A Whole-Transcriptomic Analysis of Canine Oral Melanoma: A Chance to Disclose the Radiotherapy Effect and Outcome-Associated Gene Signature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Recruitment, Clinico-Pathological Features, Therapy, and Sample Acquisition
2.2. RNA Isolation from Biopsies and Blood Samples
2.3. RNA-Seq Library Preparation and Sequencing
2.4. Differential Expression Analysis and Functional Analysis
2.5. Spearman’s Correlation
2.6. Statistical Analysis
3. Results
3.1. Clinico-Pathological Features
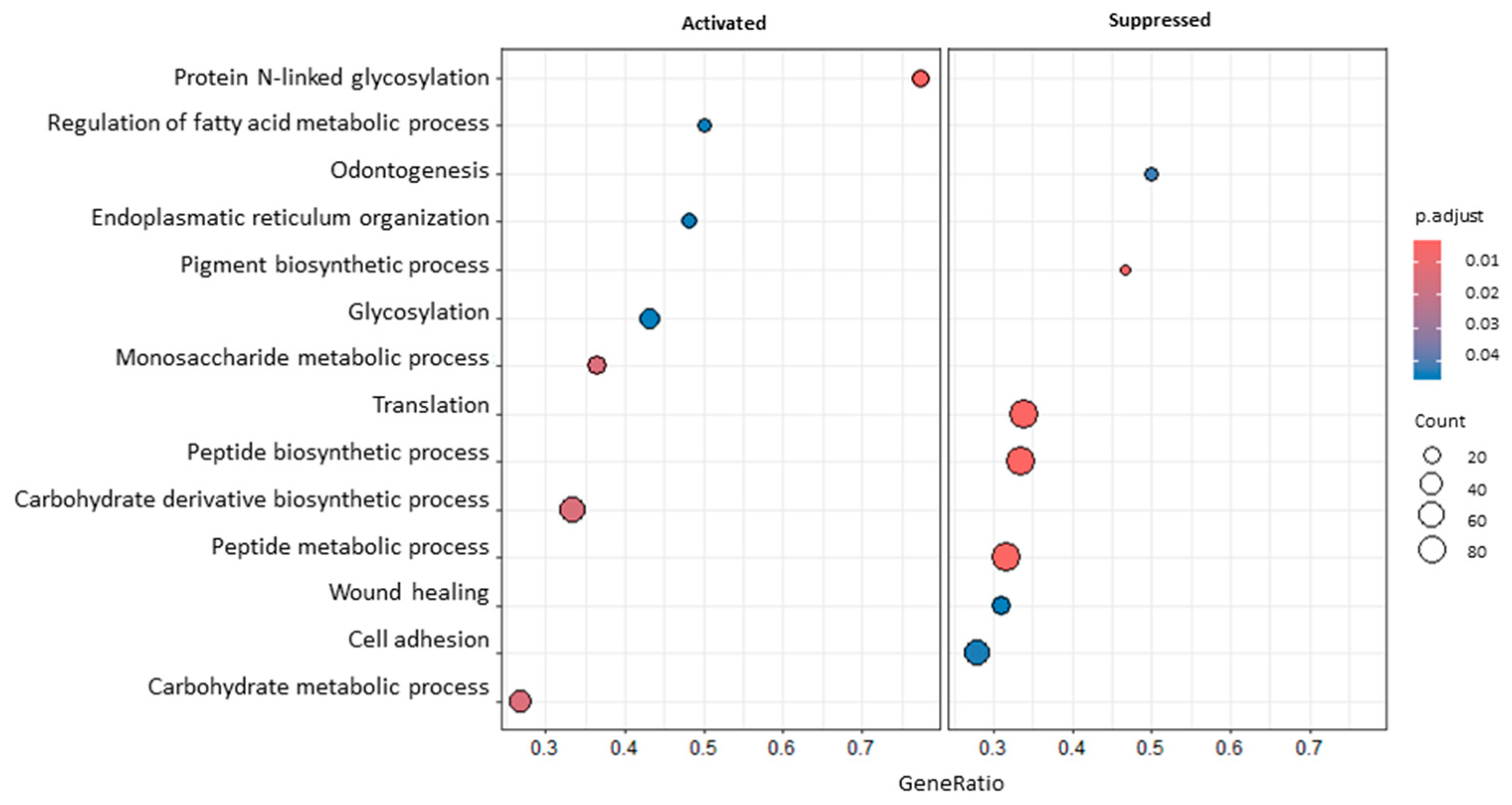
3.2. Differential Expression Analysis and Functional Analysis
3.3. Spearman’s Correlation
4. Discussion
4.1. Transcriptomic Effect of RT on Blood and Tumour Samples
4.2. Transcriptomic Differences Associated with Overall Survival Time
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gillard, M.; Cadieu, E.; De Brito, C.; Abadie, J.; Vergier, B.; Devauchelle, P.; Degorce, F.; Dréano, S.; Primot, A.; Dorso, L.; et al. Naturally Occurring Melanomas in Dogs as Models for Non-UV Pathways of Human Melanomas. Pigment. Cell Melanoma Res. 2014, 27, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, B.; Adissu, H.A.; Wei, B.R.; Michael, H.T.; Merlino, G.; Simpson, R.M. Naturally Occurring Canine Melanoma as a Predictive Comparative Oncology Model for Human Mucosal and Other Triple Wild-Type Melanomas. Int. J. Mol. Sci. 2018, 19, 394. [Google Scholar] [CrossRef] [PubMed]
- van der Weyden, L.; Brenn, T.; Patton, E.E.; Wood, G.A.; Adams, D.J. Spontaneously Occurring Melanoma in Animals and Their Relevance to Human Melanoma. J. Pathol. 2020, 252, 4–21. [Google Scholar] [CrossRef] [PubMed]
- van der Weyden, L.; Patton, E.E.; Wood, G.A.; Foote, A.K.; Brenn, T.; Arends, M.J.; Adams, D.J. Cross-species Models of Human Melanoma. J. Pathol. 2016, 238, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, J.L.; Selmic, L.E.; Worley, D.R.; Ehrhart, N.P.; Withrow, S.J. Outcome Following Curative-Intent Surgery for Oral Melanoma in Dogs: 70 Cases (1998–2011). J. Am. Vet. Med. Assoc. 2014, 245, 1266–1273. [Google Scholar] [CrossRef]
- Kim, W.S.; Vinayak, A.; Powers, B. Comparative Review of Malignant Melanoma and Histologically Well-Differentiated Melanocytic Neoplasm in the Oral Cavity of Dogs. Vet. Sci. 2021, 8, 261. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.E.; Ferreira, Ê.; de Oliveira Massoco, C.; Strauss, B.E.; Fávaro, W.J.; Durán, N.; Oyafuso da Cruz, N.; dos Santos Cunha, S.C.; Castro, J.L.C.; Rangel, M.M.M.; et al. Current Status of Canine Melanoma Diagnosis and Therapy: Report from a Colloquium on Canine Melanoma Organized by ABROVET (Brazilian Association of Veterinary Oncology). Front. Vet. Sci. 2021, 8, 707025. [Google Scholar] [CrossRef] [PubMed]
- Di Palma, S.; McConnell, A.; Verganti, S.; Starkey, M. Review on Canine Oral Melanoma: An Undervalued Authentic Genetic Model of Human Oral Melanoma? Vet. Pathol. 2021, 58, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Kaina, B.; Beltzig, L.; Strik, H. Temozolomide—Just a Radiosensitizer? Front. Oncol. 2022, 12, 912821. [Google Scholar] [CrossRef]
