Genetic Variations of MSTN and Callipyge in Tibetan Sheep: Implications for Early Growth Traits
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Sample Collection and Primer Design
2.3. SNP Identification and Sequencing
2.4. Population Genetic Index Calculation
2.5. Statistical Analysis
3. Results
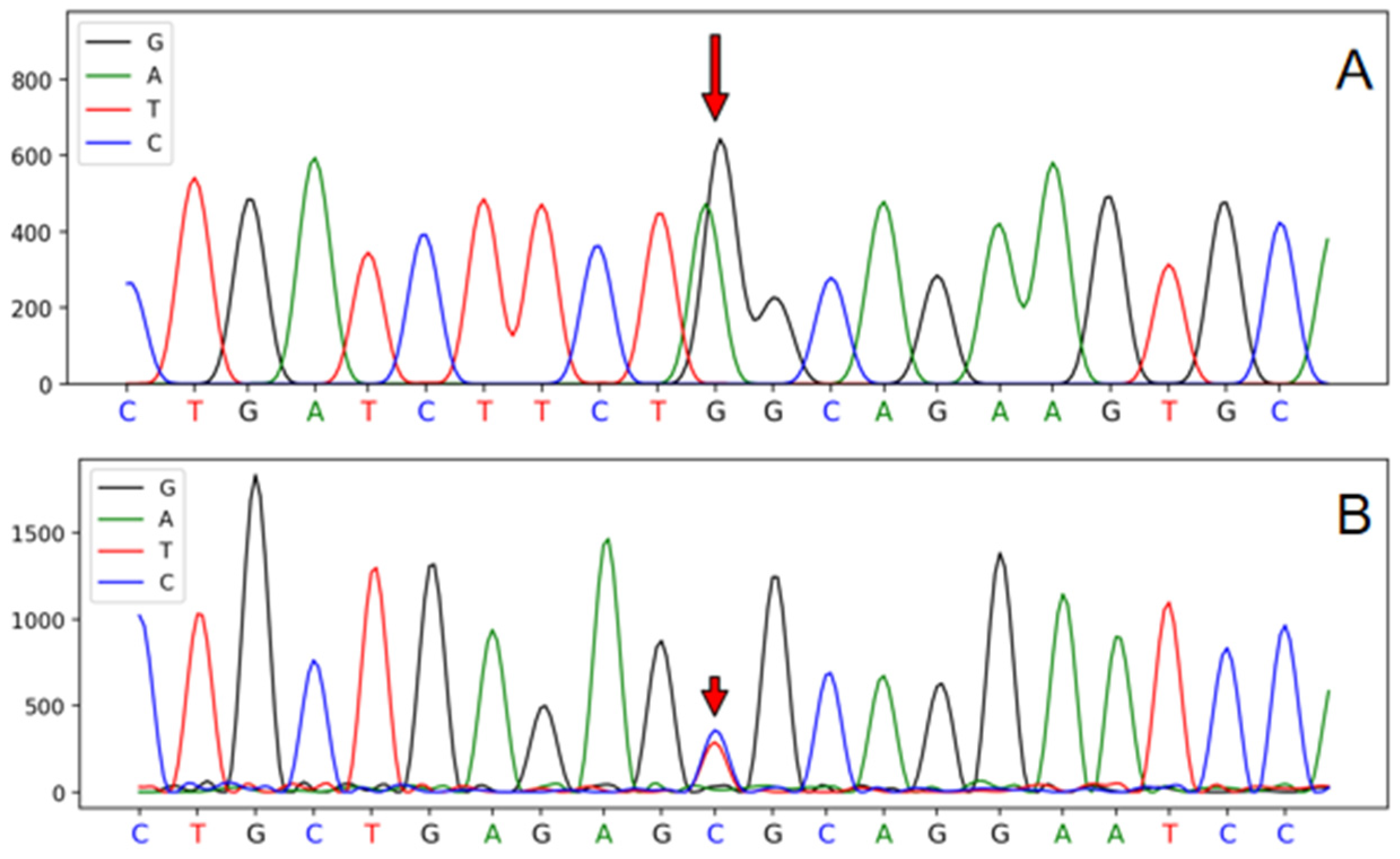
3.1. Polymorphism in Genes
3.2. Population Genetic Analysis
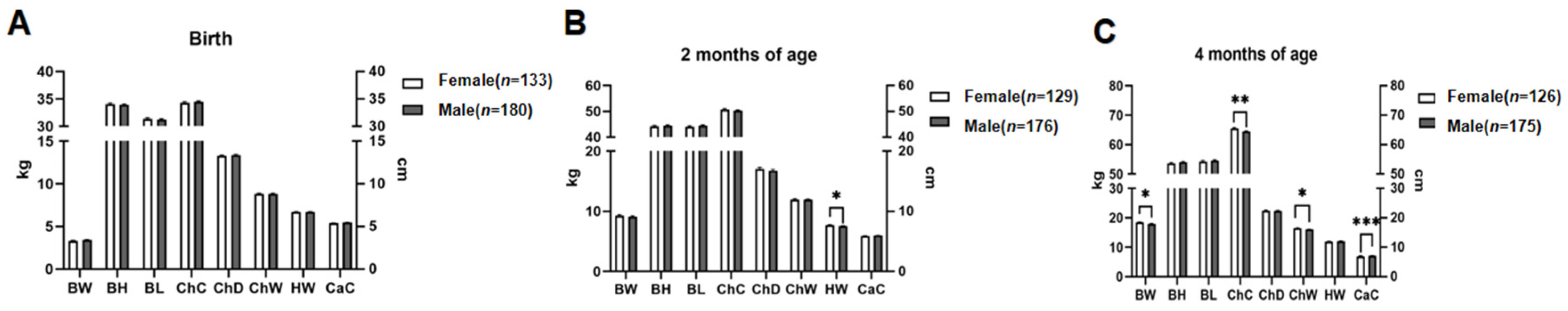
3.3. Association Analyses of Sex with Growth Traits
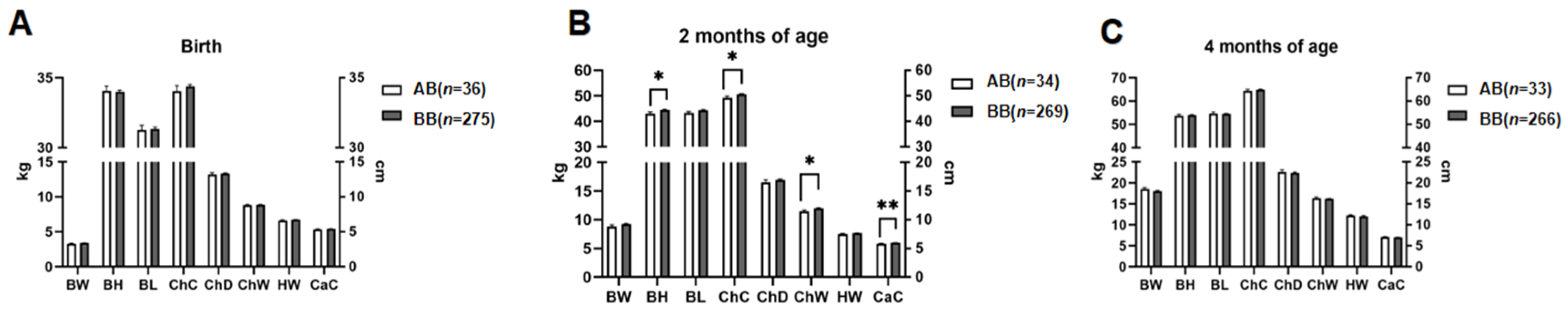
3.4. Analyses of SNPs Associated with Growth Traits
3.5. Analysis of Interaction Associations between Sex and SNPs with Growth Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.Q.; Li, Y.J.; Luo, Y.Z. Bacterial community in the rumen of Tibetan sheep and Gansu alpine fine-wool sheep grazing on the Qinghai-Tibetan Plateau, China. J. Gen. Appl. Microbiol. 2017, 63, 122–130. [Google Scholar] [CrossRef]
- Wang, X.; Xu, T.; Zhang, X.; Geng, Y.; Kang, S.; Xu, S. Effects of Dietary Protein Levels on Growth Performance, Carcass Traits, Serum Metabolites, and Meat Composition of Tibetan Sheep during the Cold Season on the Qinghai-Tibetan Plateau. Animals 2020, 10, 801. [Google Scholar] [CrossRef]
- Li, J.; Zhu, W.; Luo, M.; Ren, H.; Tang, L.; Liao, H.; Wang, Y. Molecular cloning, expression and purification of lactoferrin from Tibetan sheep mammary gland using a yeast expression system. Protein Expr. Purif. 2015, 109, 35–39. [Google Scholar] [CrossRef]
- Li, L.L. Study on the Effect of Mixed Forage Silage and Supplemental Feeding on Euler-Type Tibetan Sheep in Qingnan Pastoral Area; Chinese Academy of Agricultural Sciences: Beijing, China, 2017. [Google Scholar]
- Zhang, Y.H. Breed characteristics, production performance and grazing technology of Tibetan sheep. Contemp. Livest. Poult. Breed. 2017, 9, 15. [Google Scholar] [CrossRef]
- Wang, G.F.; Li, J.; Jiang, Q.; Ren, H.X.; Wang, L.; Li, S.Y.; Zhou, P. Growth performance and blood parameters of Hu sheep in southwest China. Heilongjiang Anim. Sci. Vet. Med. 2018, 11, 122–124. [Google Scholar]
- Xu, P. Application of Early Weaning of Lambs; Gansu Agricultural University: Lanzhou, China, 2008. [Google Scholar]
