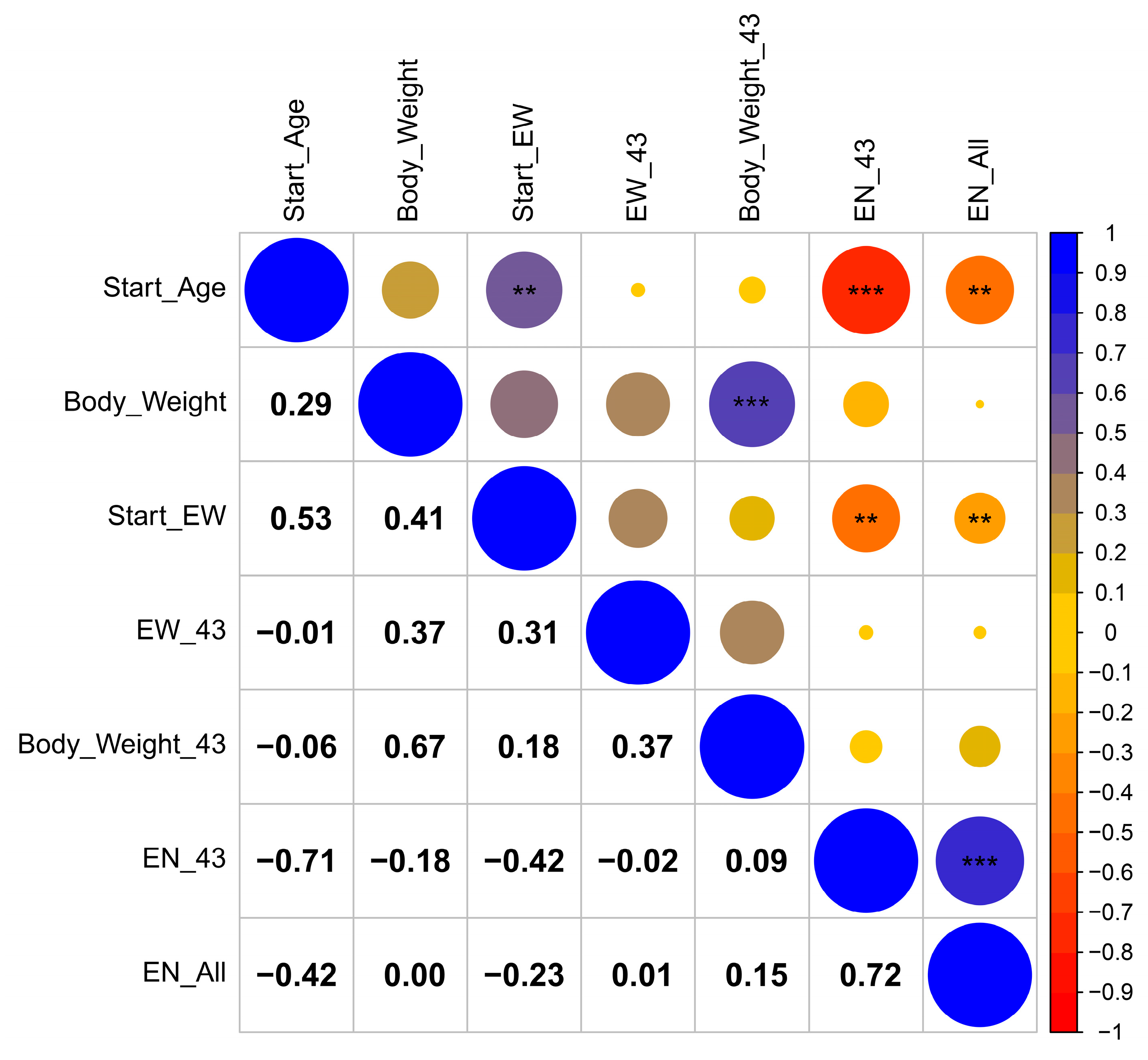
3.2. Correlation Analysis
3.3. Analysis of Variance
3.4. Genetic Parameter Statistics
3.5. Density Distribution of SNPs
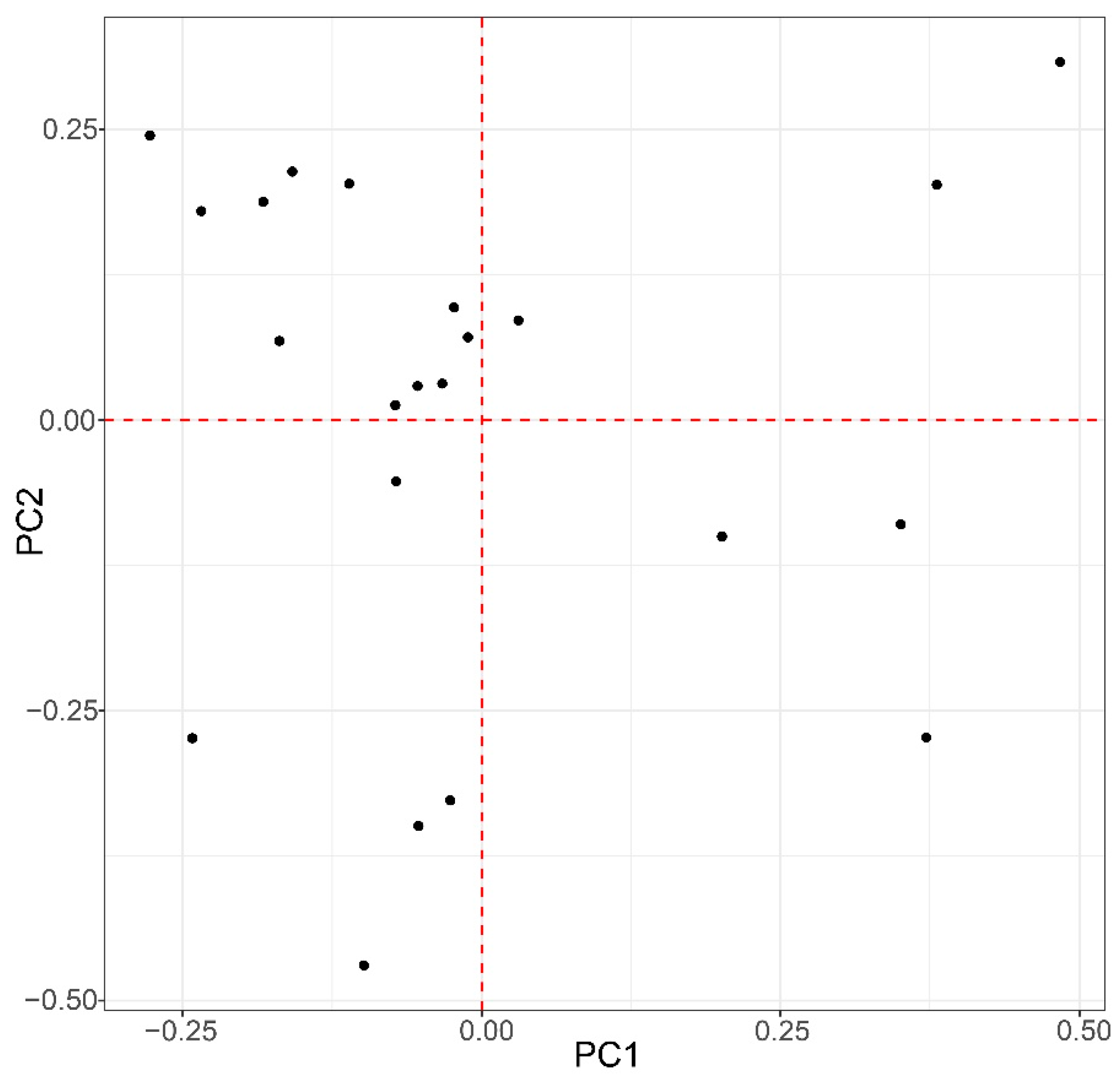
3.6. Principal Component Analysis
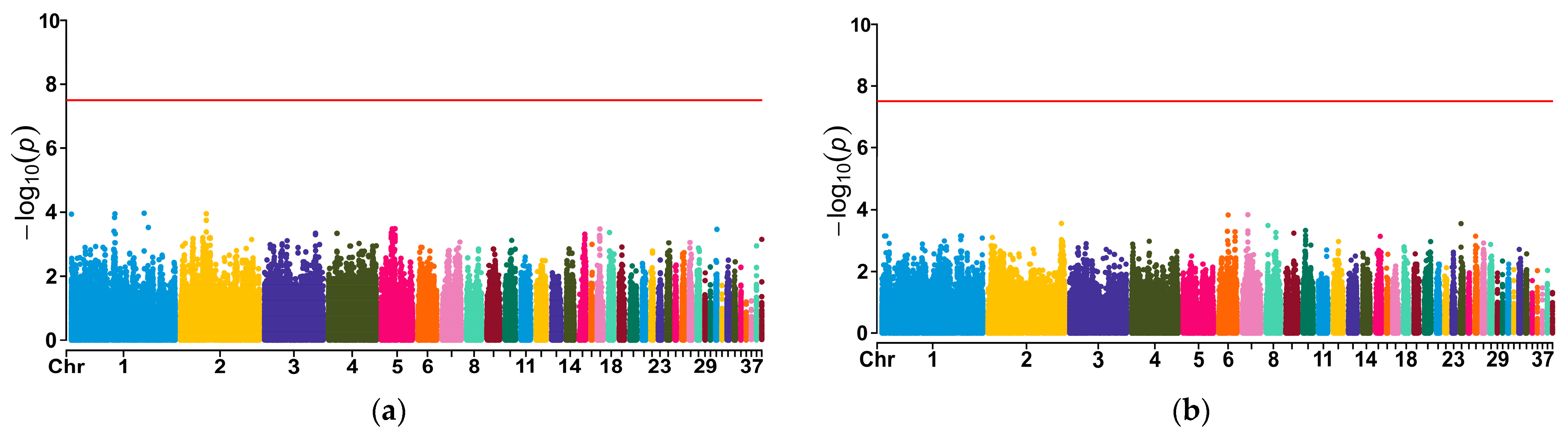
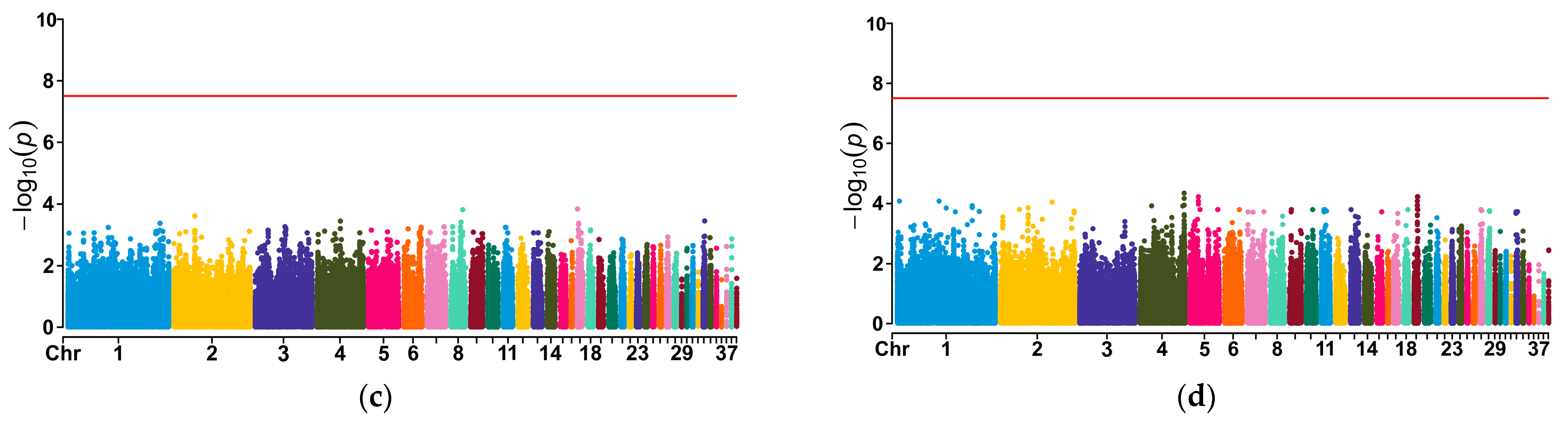
3.7. Association Analysis
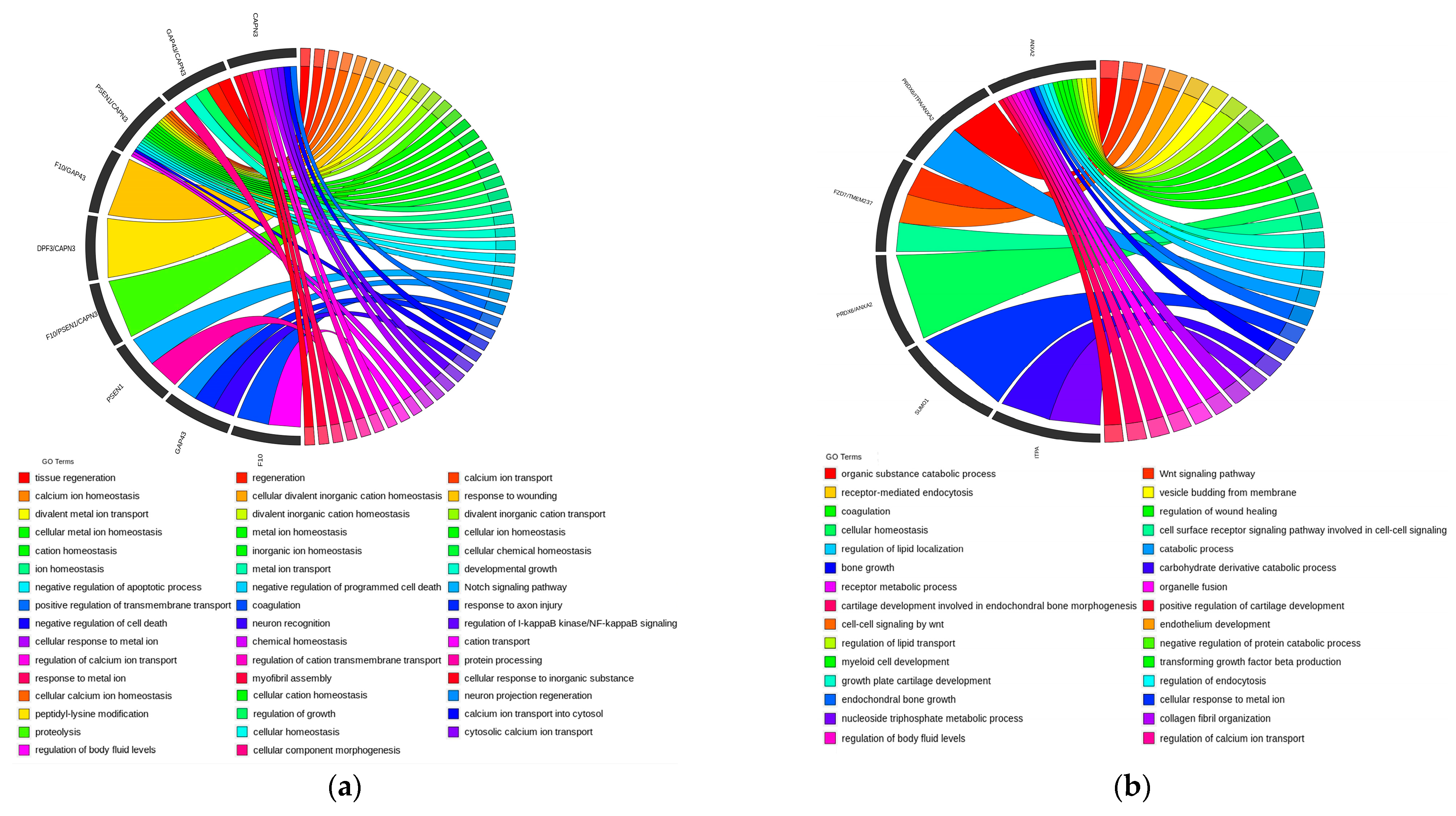
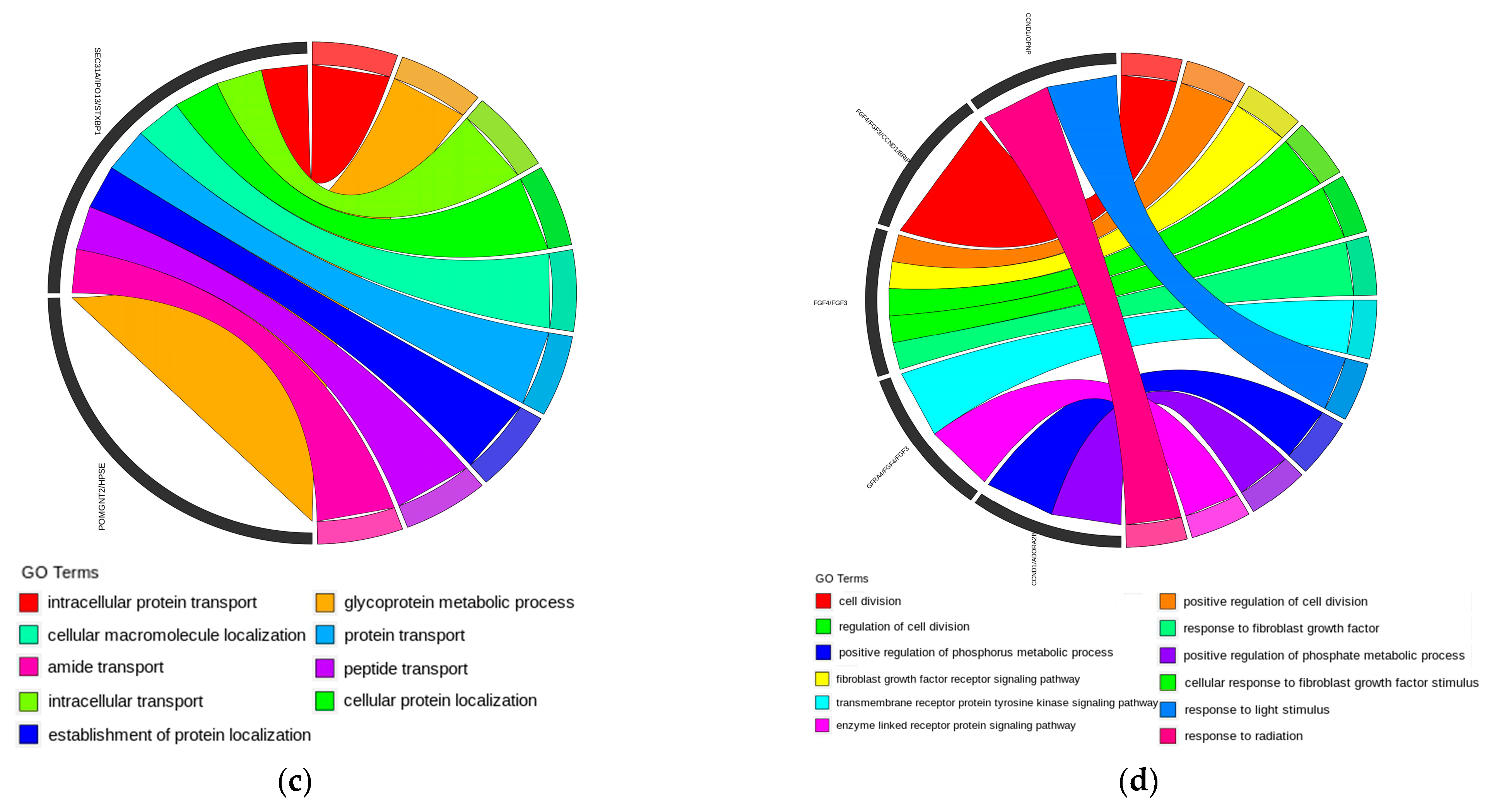
3.8. Enrichment Analysis
4. Discussion
4.1. Egg Production Analysis
4.2. Genetic Evaluation
4.3. Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Yang, N.; Yan, Y.Y.; Li, G.Q.; Liu, A.Q.; Wu, G.Q.; Sun, C.J. Genome-wide association analysis of egg production performance in chickens across the whole laying period. BMC Genom. 2019, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, G.R.; Mendonça, M.d.O.; Weitzel, L.C.d.C.; Bittencourt, T.M.; de Matos, A.S.; Valentim, J.k.; de Araújo, M.L.; de Oliveira, A.Q.C. Physical quality of eggs of four strains of poultry. Acta Sci. Anim. Sci. 2021, 43, e52738. [Google Scholar] [CrossRef]
- Kaneko, K.; Okano, K.; Fujihara, N. Studies on egg laying performance of the Gifu native fowl. Jpn. Poult. Sci. 1997, 34, 200–204. [Google Scholar] [CrossRef]
- Chandan, P.; Prince, L.L.L.; Bhattacharya, T.K.; Rajkumar, U.; Chatterjee, R.N. Estimation of heritability and genetic correlation of egg production traits using animal model in commercial layer. Indian J. Anim. Sci. 2019, 89, 1269–1273. [Google Scholar] [CrossRef]
- Ates, C.; Cimrin, T.; Alasahan, S. Pre- and post-incubation values of different weight groups of egg laid by chukar partridges (Alectoris chukar). Mustafa Kemal Univ. J. Agric. Sci. 2021, 26, 193–199. [Google Scholar] [CrossRef]
- Malau-Aduli, A.E.O.; Bawa, G.; Joel, K. Factors affecting egg production and layer bird mortality in private poultry farms in the subhumid zone of Nigeria. Anim. Sci. J. 2003, 74, 239–242. [Google Scholar] [CrossRef]
- Ghazikhani Shad, A.; Nejati Javaremi, A.; Mehrabani Yeganeh, H. Animal Model Estimation of Genetic Parameters for Most Important Economic Traits in Iranian Native Fowls. Pak. J. Biol. Sci. 2007, 10, 2787–2789. [Google Scholar] [PubMed]