- Marconato, L.; Rohrer Bley, C.; Leone, V.F.; Finotello, R. An Open-Label Dose Escalation Study Evaluating Tolerability and Safety of a Single 5-Days Course of Temozolomide in Dogs with Advanced Cancer. Vet. Comp. Oncol. 2020, 18, 838–842. [Google Scholar] [CrossRef]
- McAleavey, P.G.; Walls, G.M.; Chalmers, A.J. Radiotherapy-Drug Combinations in the Treatment of Glioblastoma: A Brief Review. CNS Oncol. 2022, 11, CNS86. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Erturk, K. Single-Agent Temozolomide May Be an Effective Option for Late Adjuvant Therapy in Patients with Melanoma. J. Oncol. Pharm. Pract. 2021, 27, 40–45. [Google Scholar] [CrossRef]
- Cancedda, S.; Rohrer Bley, C.; Aresu, L.; Dacasto, M.; Leone, V.F.; Pizzoni, S.; Gracis, M.; Marconato, L. Efficacy and Side Effects of Radiation Therapy in Comparison with Radiation Therapy and Temozolomide in the Treatment of Measurable Canine Malignant Melanoma. Vet. Comp. Oncol. 2016, 14, e146–e157. [Google Scholar] [CrossRef]
- Dolera, M.; Malfassi, L.; Bianchi, C.; Carrara, N.; Finesso, S.; Marcarini, S.; Mazza, G.; Pavesi, S.; Sala, M.; Urso, G. Frameless Stereotactic Radiotherapy Alone and Combined with Temozolomide for Presumed Canine Gliomas. Vet. Comp. Oncol. 2018, 16, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Almela, R.M.; Ansón, A. A Review of Immunotherapeutic Strategies in Canine Malignant Melanoma. Vet. Sci. 2019, 6, 15. [Google Scholar] [CrossRef]
- Pazzi, P.; Steenkamp, G.; Rixon, A.J. Treatment of Canine Oral Melanomas: A Critical Review of the Literature. Vet. Sci. 2022, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Garden, O.A.; Volk, S.W.; Mason, N.J.; Perry, J.A. Companion Animals in Comparative Oncology: One Medicine in Action. Vet. J. 2018, 240, 6–13. [Google Scholar] [CrossRef]
- Giuliano, A. Companion Animal Model in Translational Oncology; Feline Oral Squamous Cell Carcinoma and Canine Oral Melanoma. Biology 2021, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Prouteau, A.; André, C. Canine Melanomas as Models for Human Melanomas: Clinical, Histological, and Genetic Comparison. Genes 2019, 10, 501. [Google Scholar] [CrossRef]
- Boss, M.K. Canine Comparative Oncology for Translational Radiation Research. Int. J. Radiat. Biol. 2022, 98, 496–505. [Google Scholar] [CrossRef]
- Harrison, B.M.; Loukopoulos, P. Genomics and Transcriptomics in Veterinary Oncology (Review). Oncol. Lett. 2021, 21, 336. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.L.; Fenger, J.M.; London, C.A. Dogs as a Model for Cancer. Annu. Rev. Anim. Biosci. 2016, 4, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.J. Canine Oral Melanoma. Clin. Tech. Small Anim. Pract. 2007, 22, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.M.; Bastian, B.C.; Michael, H.T.; Webster, J.D.; Prasad, M.L.; Conway, C.M.; Prieto, V.M.; Gary, J.M.; Goldschmidt, M.H.; Esplin, D.G.; et al. Sporadic Naturally Occurring Melanoma in Dogs as a Preclinical Model for Human Melanoma. Pigment. Cell Melanoma Res. 2014, 27, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, J.; Guo, J.; Si, L.; Bai, X. Evolving Treatment Approaches to Mucosal Melanoma. Curr. Oncol. Rep. 2022, 24, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Qi, Z.; Zhang, L.; Guo, J.; Si, L. Immunotherapy in Acral and Mucosal Melanoma: Current Status and Future Directions. Front. Immunol. 2021, 12, 680407. [Google Scholar] [CrossRef] [PubMed]