- Al-Mamun, H.A.; Kwan, P.; Clark, S.A.; Ferdosi, M.H.; Tellam, R.; Gondro, C. Genome-wide association study of body weight in Australian Merino sheep reveals an orthologous region on OAR6 to human and bovine genomic regions affecting height and weight. Genet. Sel. Evol. 2015, 47, 66. [Google Scholar] [CrossRef]
- La, Y.; Zhang, X.; Li, F.; Zhang, D.; Li, C.; Mo, F.; Wang, W. Molecular Characterization and Expression of SPP1, LAP3 and LCORL and Their Association with Growth Traits in Sheep. Genes 2019, 10, 616. [Google Scholar] [CrossRef]
- Salabi, F.; Nazari, M.; Chen, Q.; Nimal, J.; Tong, J.; Cao, W.G. Myostatin knockout using zinc-finger nucleases promotes proliferation of ovine primary satellite cells in vitro. J. Biotechnol. 2014, 192, 268–280. [Google Scholar] [CrossRef]
- Du, C.; Zhou, X.; Zhang, K.; Huang, S.; Wang, X.; Zhou, S.; Chen, Y. Inactivation of the MSTN gene expression changes the composition and function of the gut microbiome in sheep. BMC Microbiol. 2022, 22, 273. [Google Scholar] [CrossRef]
- Grobet, L.; Martin, L.J.; Poncelet, D.; Pirottin, D.; Brouwers, B.; Riquet, J.; Schoeberlein, A.; Dunner, S.; Ménissier, F.; Massabanda, J.; et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat. Genet. 1997, 17, 71–74. [Google Scholar] [CrossRef]
- Lee, S.J.; McPherron, A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA 2001, 98, 9306–9311. [Google Scholar] [CrossRef]
- Mcpherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-psuperfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Yu, L.; Tang, H.; Wang, J.; Wu, Y.; Zou, L.; Jiang, Y.; Wu, C.; Li, N. Polymorphisms in the 5′ regulatory region of myostatin gene are associated with early growth traits in Yorkshire pigs. Sci. China C Life Sci. 2007, 50, 642–647. [Google Scholar] [CrossRef]
- Boman, I.A.; Klemetsdal, G.; Blichfeldt, T.; Nafstad, O.; Våge, D.I. A frameshift mutation in the coding region of the myostatin gene (MSTN) affects carcass conformation and fatness in Norwegian white sheep (Ovis aries). Anim. Genet. 2009, 40, 418–422. [Google Scholar] [CrossRef]
- Zhang, G.X.; Zhao, X.H.; Wang, J.Y.; Ding, F.X.; Zhang, L. Effect of an exon 1 mutation in the myostatin gene on the growth traits of the Bian chicken. Anim. Genet. 2011, 43, 458–459. [Google Scholar] [CrossRef]
