Transcriptome Analysis Reveals Genes and Pathways Associated with Drought Tolerance of Early Stages in Sweet Potato (Ipomoea batatas (L.) Lam.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Drought-Related Physiological Chemical Contents and Yield-Related Indexes
2.3. Total RNA Isolation and Library Construction
2.4. Analysis of Differentially Expressed Genes
2.5. Gene Ontology Term Enrichment Analysis and KEGG Enrichment Analysis
3. Results
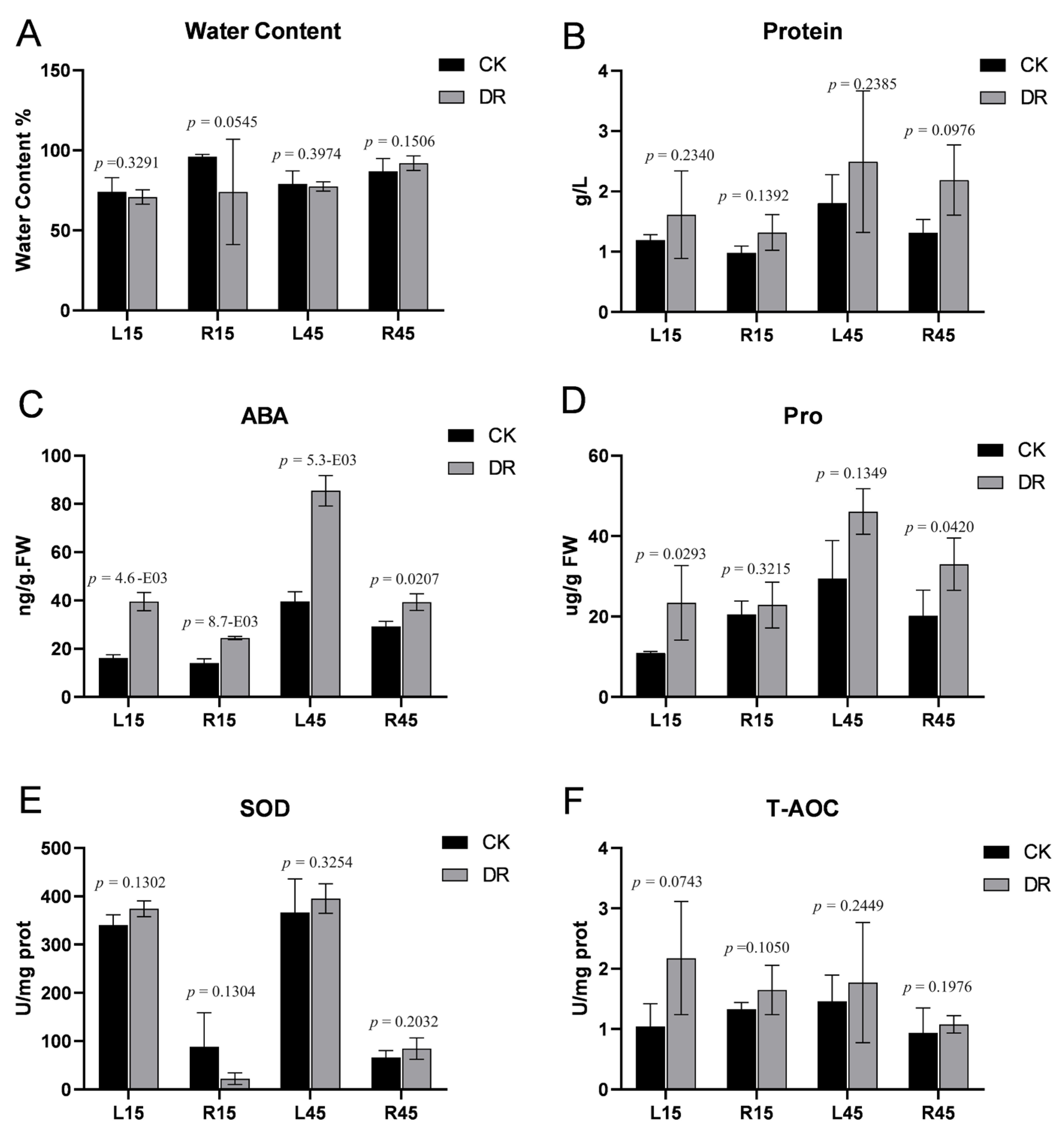
3.1. Physiological Responses of Tuberous Roots and Leaves under Drought Stress
3.2. Transcriptome Sequencing, Quality Filtering, and Assembly