- de Freitas, L.A.; Savegnago, R.P.; Grupioni, N.V.; Ramos, S.B.; Stafuzza, N.B.; de Figueiredo, E.A.P.; Schmidt, G.S.; Ledur, M.C.; Munari, D.P. Reduced-rank estimation of genetic parameters for egg production traits and cluster analyses with predicted breeding values. Acta Agric. Scand. Sect. A—Anim. Sci. 2018, 68, 81–86. [Google Scholar] [CrossRef]
- Niknafs, S.; Nejati-Javaremi, A.; Mehrabani-Yeganeh, H.; Fatemi, S.A. Estimation of genetic parameters for body weight and egg production traits in Mazandaran native chicken. Trop. Anim. Health Prod. 2012, 44, 1437–1443. [Google Scholar] [CrossRef]
- Gao, J.F.; Xu, W.W.; Zeng, T.; Tian, Y.; Wu, C.Q.; Liu, S.Z.; Zhao, Y.; Zhou, S.H.; Lin, X.Q.; Cao, H.G.; et al. Genome-Wide Association Study of Egg-Laying Traits and Egg Quality in LingKun Chickens. Front. Vet. Sci. 2022, 9, 877739. [Google Scholar] [CrossRef]
- Goto, T.; Tsudzuki, M. Genetic Mapping of Quantitative Trait Loci for Egg Production and Egg Quality Traits in Chickens: A Review. J. Poult. Sci. 2017, 54, 1–12. [Google Scholar] [CrossRef]
- Du, Y.; Liu, L.; He, Y.; Dou, T.; Jia, J.; Ge, C. Endocrine and genetic factors affecting egg laying performance in chickens: A review. Br. Poult. Sci. 2020, 61, 538–549. [Google Scholar] [CrossRef]
- Gao, G.L.; Gao, D.F.; Zhao, X.Z.; Xu, S.S.; Zhang, K.S.; Wu, R.; Yin, C.H.; Li, J.; Xie, Y.H.; Hu, S.L.; et al. Genome-Wide Association Study-Based Identification of SNPs and Haplotypes Associated With Goose Reproductive Performance and Egg Quality. Front. Genet. 2021, 12, 602583. [Google Scholar] [CrossRef] [PubMed]
- Azmal, S.A.; Bhuiyan, A.A.; Omar, A.I.; Ma, S.; Sun, C.H.; Han, Z.D.; Zhang, M.K.; Zhao, S.H.; Li, S.J. Novel Polymorphisms in RAPGEF6 Gene Associated with Egg-Laying Rate in Chinese Jing Hong Chicken using Genome-Wide SNP Scan. Genes 2019, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.L.; Liu, Q.X.; Jiang, Z.Y.; Song, Y.; Yi, S.J.; Qiu, J.H.; Hao, G.J.; Sun, S.H. Characteristics of Salmonella from Chinese Native Chicken Breeds Fed on Conventional or Antibiotic-Free Diets. Front. Vet. Sci. 2021, 8, 607491. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.G.; Xu, X.L.; Cao, H.Y.; Zhou, W.; Yin, Z.Z. Effect of age at first egg on reproduction performance and characterization of the hypothalamo–pituitary–gonadal axis in chickens. Poult. Sci. 2021, 100, 101325. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.R.; Lin, C.Y.; Cheng, Y.S.; Lin, E.Y.; Huang, S.P.; Hung, K.H.; Liang, H.M. Genetic parameters for body weight and egg production traits in Taiwan native chicken homozygous for the heat shock protein 70 gene. Asian J. Agric. Biol. 2018, 6, 396–402. [Google Scholar]