- Li, H.; Wang, G.; Hao, Z.; Zhang, G.; Qing, Y.; Liu, S.; Qing, L.; Pan, W.; Chen, L.; Liu, G.; et al. Generation of biallelic knock-out sheep via gene-editing and somatic cell nuclear transfer. Sci. Rep. 2016, 6, 33675. [Google Scholar] [CrossRef]
- Clop, A.; Marcq, F.; Takeda, H.; Pirottin, D.; Tordoir, X.; Bibé, B.; Bouix, J.; Caiment, F.; Elsen, J.M.; Eychenne, F.; et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat. Genet. 2006, 38, 813–818. [Google Scholar] [CrossRef]
- Ren, Z.Z.; He, X.Y.; Chu, M.X. Research Progress of Callipyge in Sheep. Chin. Herbiv. Sci. 2023, 43, 52–54. [Google Scholar]
- Li, J.; Greenwood, P.L.; Cockett, N.E.; Hadfield, T.S.; Vuocolo, T.; Byrne, K.; White, J.D.; Tellam, R.L.; Schirra, H.J. Impacts of the Callipyge mutation on ovine plasma metabolites and muscle fibre type. PLoS ONE 2014, 9, 99726. [Google Scholar] [CrossRef]
- Charlier, C.; Segers, K.; Wagenaar, D.; Karim, L.; Berghmans, S.; Jaillon, O.; Shay, T.; Weissenbach, J.; Cockett, N.; Gyapay, G.; et al. Humanovine comparative sequencing of a 250kilo base imprinted domaien compassing the Callipyge (clpg) gene and identification of six imprinted transcripts:DLK1,DAT GTL2,PEG11, antiPEG11 and MEG8. Genome Res. 2001, 11, 850–862. [Google Scholar] [CrossRef]
- Georgiades, P.; Watkins, M.; Surani, M.A.; Ferguson-Smith, A.C. Parental origin-pecific developmental defects in mice with uniparental disomy for chromosome 12. Development 2000, 127, 4719–4728. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Redrup, L. Genetic imprinting:conflict at the Callipyge locus. Curr. Biol. 2005, 15, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C. Towards the molecular understanding of the polar over dominance phenomenon associated with the Callipyge phenotype in sheep. Bull. Mem. Acad. R. Med. Belg. 2004, 159, 490–496. [Google Scholar] [PubMed]