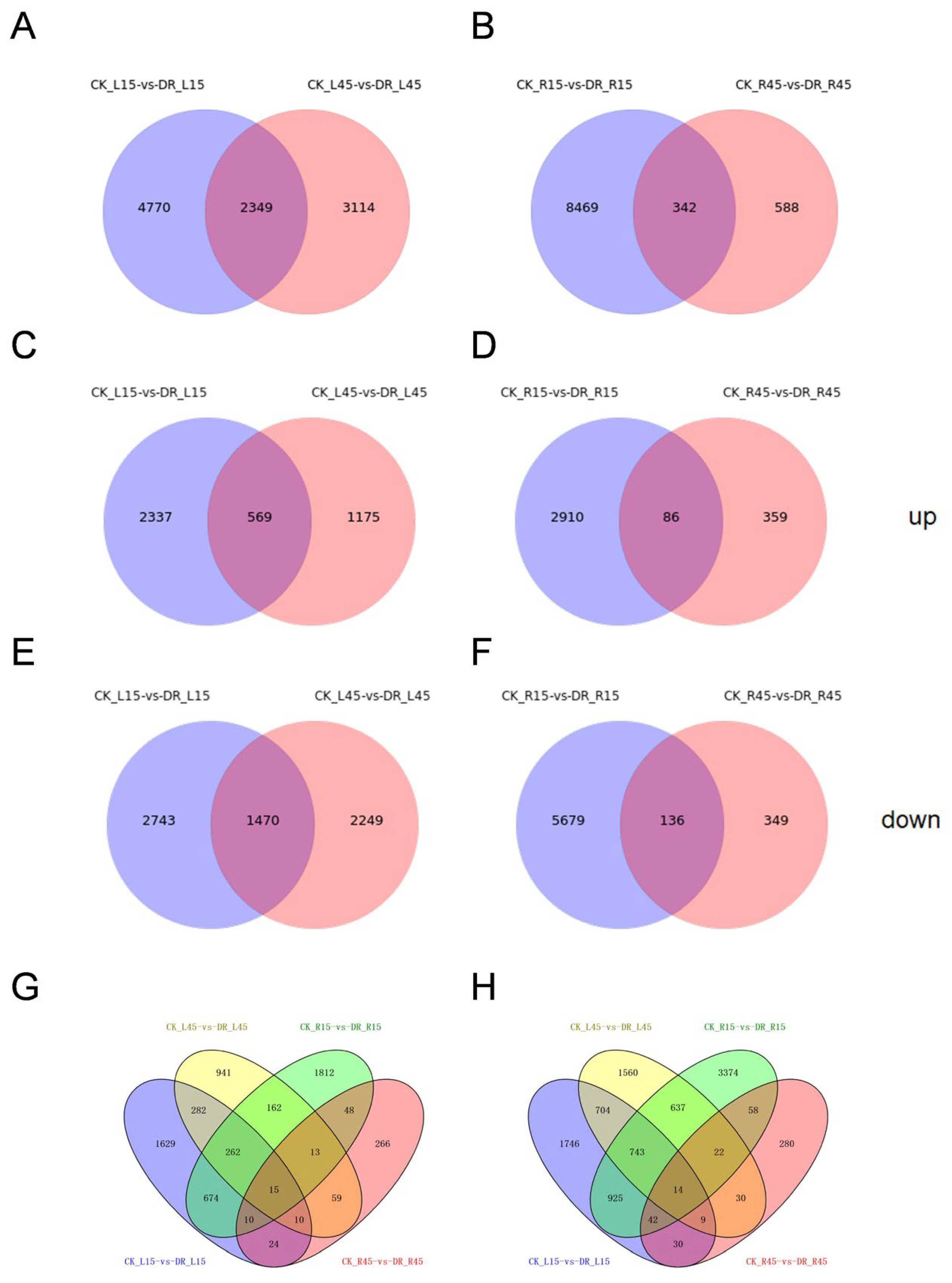
3.3. Comparison of Differentially Expressed Genes among All Four Libraries between Drought Versus Corresponding Control Samples
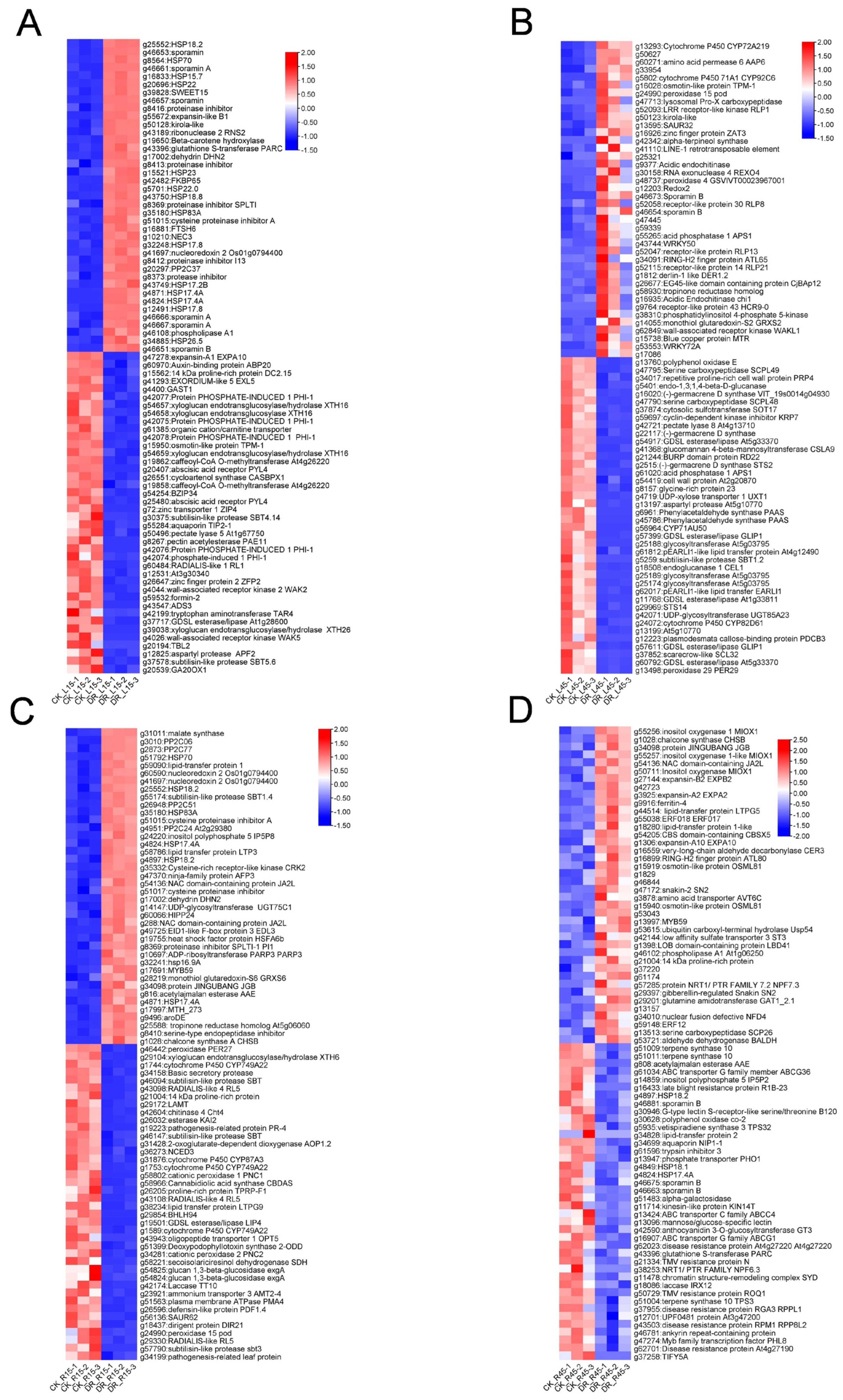
3.4. Functional Annotation and Classification of DEGs
3.5. Differentially Expressed Transcriptional Factors
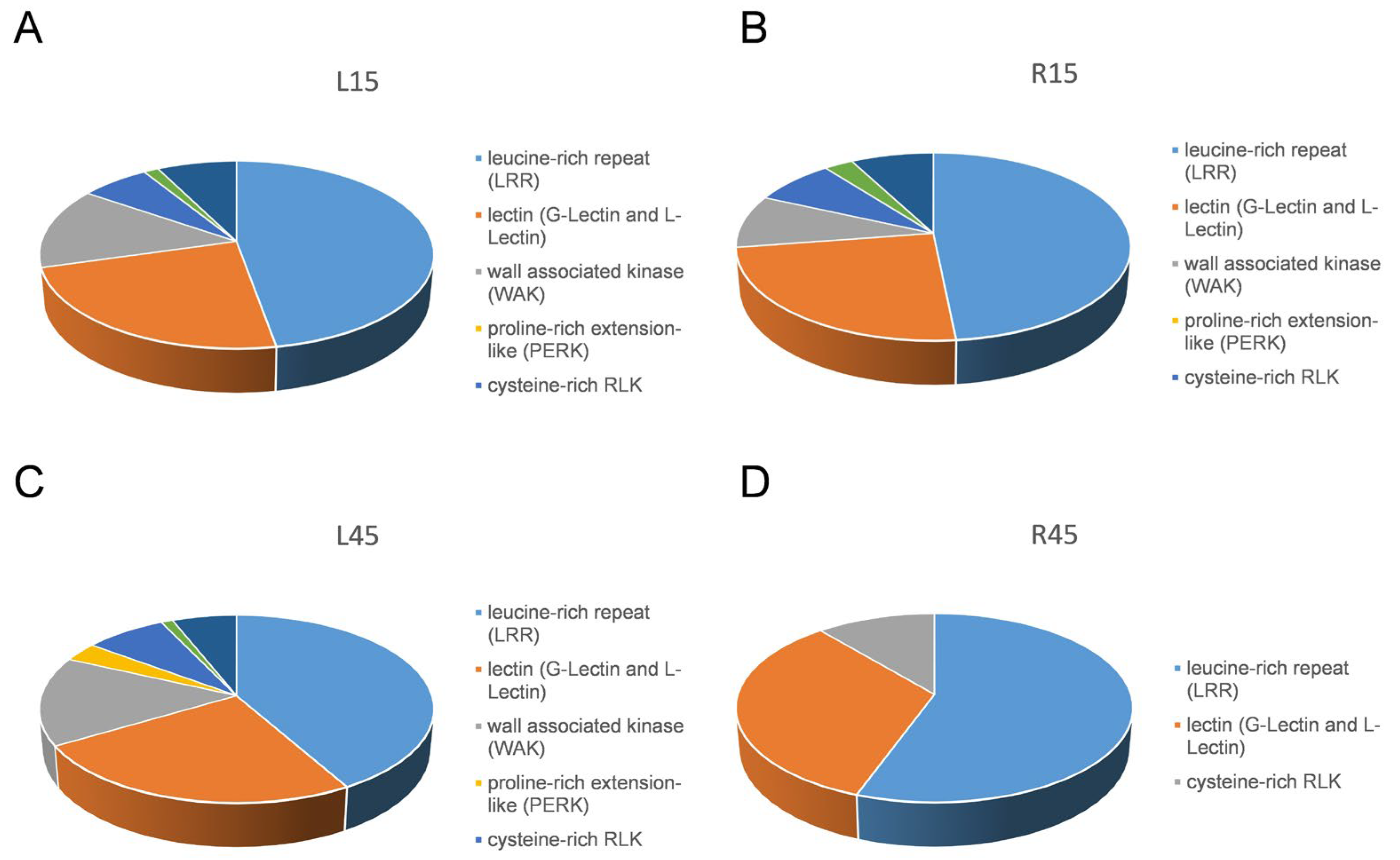
3.6. Receptor-like Kinases Differentially Expressed in Response to Drought
4. Discussion
4.1. Pathways of Drought Tolerance in Different Tissues and Developmental Stages
4.2. ABA Biosynthesis and Signaling Pathway
4.3. Plant–Pathogen Interaction
4.4. Osmotic Homeostasis
4.5. Osmotin-like Protein TPM1
4.6. Aquaporin
4.7. Damage Control and Repair
Heat Shock Proteins
4.8. Sporamin
4.9. LEA Proteins
4.10. Proteins Required for Detoxification
4.11. Lipid Metabolism
GDSL Esterase/Lipase
4.12. Growth Inhibition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Rico-Medina, A.; Cano-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Palta, J.; Vadez, V. Review: An integrated framework for crop adaptation to dry environments: Responses to transient and terminal drought. Plant Sci. 2016, 253, 58–67. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and Drought Stress Signal Transduction in Plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Amagloh, F.C.; Yada, B.; Tumuhimbise, G.A.; Amagloh, F.K.; Kaaya, A.N. The Potential of Sweetpotato as a Functional Food in Sub-Saharan Africa and Its Implications for Health: A Review. Molecules 2021, 26, 2971. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Wang, L.; Wu, Y.J.; Chen, X.L.; Kong, X.; Gong, J.Y.; Wang, J.F.; Yi, Y.; Xiong, H. Molecular and Microecological Mechanisms of Plant Responses to Drought Stress. Mol. Plant Breed. 2023, 7, 50. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20230406.1634.006.html (accessed on 6 May 2024). (In Chinese with English abstract).