- Bogdanski, F.A.; Silveira, R.M.F.; Rovadoscki, G.A.; Franzo, V.; Gervásio, I.C.; Escobar, D.Y.O.; Dauria, B.D.; Meira, A.N.; Mourao, L.M.B.; Coutinho, L.L.; et al. Genetic parameters for production, quality, and colors from eggs in Brazilian lineages of chickens. Trop. Anim. Health Prod. 2023, 55, 148. [Google Scholar] [CrossRef] [PubMed]
- Wolc, A.; Arango, J.; Settar, P.; O‘Sullivan, N.P.; Olori, V.E.; White, I.M.S.; Hill, W.G.; Dekkers, J.C.M. Genetic parameters of egg defects and egg quality in layer chickens. Poult. Sci. 2012, 9, 1292–1298. [Google Scholar] [CrossRef]
- Tongsiri, S.; Jeyaruban, G.M.; Hermesch, S.; vanderWerf, J.H.J.; Li, L.; Chormai, T. Genetic parameters and inbreeding effects for production traits of Thai native chickens. Asian-Australas. J. Anim. Sci. 2019, 32, 930–938. [Google Scholar] [CrossRef]
- Noda, K.; Miyakawa, H.; Nakamura, A.; Mizuno, K.; Umezawa, Y. Genetic parameter estimates for egg weight and its related traits in Japanese quail. Jpn. Poult. Sci. 2003, 40, J66–J70. [Google Scholar]
- Begli, H.E.; Wood, B.J.; Abdalla, E.A.; Balzani, A.; Willems, O.; Schenkel, F.; Harlander-Matauschek, A.; Baes, C.F. Genetic parameters for clutch and broodiness traits in turkeys (Meleagris Gallopavo) and their relationship with body weight and egg production. Poult. Sci. 2019, 98, 6263–6269. [Google Scholar] [CrossRef] [PubMed]
- Lacin, E.; Yildiz, A.; Esenbuga, N.; Macit, M. Effects of differences in the initial body weight of groups on laying performance and egg quality parameters of Lohmann laying hens. Czech J. Anim. Sci. 2008, 53, 466–471. [Google Scholar] [CrossRef]
- Karami, K.; Zerehdaran, S.; Barzanooni, B.; Lotfi, E. Genetic analyses of partial egg production in Japanese quail using multi-trait random regression models. Br. Poult. Sci. 2017, 58, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Nassar, F.S.; El-Koury, E.M.; Abdou, A.M. Effect of selection for high live body weight on heritability, phenotypic and genetic correlation estimates for productive performance in local broiler breeders. Biosci. Res. 2019, 15, 3357–3366. [Google Scholar]