- Cockett, N.E.; Jackson, S.P.; Shay, T.L.; Farnir, F.; Berghmans, S.; Snowder, G.D.; Nielsen, D.M.; Georges, M. Polar Overdominance at the Ovine Callipyge Locus. Science 1996, 273, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, C.A.; Shay, T.L.; Georges, M.; Beever, J.E.; Berghmans, S.; Cockett, N.E. Differential expression of the GTL2 gene within the Callipyge region of ovine chromosome 18. Anim. Genet. 2001, 32, 248–256. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, L.P.; Yang, L.; Wu, J.P.; Ha, Z.J.; Yu, L.H.; Li, W.W.; Wu, X.J. Study on correlation between Callipyge gene polymorphisms and growth traits in Oula Tibetan sheep. J. Gansu Agric. Univ. 2010, 45, 1–4+9. [Google Scholar] [CrossRef]
- Hu, S.J.; Zhang, S.Z.; Yuan, Z.H.; Xuan, J.L.; Ma, X.M.; Zhang, L.; Zhao, F.P.; Wei, C.H.; Du, L.X. Polymorphisms of CLPG and MSTN genes in sheep and their association with growth traits. China Anim. Husb. Vet. Med. 2016, 43, 1285–1293. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef]
- Nei, M.; Roychoudhury, A.K. Sampling variances of heterozygosity and genetic distance. Genetics 1974, 76, 379–390. [Google Scholar] [CrossRef]
- Ortega, M.S.; Denicol, A.C.; Cole, J.B.; Null, D.J.; Hansen, P.J. Use of single nucleotide polymorphisms in candidate genes associated with daughter pregnancy rate for prediction of genetic merit for reproduction in Holstein cows. Anim. Genet. 2016, 47, 288–297. [Google Scholar] [CrossRef]
- Marcq, F.; Larzul, C.; Marot, V.; Bouix, J.; Eychenne, F.; Laville, É.; Bibé, B.; Leroy, P.; Georges, M.; Elsenet, J. Preliminary results of a whole-genome scan targeting QTL for carcass traits in a Texel×Romanov intercross. Agric. Food Sci. 2002, 34, 371. [Google Scholar] [CrossRef]
- Fahrenkrug, S.C.; Freking, B.A.; Rexroad, C.E.; Leymaster, K.A.; Kappes, S.M.; Smith, T.P. Comparative mapping of the ovine clpg locus. Mamm. Genome 2000, 11, 871–876. [Google Scholar] [CrossRef]
- Wang, S.; Ma, T.; Zhao, G.; Zhang, N.; Tu, Y.; Li, F.; Cui, K.; Bi, Y.; Ding, H.; Diao, Q. Effect of Age and Weaning on Growth Performance, Rumen Fermentation, and Serum Parameters in Lambs Fed Starter with Limited Ewe-Lamb Interaction. Animals 2019, 9, 825. [Google Scholar] [CrossRef]
- Harmata, A.J.; Ma, Y.; Sanchez, C.J.; Zienkiewicz, K.J.; Elefteriou, F.; Wenke, J.C.; Guelcher, S.A. D-amino acid inhibits biofilm but not new bone formation in an ovine model. Clin. Orthop. Relat. Res. 2015, 473, 3951–3961. [Google Scholar] [CrossRef]