- Flory, A.; Kruglyak, K.M.; Tynan, J.A.; McLennan, L.M.; Rafalko, J.M.; Fiaux, P.C.; Hernandez, G.E.; Marass, F.; Nakashe, P.; Ruiz-Perez, C.A.; et al. Clinical Validation of a Next-Generation Sequencing-Based Multi-Cancer Early Detection “Liquid Biopsy” Blood Test in over 1000 Dogs Using an Independent Testing Set: The CANcer Detection in Dogs (CANDiD) Study. PLoS ONE 2022, 17, e0266623. [Google Scholar] [CrossRef]
- Prouteau, A.; Mottier, S.; Primot, A.; Cadieu, E.; Bachelot, L.; Botherel, N.; Cabillic, F.; Houel, A.; Cornevin, L.; Kergal, C.; et al. Canine Oral Melanoma Genomic and Transcriptomic Study Defines Two Molecular Subgroups with Different Therapeutical Targets. Cancers 2022, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- International Human Genome Sequencing Consortium. Finishing the Euchromatic Sequence of the Human Genome. Nature 2004, 431, 931–945. [Google Scholar] [CrossRef]
- Lindblad-Toh, K.; Wade, C.M.; Mikkelsen, T.S.; Karlsson, E.K.; Jaffe, D.B.; Kamal, M.; Clamp, M.; Chang, J.L.; Kulbokas, E.J.; Zody, M.C.; et al. Genome Sequence, Comparative Analysis and Haplotype Structure of the Domestic Dog. Nature 2005, 438, 803–819. [Google Scholar] [CrossRef]
- Chibuk, J.; Flory, A.; Kruglyak, K.M.; Leibman, N.; Nahama, A.; Dharajiya, N.; van den Boom, D.; Jensen, T.J.; Friedman, J.S.; Shen, M.R.; et al. Horizons in Veterinary Precision Oncology: Fundamentals of Cancer Genomics and Applications of Liquid Biopsy for the Detection, Characterization, and Management of Cancer in Dogs. Front. Vet. Sci. 2021, 8, 664718. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Koren, S.; Rhie, A.; Rautiainen, M.; Bzikadze, A.V.; Mikheenko, A.; Vollger, M.R.; Altemose, N.; Uralsky, L.; Gershman, A.; et al. The Complete Sequence of a Human Genome. Science 2022, 376, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, V.; Hitte, C.; Kidd, J.M.; Masterson, P.; Murphy, T.D.; Emery, S.; Davis, B.; Buckley, R.M.; Liu, Y.H.; Zhang, X.Q.; et al. Dog10K_Boxer_Tasha_1.0: A Long-Read Assembly of the Dog Reference Genome. Genes 2021, 12, 847. [Google Scholar] [CrossRef]
- Pavlopoulou, A.; Bagos, P.G.; Koutsandrea, V.; Georgakilas, A.G. Molecular Determinants of Radiosensitivity in Normal and Tumor Tissue: A Bioinformatic Approach. Cancer Lett. 2017, 403, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Berglund, A.; Schell, M.J.; Mihaylov, I.; Fulp, W.J.; Yue, B.; Welsh, E.; Caudell, J.J.; Ahmed, K.; Strom, T.S.; et al. A Genome-Based Model for Adjusting Radiotherapy Dose (GARD): A Retrospective, Cohort-Based Study. Lancet Oncol. 