- Rahim, A.; Kumar, S.; Jagadeesan, K.; Ullah, S.; Debnath, J.; Yadav, R.; Bhanja, S.K. Genetic analysis of growth, production and reproduction traits after long-term selection in Rhode Island Red chicken. Indian J. Anim. Res. 2016, 50, 646–651. [Google Scholar] [CrossRef]
- Ledur, M.C.; Liljedahl, L.E.; McMillan, I.; Asselstine, L.; Fairfull, R.W. Genetic effects of aging on egg quality traits in the first laying cycle of White Leghorn strains and strain crosses. Poult. Sci. 2002, 81, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, U.; Prince, L.L.L.; Haunshi, S.; Paswan, C.; Reddy, B.L.N. Evaluation of Vanaraja female line chicken for growth, production, carcass and egg quality traits. Indian J. Anim. Sci. 2021, 90, 603–609. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.P.; Singh, S.K.; Singh, H. Genetic trend in growth and reproductive traits of synthetic broiler dam line pullet. Indian J. Poult. Sci. 2003, 38, 13–16. [Google Scholar]
- Luo, P.T.; Yang, R.Q.; Yang, N. Estimation of genetic parameters for cumulative egg numbers in a broiler dam line by using a random regression model. Poult. Sci. 2007, 86, 30–36. [Google Scholar] [CrossRef]
- Hu, Y.H.; Poivey, J.P.; Rouvier, R.; Liu, S.C.; Tai, C. Heritabilities and genetic correlations of laying performance in Muscovy ducks selected in Taiwan. Br. Poult. Sci. 2004, 45, 180–185. [Google Scholar] [CrossRef]
- Savas, T.; Preisinger, R.; Roehe, R.; Kalm, E. Genetic parameters and optimal test period for egg production from part-period egg production traits in laying hens. Arch. Anim. Breed. 1998, 41, 421–432. [Google Scholar]
- Barot, V.N.; Savaliya, F.P.; Hirani, N.D.; Patel, A.B.; Vataliya, P.H.; Khanna, K.; Patel, A.M.; Joshi, R.S. Genetic parameters of various economic traits in different generations of synthetic White Leghorn. Indian J. Poult. Sci. 2008, 43, 20–24. [Google Scholar]
- Xu, H.Y.; Zhang, Y.H.; Zou, Q.; Li, L.; Han, C.C.; Liu, H.H.; Hu, J.W.; Zhong, T.; Wang, Y. Characterization of the Goose CAPN3 Gene and its Expression Pattern in Muscle Tissues of Sichuan White Geese at Different Growth Stages. J. Poult. Sci. 2018, 55, 172–181. [Google Scholar] [CrossRef]
- Mac Donald, K.; Iulianella, A. The actin-cytoskeleton associating protein BASP1 regulates neural progenitor localization in the neural tube. Genesis 2021, 60, e23464. [Google Scholar] [CrossRef] [PubMed]