- Deng, B.; Zhang, F.; Wen, J.; Ye, S.; Wang, L.; Yang, Y.; Gong, P.; Jiang, S. The function of myostatin in the regulation of fat mass in mammals. Nutr. Metab. 2017, 14, 29. [Google Scholar] [CrossRef]
- Tao, M.H.; Meng, K.; Rong, X.; Qiang, H.; Nie, W.; Guo, C.H.; Feng, D.Z. Polymorphisms of MSTN gene and its association with meat quality traits in sheep. China Anim. Husb. Vet. Med. 2023, 2766–2776. [Google Scholar] [CrossRef]
- Lei, X.; Liu, C.X.; He, S.G.; Peng, X.R.; Mao, L.J.; Liu, M.J.; Qi, A. Association analysis of g+6723GA locus of MSTN gene with carcass body size and muscle weight in different parts. Grass-Fed. Livest. 2022, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.N.; Wang, Y.C.; Yang, L.C.; He, J.; Song, Y.X. Polymorphisms of MSTN gene and its association with growth traits in East Freey sheep. China Anim. Husb. Vet. Med. 2021, 4537–4544. [Google Scholar] [CrossRef]
- Ma, L.N.; Li, Y.K.; Yu, Y.; E, E.H.H.; Ma, Q. TaqMan probe SNP typing of myostatin (MSTN) gene and its association with growth traits. Heilongjiang Anim. Husb. Vet. Med. 2016, 4–6. [Google Scholar] [CrossRef]
- Hadjipavlou, G.; Matika, O.; Clop, A.; Bishop, S.C. Two single nucleotide polymorphisms in the myostatin (GDF8) gene have significant association with muscle depth of commercial Charollais sheep. Anim. Genet. 2008, 39, 346–353. [Google Scholar] [CrossRef]
- Sahu, A.R.; Jeichitra, V.; Rajendran, R.; Raja, A. Novel report on mutation in exon 3 of myostatin (MSTN) gene in Nilagiri sheep: An endangered breed of South India. Trop. Anim. Health Prod. 2019, 51, 1817–1822. [Google Scholar] [CrossRef]
- Hickford, J.G.; Forrest, R.H.; Zhou, H.; Fang, Q.; Han, J.; Frampton, C.M.; Horrell, A.L. Polymorphisms in the ovine myostatin gene (MSTN) and their association with growth and carcass traits in New Zealand Romney sheep. Anim. Genet. 2010, 41, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Grade, C.V.C.; Mantovani, C.S.; Alvares, L.E. Myostatin gene promoter:Structure, conservation and importance as a target for muscle modulation. J. Anim. Sci. Biotechnol. 