- Yan, H.; Zhang, Y.; Ahmad, M.Q.; Liu, Y.; Kou, M.; Ma, M.; Li, C.; Arisha, M.H.; Tang, W.; Wang, X.; et al. Comparative Analysis of Anthocyanin Compositions and Starch Physiochemical Properties of Purple-Fleshed Sweetpotato “Xuzishu8” in Desert Regions of China. Front. Plant Sci. 2022, 13, 841969. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, S.; Di Marco, G.; Bruno, L.; Gismondi, A.; Canini, A. Investigating the Drought and Salinity Effect on the Redox Components of Sulla Coronaria (L.) Medik. Antioxidants 2021, 10, 1048. [Google Scholar] [CrossRef]
- Rai, G.K.; Parveen, A.; Jamwal, G.; Basu, U.; Kumar, R.R.; Rai, P.K.; Sharma, J.P.; Alalawy, A.I.; Al-Duais, M.A.; Hossain, M.A.; et al. Leaf Proteome Response to Drought Stress and Antioxidant Potential in Tomato (Solanum lycopersicum L.). Atmosphere 2021, 12, 1021. [Google Scholar] [CrossRef]
- Lau, K.H.; Del Rosario Herrera, M.; Crisovan, E.; Wu, S.; Fei, Z.; Khan, M.A.; Buell, C.R.; Gemenet, D.C. Transcriptomic analysis of sweet potato under dehydration stress identifies candidate genes for drought tolerance. Plant Direct 2018, 2, e00092. [Google Scholar] [CrossRef]
- Arisha, M.H.; Aboelnasr, H.; Ahmad, M.Q.; Liu, Y.; Tang, W.; Gao, R.; Yan, H.; Kou, M.; Wang, X.; Zhang, Y.; et al. Transcriptome sequencing and whole genome expression profiling of hexaploid sweetpotato under salt stress. BMC Genom. 2020, 21, 197. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Xu, L.; Luo, Z.; Li, Z.; Zhou, G.; Gao, H.; Fang, F.; Tang, J.; Zhao, Y.; Zhou, Z.; et al. Transcriptomic analysis reveals mechanisms for the different drought tolerance of sweet potatoes. Front. Plant Sci. 2023, 14, 1136709. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Y.; Fu, Z.G. Advance of research on drought resistant physiology and cultivation techniques of sweet potato. Agric. Res. Arid. Areas 2004, 22, 128–131, (In Chinese with English abstract). [Google Scholar]
- Zhang, L.; Gao, W.; Cao, Z.; He, L.S.; Tan, G.Y.; Wang, B.M. Immunolocalization and Quantitation of ABA and IAA in the Organs of Wheat (Triticum aestivum L.) Under Drought Stress. Sci. Agric. Sin. 2014, 47, 2940–2948. [Google Scholar]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7, gix120. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Nie, H.; Wang, Y.; Wang, X.; Jarret, R.; Zhao, J.; Wang, H.; Yang, J. Exploring and exploiting genetics and genomics for sweetpotato improvement: Status and perspectives. Plant Commun. 2022, 3, 100332. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Moeinzadeh, M.H.; Kuhl, H.; Helmuth, J.; Xiao, P.; Haas, S.; Liu, G.; Zheng, J.; Sun, Z.; Fan, W.; et al. Haplotype-resolved sweet potato genome traces back its hexaploidization history. Nat. Plants 2017, 3, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Oelmüller, R. Emerging Roles of Receptor-like Protein Kinases in Plant Response to Abiotic Stresses. Int. J. Mol. Sci. 2023, 24, 14762. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.Z. Influence of soil aridity on the growth, development and yield of sweet potato (Ipomoea batatas L.). Acta Agric. Boreali-Sin. 1995, 02, 106–110, (In Chinese with English abstract). [Google Scholar]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2021, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Viktorova, J.; Krasny, L.; Kamlar, M.; Novakova, M.; Mackova, M.; Macek, T. Osmotin, a pathogenesis-related protein. Curr. Protein Pept. Sci. 2012, 13, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Arisha, M.H.; Zhang, Z.; Yan, H.; Kou, M.; Song, W.; Li, C.; Gao, R.; Ma, M.; Wang, X.; et al. Comparative transcriptomic and proteomic analysis reveals common molecular factors responsive to heat and drought stresses in sweetpotaoto (Ipomoea batatas). Front. Plant Sci. 2022, 13, 1081948. [Google Scholar] [CrossRef]
- Kaldenhoff, R.; Fischer, M. Aquaporins in plants. Acta Physiol. 2006, 187, 169–176. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.W.; Chen, J.C.; Lin, M.I.; Chen, Y.M.; Lin, C.Y. Functional activity of sporamin from sweet potato (Ipomoea batatas Lam.): A tuber storage protein with trypsin inhibitory activity. Plant Mol. Biol. 1997, 33, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, R.; Yeh, K.-W. Multiple biological functions of sporamin related to stress tolerance in sweet potato (Ipomoea batatas Lam). Biotechnol. Adv. 2012, 30, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.C.; Chen, Y.C.; Chen, H.J.; Lin, Y.H.; Yang, L.L.; Lee, M.H. Antioxidant activities of trypsin inhibitor, a 33 KDa root storage protein of sweet potato (Ipomoea batatas (L.) Lam cv. Tainong 57). J. Agric. Food Chem. 2001, 49, 2978–2981. [Google Scholar] [CrossRef]
- Hand, S.C.; Menze, M.A.; Toner, M.; Boswell, L.; Moore, D. LEA Proteins During Water Stress: Not Just for Plants Anymore. Annu. Rev. Physiol. 2011, 73, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Wang, L.; Wu, R.; Phillips, J.; Deng, X. LEA 4 group genes from the resurrection plant Boea hygrometrica confer dehydration tolerance in transgenic tobacco. Plant Sci. 2009, 176, 90–98. [Google Scholar] [CrossRef]
- Athar, H.-R.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A.; et al. Salt stress proteins in plants: An overview. Front. Plant Sci. 2022, 13, 999058. [Google Scholar] [CrossRef] [PubMed]
- Aparato, V.P.M.; Suh, D.Y. The functions and applications of GDSL esterase/lipase proteins in agriculture: A review. JSFA Rep. 2022, 2, 304–312. [Google Scholar] [CrossRef]
- Huang, K.C.; Lin, W.C.; Cheng, W.H. Salt hypersensitive mutant 9, a nucleolar APUM23 protein, is essential for salt sensitivity in association with the ABA signaling pathway in Arabidopsis. BMC Plant Biol. 2018, 18, 40. [Google Scholar] [CrossRef]
- Li, W.; Zhao, F.; Fang, W.; Xie, D.; Hou, J.; Yang, X.; Zhao, Y.; Tang, Z.; Nie, L.; Lv, S. Identification of early salt stress responsive proteins in seedling roots of upland cotton (Gossypium hirsutum L.) employing iTRAQ-based proteomic technique. Front. Plant Sci. 2015, 6, 732. [Google Scholar] [CrossRef]

| Sample | Clean Reads Pairs | Clean Base (bp) | Length | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| CK_L15-1 | 26,937,669 | 8,081,300,700 | 150; 150 | 98.19; 97.09 | 94.74; 91.89 | 47.74; 47.70 |
| CK_L15-2 | 33,997,884 | 10,199,365,200 | 150; 150 | 98.19; 97.66 | 94.74; 93.26 | 48.61; 48.60 |