- Andreas, W.; Julie, S.; Pico, C. The motility-associated proteins GAP-43, MARCKS, and CAP-23 share unique targeting and surface activity-inducing properties. Exp. Cell Res. 1997, 236, 103–116. [Google Scholar] [CrossRef]
- Bagaria, J.; Bagyinszky, E.; An, S.S.A. Genetics, Functions, and Clinical Impact of Presenilin-1 (PSEN1) Gene. Int. J. Mol. Sci. 2022, 23, 10970. [Google Scholar] [CrossRef]
- Perini, F.; Cendron, F.; Wu, Z.; Sevane, N.; Li, Z.Q.; Huang, C.H.; Smith, J.; Lasagna, E.; Cassandro, M.; Penasa, M. Genomics of Dwarfism in Italian Local Chicken Breeds. Genes 2023, 14, 633. [Google Scholar] [CrossRef]
- Qiao, X.B.; Zhou, W.D.; Zhang, S.E.; Gong, Y.Q.; Kang, L.; Jiang, Y.L. Identification of nucleotide polymorphisms in the key promoter region of chicken annexins A2 gene associatied with egg laying traits. Anim. Biotechnol. 2022, 34, 1413–1421. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Chen, X.; Mao, Y.; Kang, L.; Ma, X.L.; Jiang, Y.L. Characterization of annexin A2 in chicken follicle development: Evidence for its involvement in angiogenesis. Anim. Reprod. Sci. 2015, 161, 104–111. [Google Scholar] [CrossRef]
- Gu, S.M.; Yu, E.; Kim, Y.E.; Yoon, S.S.; Lee, D.; Hong, J.T.; Yun, J. Peroxiredoxin 6 Overexpression Induces Anxiolytic and Depression-Like Behaviors by Regulating the Serotonergic Pathway in Mice. Biomol. Ther. 2022, 30, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Peroxiredoxin 6: A Bifunctional Enzyme with Glutathione Peroxidase and Phospholipase A2 Activities. Antioxid Redox Signal 2011, 15, 831–844. [Google Scholar] [CrossRef]
- Tan, M.; Asad, M.; Heong, V.; Wong, M.K.; Tan, T.Z.; Ye, J.R.; Kuay, K.T.; Thiery, J.P.; Scott, C.; Huang, R.Y.J. The FZD7-TWIST1 axis is responsible for anoikis resistance and tumorigenesis in ovarian carcinoma. Mol. Oncol. 2019, 13, 757–780. [Google Scholar] [CrossRef]
- Liebelt, F.; Vertegaal, A.C.O. Ubiquitin-dependent and independent roles of SUMO in proteostasis. Am. J. Phusiology-Cell Physiol. 2016, 311, C284–C296. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.W.; Hsieh, J.H.; Wang, S.W.; Yeh, T.Y.; Chang, M.F.; Tang, T.Y.; Chao, C.C.; Feng, F.P.; Hsieh, S.T. Nonpermissive Skin Environment Impairs Nerve Regeneration in Diabetes via Sec31a. Ann. Neurol. 2022, 91, 821–833. [Google Scholar] [CrossRef]