2019, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Niu, Y.; Zhou, J.; Yu, H.; Kou, Q.; Lei, A.; Zhao, X.; Yan, H.; Cai, B.; Shen, Q.; et al. Multiplex gene editing via CRISPR/Cas9 exhibits desirable muscle hypertrophy without detectable off-target effects in sheep. Sci. Rep. 2016, 6, 32271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Kalds, P.; Luo, Q.; Sun, K.; Zhao, X.; Gao, Y.; Cai, B.; Huang, S.; Kou, Q.; Petersen, B.; et al. Optimized Cas9:sgRNA delivery efficiently generates biallelic MSTN knockout sheep without affecting meat quality. BMC Genom. 2022, 23, 348. [Google Scholar] [CrossRef]
- Kijas, J.W.; McCulloch, R.; Edwards, J.E.; Oddy, V.H.; Lee, S.H.; van der Werf, J. Evidence for multiple alleles effecting muscling and fatness at the ovine GDF8 locus. BMC Genet. 2007, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Chen, R.J.; Mao, Y.J.; Huang, D.L.; Yang, Z.P. Analysis on SNPs of Callipyge gene and Meg3.1 and DLK1-INTR1.1 loci in four sheep. Anim. Husb. Vet. Med. 2008, 4, 11–15. [Google Scholar] [CrossRef]
- Yan, Y.B. Study on the Correlation between SNPs and Five Microsatellite Loci Polymorphisms of CLPG Gene and Hind Rump Development in Meat Sheep; Gansu Agricultural University: Lanzhou, China, 2008. [Google Scholar]
- Freking, B.A.; Murphy, S.K.; Wylie, A.A.; Rhodes, S.J.; Keele, J.W.; Leymaster, K.A.; Jirtle, R.L.; Smith, T.P. Identification of the single base change causing the Callipyge muscle hypertrophy phenotype, the only known example of polar overdominance in mammals. Genome Res. 2002, 12, 1496–1506. [Google Scholar] [CrossRef]
- Dai, R.A. Preliminary Study on the Relationship between CLPG and BMP15 Gene Polymorphisms and the Production Performance of Seven Sheep Populations in Northern Xinjiang; Nanjing Agricultural University: Nanjing, China, 2004. [Google Scholar]
- Meyer, H.H.; Busboom, J.R.; Abdulkhaliq, A. Carcass and cooked meat traits of lambs sired by Dorset rams heterozygous for the Callipyge gene, Suffolk rams and Texel rams Proc. West. Sect. Am. Soc. Anim. Sci. 1995, 46, 199. [Google Scholar]

| Primer Names | Primer Sequences (5′–3′) | Size (bp) | Tm (°C) |
|---|---|---|---|
| Callipyge | F:TGAAAACGTGAACCCAGAAGC | 493 | 60 |
| R: GGCAGGAGAGACGGTTAAT | |||
| MSTN-Exon1 | F: ATCACAGATCCCGACGACAC | 704 | 60 |