| CK_L15-3 | 30,294,698 | 9,088,409,400 | 150; 150 | 98.19; 98.01 | 94.73; 94.09 | 48.67; 48.66 |
| CK_L45-1 | 27,583,944 | 8,275,183,200 | 150; 150 | 98.17; 97.74 | 94.70; 93.42 | 47.73; 47.72 |
| CK_L45-2 | 26,057,986 | 7,817,395,800 | 150; 150 | 98.12; 97.31 | 94.54; 92.39 | 46.80; 46.77 |
| CK_L45-3 | 29,755,864 | 8,926,759,200 | 150; 150 | 98.11; 97.43 | 94.52; 92.70 | 47.48; 47.47 |
| CK_R15-1 | 28,985,437 | 8,695,631,100 | 150; 150 | 98.19; 98.05 | 94.72; 94.14 | 46.18; 46.16 |
| CK_R15-2 | 27,361,580 | 8,208,474,000 | 150; 150 | 98.15; 97.31 | 94.64; 92.42 | 46.43; 46.41 |
| CK_R15-3 | 24,953,901 | 7,486,170,300 | 150; 150 | 98.15; 97.82 | 94.65; 93.61 | 46.09; 46.05 |
| CK_R45-1 | 29,480,386 | 8,844,115,800 | 150; 150 | 98.18; 97.78 | 94.69; 93.54 | 48.82; 48.77 |
| CK_R45-2 | 25,654,529 | 7,696,358,700 | 150; 150 | 98.17; 97.87 | 94.65; 93.75 | 48.71; 48.68 |
| CK_R45-3 | 27,392,172 | 8,217,651,600 | 150; 150 | 98.19; 97.21 | 94.72; 92.21 | 49.10; 49.05 |
| DR_L15-1 | 29,058,008 | 8,717,402,400 | 150; 150 | 98.16; 97.45 | 94.65; 92.74 | 47.22; 47.19 |
| DR_L15-2 | 26,690,924 | 8,007,277,200 | 150; 150 | 98.16; 97.70 | 94.64; 93.31 | 47.38; 47.34 |
| DR_L15-3 | 27,290,514 | 8,187,154,200 | 150; 150 | 98.16; 97.23 | 94.67; 92.27 | 47.63; 47.61 |
| DR_L45-1 | 26,082,650 | 7,824,795,000 | 150; 150 | 98.13; 97.59 | 94.58; 93.05 | 47.26; 47.22 |
| DR_L45-2 | 24,928,074 | 7,478,422,200 | 150; 150 | 98.12; 97.70 | 94.56; 93.34 | 47.10; 47.06 |
| DR_L45-3 | 26,047,394 | 7,814,218,200 | 150; 150 | 98.14; 98.12 | 94.65; 94.37 | 47.33; 47.30 |
| DR_R15-1 | 27,467,948 | 8,240,384,400 | 150; 150 | 98.18; 97.81 | 94.70; 93.57 | 46.40; 46.36 |
| DR_R15-2 | 28,567,133 | 8,570,139,900 | 150; 150 | 98.11; 97.83 | 94.54; 93.64 | 46.29; 46.27 |
| DR_R15-3 | 31,338,669 | 9,401,600,700 | 150; 150 | 98.17; 97.87 | 94.67; 93.74 | 46.12; 46.10 |
| DR_R45-1 | 28,006,857 | 8,402,057,100 | 150; 150 | 98.17; 97.86 | 94.69; 93.74 | 48.93; 48.87 |
| DR_R45-2 | 26,002,898 | 7,800,869,400 | 150; 150 | 98.19; 97.30 | 94.75; 92.42 | 49.24; 49.22 |
| DR_R45-3 | 23,126,099 | 6,937,829,700 | 150; 150 | 98.21; 97.93 | 94.77; 93.88 | 48.55; 48.53 |
| Group | Total | Up | Down |
|---|---|---|---|
| CK_L15-vs-DR_L15 | 7119 | 2906 | 4213 |
| CK_R15-vs-DR_R15 | 8811 | 2996 | 5815 |
| CK_L45-vs-DR_L45 | 5463 | 1744 | 3719 |
| CK_R45-vs-DR_R45 | 930 | 445 | 485 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, P.; Kong, F.; Han, Y.; Liu, X.; Xia, J. Transcriptome Analysis Reveals Genes and Pathways Associated with Drought Tolerance of Early Stages in Sweet Potato (Ipomoea batatas (L.) Lam.). Genes 2024, 15, 948. https://doi.org/10.3390/genes15070948
Cheng P, Kong F, Han Y, Liu X, Xia J. Transcriptome Analysis Reveals Genes and Pathways Associated with Drought Tolerance of Early Stages in Sweet Potato (Ipomoea batatas (L.) Lam.). Genes. 2024; 15(7):948. https://doi.org/10.3390/genes15070948
Chicago/Turabian StyleCheng, Peng, Fanna Kong, Yang Han, Xiaoping Liu, and Jiaping Xia. 2024. "Transcriptome Analysis Reveals Genes and Pathways Associated with Drought Tolerance of Early Stages in Sweet Potato (Ipomoea batatas (L.) Lam.)" Genes 15, no. 7: 948. https://doi.org/10.3390/genes15070948