- Liu, X.J.; Lin, W.B.; Shi, X.Y.; Davies, R.G.; Wagstaff, K.M.; Tao, T.; Jans, D.A. PKA-site phosphorylation of importin13 regulates its subcellular localization and nuclear transport function. Biochem. J. 2018, 475, 2699–2712. [Google Scholar] [CrossRef]
- Yang, J.Y.; Halmo, S.M.; Praissman, J.; Chapla, D.; Singh, D.; Wells, L.; Moremen, K.W.; Lanzilotta, W.N. Crystal structures of β-1,4-N-acetylglucosaminyltransferase 2: Structural basis for inherited muscular dystrophies. Acta Crystallogr. Sect. D-Struct. Biol. 2021, 77, 486–495. [Google Scholar] [CrossRef]
- Shaik, A.P.; Alsaeed, A.H.; Sultana, A. A Phylogenetic analysis of Heparanase (HPSE) gene. Bioinformation 2012, 8, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liswaniso, S.; Shan, X.S.; Zhao, J.H.; Chimbaka, I.M.; Xu, R.F.; Qin, N. The opposite effects of VGLL1 and VGLL4 genes on granulosa cell proliferation and apoptosis of hen ovarian prehierarchical follicles. Theriogenology 2022, 181, 95–104. [Google Scholar] [CrossRef]
- Mizushima, S.; Sasanami, T.; Ono, T.; Matsuzaki, M.; Kansaku, N.; Kuroiwa, A. Cyclin D1 gene expression is essential for cell cycle progression from the maternal-to-zygotic transition during blastoderm development in Japanese quail. Dev. Biol. 2021, 476, 249–258. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Geng, D.; He, J.; Zhao, Y.; Yu, L. Clinical significance of fibroblast growth factor receptor-3 mutations in bladder cancer: A systematic review and meta-analysis. Genet. Mol. Res. 2014, 13, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Takyu, R.; Morimoto, H.; Toei, S.; Sakon, H.; Goto, S.; Moriya, S.; Kono, T. Cell proliferation potency is independent of FGF4 signaling in trophoblast stem cells derived from androgenetic embryos. J. Reprod. Dev. 2016, 62, 51–58. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, I.G.D.; Jorge, E.C.; Copola, A.G.L.; Bertassoli, B.M.; de Goes, A.M.; Silva, G.A.B. FGF2, FGF3 and FGF4 expression pattern during molars odontogenesis in Didelphis albiventris. Acta Histochem. 2017, 119, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.F.; Zhang, L.J.; Guo, F.; Wu, X.Y.; Liu, Y. MiR-195-5p inhibits proliferation and invasion of nerve cells in Hirschsprung disease by targeting GFRA4. Mol. Cell. Biochem. 2021, 476, 2061–2073. [Google Scholar] [CrossRef]
- Wen, J.M.; Grenz, A.; Zhang, Y.J.; Dai, Y.B.; Kellems, R.E.; Blackburn, M.R.; Eltzschig, H.K.; Xia, Y. A2B adenosine receptor contributes to penile erection via PI3K/AKT signaling cascade-mediated eNOS activation. Faseb J. 2011, 25, 2823–2830. [Google Scholar] [CrossRef]