| R: CTCTTTGCCCTCCTCCTTAC | |||
| MSTN-Exon2 | F: CATAGATTGACATGGAGGCG | 601 | 60 |
| R: TTTATTGGGTACAGGGCTAC | |||
| MSTN-Exon3 | F: CCATAAAGGCAGAATCAAGC | 736 | 60 |
| R: TGTTGTGATGGTTAAATGCC |
| SNP | Gene Frequency | N | Ho | He | PIC | Ne | HW | |
|---|---|---|---|---|---|---|---|---|
| A | B | |||||||
| MSTN | 0.058 | 0.942 | 311 | 0.116 | 0.109 | 0.221 | 1.122 | ns |
| Callipyge | 0.861 | 0.139 | 312 | 0.234 | 0.240 | 0.404 | 1.316 | ns |
| State | SNP | Trait | Genotype | Female | Male | p-Value |
|---|---|---|---|---|---|---|
| Birth | MSTN | BW | AB | 3.12 ± 0.10 b | 3.51 ± 0.13 a | 0.020 |
| BB | 3.37 ± 0.05 | 3.40 ± 0.04 | 0.587 | |||
| p-value | 0.044 | |||||
| 2 months | Callipyge | CaC | AA | 5.88 ± 0.04 B | 6.04 ± 0.03 A | 0.003 |
| AB | 5.99 ± 0.07 | 5.85 ± 0.06 | 0.123 | |||
| BB | 6.03 ± 0.22 | 5.70 ± 0.19 | 0.249 | |||
| p-value | 0.007 | |||||
| Gene | Breed | SNP |
|---|---|---|
| MSTN | Dubo sheep, Tan sheep, small-tailed Han sheep | rs129059715 |
| Texel × Altai crossbred sheep | g.6723G>A | |
| East Friensian sheep | 3′-UTR-272, 5-UTR-176 | |
| Tan sheep | rs417816017 | |
| Charollais sheep | g.2449G>C | |
| Nilagiri sheep | g.5622G>C | |
| New Zealand Romney sheep | g.6223G | |
| Callipyge | Tausset sheep | A→G (at 211 bp in AF401294 amplified region) |
| Tibetan sheep | C→T (at 176 bp in AF401294 amplified region) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Li, X.; Liu, D.; Wang, L.; Pei, Q.; Han, B.; Zhang, Z.; Tian, D.; Wang, S.; Zhao, J.; et al. Genetic Variations of MSTN and Callipyge in Tibetan Sheep: Implications for Early Growth Traits. Genes 2024, 15, 921. https://doi.org/10.3390/genes15070921
Zhao K, Li X, Liu D, Wang L, Pei Q, Han B, Zhang Z, Tian D, Wang S, Zhao J, et al. Genetic Variations of MSTN and Callipyge in Tibetan Sheep: Implications for Early Growth Traits. Genes. 2024; 15(7):921. https://doi.org/10.3390/genes15070921
Chicago/Turabian StyleZhao, Kai, Xue Li, Dehui Liu, Lei Wang, Quanbang Pei, Buying Han, Zian Zhang, Dehong Tian, Song Wang, Jincai Zhao, and et al. 2024. "Genetic Variations of MSTN and Callipyge in Tibetan Sheep: Implications for Early Growth Traits" Genes 15, no. 7: 921. https://doi.org/10.3390/genes15070921