| Groups | AFE | BW-43 | BWFE |
|---|---|---|---|
| 1 | 147–165 d | 1010–1380 g | 1060–1270 g |
| 2 | 166–183 d | 1380–1750 g | 1270–1480 g |
| 3 | 184–201 d | 1750–2120 g | 1480–1690 g |
| 4 | 202–220 d | 2120–2490 g | 1690–1890 g |
| 5 | / | / | 1890–2100 g |
| Traits | Age | n | Mean | SD | 95% Confidence Intervals | |
|---|---|---|---|---|---|---|
| Upper Limit | Lower Limit | |||||
| Start-EW | 1 | 569 | 35.22 | 0.16 | 34.92 | 35.53 |
| 2 | 1235 | 37.80 | 0.11 | 37.59 | 38.01 | |
| 3 | 652 | 41.10 | 0.15 | 40.81 | 41.39 | |
| 4 | 125 | 42.27 | 0.33 | 41.61 | 42.92 | |
| EW-43 | 1 | 569 | 49.92 | 0.17 | 49.59 | 50.24 |
| 2 | 1235 | 50.36 | 0.11 | 50.14 | 50.58 | |
| 3 | 652 | 49.93 | 0.16 | 49.63 | 50.24 | |
| 4 | 125 | 49.69 | 0.35 | 48.99 | 50.38 | |
| EN-43 | 1 | 569 | 129.81 | 0.61 | 128.62 | 131.00 |
| 2 | 1235 | 116.53 | 0.41 | 115.72 | 117.34 | |
| 3 | 652 | 98.30 | 0.57 | 97.19 | 99.41 | |
| 4 | 125 | 83.13 | 1.30 | 80.59 | 85.67 | |
| EN-All | 1 | 569 | 206.62 | 1.57 | 203.55 | 209.69 |
| 2 | 1235 | 191.06 | 1.06 | 188.97 | 193.14 | |
| 3 | 652 | 166.68 | 1.46 | 163.81 | 169.55 | |
| 4 | 125 | 147.13 | 3.34 | 140.58 | 153.68 | |
| Age at First Egg | n | Start-EW | EW-43 | EN-43 | EN-All |
|---|---|---|---|---|---|
| 1 | 569 | 35.22 ± 0.15 d | 49.92 ± 0.17 ab | 129.81 ± 0.61 a | 206.62 ± 1.57 a |
| 2 | 1235 | 37.80 ± 0.11 c | 50.36 ± 0.11 a | 116.53 ± 0.41 b | 191.06 ± 1.06 b |
| 3 | 652 | 41.10 ± 0.15 b | 49.93 ± 0.16 ab | 98.30 ± 0.57 c | 166.68 ± 1.46 c |
| 4 | 125 | 42.27 ± 0.33 a | 49.69 ± 0.35 b | 83.13 ± 1.30 d | 147.13 ± 3.34 d |
| Body Weight at First Egg | n | Start-EW | EW-43 | EN-43 | EN-All |
|---|---|---|---|---|---|
| 1 | 93 | 34.44 ± 0.41 e | 47.80 ± 0.38 e | 116.70 ± 1.98 a | 180.60 ± 4.23 a |
| 2 | 732 | 36.52 ± 0.15 d | 48.53 ± 0.14 d | 116.77 ± 0.71 a | 185.49 ± 1.51 a |
| 3 | 1143 | 38.41 ± 0.12 c | 50.27 ± 0.11 c | 113.53 ± 0.56 a | 187.66 ± 1.21 a |
| 4 | 498 | 40.32 ± 0.18 b | 51.79 ± 0.17 b | 108.80 ± 0.86 b | 185.52 ± 1.83 a |
| 5 | 115 | 42.44 ± 0.37 a | 53.47 ± 0.34 a | 104.19 ± 1.78 c | 183.74 ± 3.81 a |
| Body Weight at 43 Weeks | n | Start-EW | EW-43 | EN-43 | EN-All |
|---|---|---|---|---|---|
| 1 | 331 | 37.22 ± 0.24 c | 48.17 ± 0.20 d | 108.98 ± 1.06 b | 166.56 ± 2.20 b |
| 2 | 1635 | 38.13 ± 0.11 c | 49.72 ± 0.09 c | 113.31 ± 0.48 ab | 188.22 ± 0.99 a |
| 3 | 573 | 39.17 ± 0.18 b | 52.15 ± 0.16 b | 115.44 ± 0.81 a | 191.81 ± 1.67 a |
| 4 | 41 | 40.44 ± 0.67 a | 53.82 ± 0.58 a | 114.15 ± 3.01 a | 188.07 ± 6.26 a |
| Item | Autumn | Winter | Spring | |
|---|---|---|---|---|
| Autumn | Pearson Correlation | 1 | 0.165 ** | 0.094 ** |
| Significance (two-tailed) | <0.001 | <0.001 | ||
| n | 2980 | 2980 | 2980 | |
| Winter | Pearson Correlation | 0.165 ** | 1 | 0.597 ** |
| Significance (two-tailed) | <0.001 | <0.001 | ||
| n | 2980 | 3149 | 3149 | |
| Spring | Pearson Correlation | 0.094 ** | 0.597 ** | 1 |
| Significance (two-tailed) | <0.001 | <0.001 | ||
| n | 2980 | 3149 | 3149 | |
| Traits | Heritability | Heritability Standard Error |
|---|---|---|
| Start-EW | 0.299 | 0.049 |
| EW-43 | 0.213 | 0.048 |
| EN-43 | 0.132 | 0.040 |
| EN-All | 0.162 | 0.047 |
| Item | Start-EW | EW-43 | EN-43 | EN-All |
|---|---|---|---|---|
| Start-EW | 1.00 | 0.52 | −0.31 | −0.30 |
| EW-43 | 0.52 | 1.00 | −0.23 | −0.15 |
| EN-43 | −0.31 | −0.23 | 1.00 | 0.67 |
| EN-All | −0.30 | −0.15 | 0.67 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Lu, X.; Li, G.; Zhang, H.; Zhou, C.; Yin, J.; Han, W.; Yang, H. Genetic Analysis of Egg Production Traits in Luhua Chickens: Insights from a Multi-Trait Animal Model and a Genome-Wide Association Study. Genes 2024, 15, 796. https://doi.org/10.3390/genes15060796
Yang Q, Lu X, Li G, Zhang H, Zhou C, Yin J, Han W, Yang H. Genetic Analysis of Egg Production Traits in Luhua Chickens: Insights from a Multi-Trait Animal Model and a Genome-Wide Association Study. Genes. 2024; 15(6):796. https://doi.org/10.3390/genes15060796
Chicago/Turabian StyleYang, Qianwen, Xubin Lu, Guohui Li, Huiyong Zhang, Chenghao Zhou, Jianmei Yin, Wei Han, and Haiming Yang. 2024. "Genetic Analysis of Egg Production Traits in Luhua Chickens: Insights from a Multi-Trait Animal Model and a Genome-Wide Association Study" Genes 15, no. 6: 796. https://doi.org/10.3390/genes15060796
APA StyleYang, Q., Lu, X., Li, G., Zhang, H., Zhou, C., Yin, J., Han, W., & Yang, H. (2024). Genetic Analysis of Egg Production Traits in Luhua Chickens: Insights from a Multi-Trait Animal Model and a Genome-Wide Association Study. Genes, 15(6), 796. https://doi.org/10.3390/genes15060796