2017, 18, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.W.; Kuo, M.T. Improving Radiotherapy in Cancer Treatment: Promises and Challenges. Oncotarget 2017, 8, 62742–62758. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Gregucci, F.; Desideri, I.; Fiore, M.; Marino, L.; Errico, A.; Di Rito, A.; Borghetti, P.; Franco, P.; Greto, D.; et al. Radiation Treatment for Adult Rare Cancers: Oldest and Newest Indication. Crit. Rev. Oncol. Hematol. 2021, 159, 103228. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, M.; Mori, T.; Ito, Y.; Murakami, M.; Sakai, H.; Yanai, T.; Maruo, K. Outcomes of Dogs Undergoing Radiotherapy for Treatment of Oral Malignant Melanoma: 111 Cases (2006–2012). J. Am. Vet. Med. Assoc. 2015, 247, 1146–1153. [Google Scholar] [CrossRef]
- Yde, S.S.; Sjoegren, P.; Heje, M.; Stolle, L.B. Mucosal Melanoma: A Literature Review. Curr. Oncol. Rep. 2018, 20, 28. [Google Scholar] [CrossRef]
- Lawrence, T.S.; Blackstock, A.W.; McGinn, C. The Mechanism of Action of Radiosensitization of Conventional Chemotherapeutic Agents. Semin. Radiat. Oncol. 2003, 13, 13–21. [Google Scholar] [CrossRef]
- Azria, D.; Bourgier, C.; Brengues, M. One Size Fits All: Does the Dogma Stand in Radiation Oncology? eBioMedicine 2016, 10, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Using DNA, Radiation Therapy Gets Personal. Science 2016, 353, 1348–1349. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.R. Precision Medicine—Right Treatment, Right Patient, Right Time, Wrong Approach? Clin. Chem. 2017, 63, 928–929. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.G.; Alexander, B.; Baumann, M.; Bratman, S.V.; Brown, J.M.; Camphausen, K.; Choyke, P.; Citrin, D.; Contessa, J.N.; Dicker, A.; et al. Combining Precision Radiotherapy with Molecular Targeting and Immunomodulatory Agents: A Guideline by the American Society for Radiation Oncology. Lancet Oncol. 2018, 19, e240–e251. [Google Scholar] [CrossRef] [PubMed]
- Guhan, S.; Klebanov, N.; Tsao, H. Melanoma Genomics: A State-of-the-art Review of Practical Clinical Applications. Br. J. Dermatol. 2021, 185, 272–281. [Google Scholar] [CrossRef]
- Hussen, B.M.; Abdullah, S.T.; Salihi, A.; Sabir, D.K.; Sidiq, K.R.; Rasul, M.F.; Hidayat, H.J.; Ghafouri-Fard, S.; Taheri, M.; Jamali, E. The Emerging Roles of NGS in Clinical Oncology and Personalized Medicine. Pathol. Res. Pract. 2022, 230, 153760. [Google Scholar] [CrossRef] [PubMed]
- Mery, B.; Vallard, A.; Rowinski, E.; Magne, N. High-Throughput Sequencing in Clinical Oncology: From Past to Present. Swiss Med. Wkly. 2019, 149, w20057. [Google Scholar] [CrossRef] [PubMed]
- Bibault, J.E.; Tinhofer, I. The Role of Next-Generation Sequencing in Tumoral Radiosensitivity Prediction. Clin. Transl. Radiat. Oncol. 2017, 3, 16–20. [Google Scholar] [CrossRef]
- Tang, Z.; Zeng, Q.; Li, Y.; Zhang, X.; Ma, J.; Suto, M.J.; Xu, B.; Yi, N. Development of a Radiosensitivity Gene Signature for Patients with Soft Tissue Sarcoma. Oncotarget 2017, 8, 27428–27439. [Google Scholar] [CrossRef]
- Tinhofer, I.; Niehr, F.; Konschak, R.; Liebs, S.; Munz, M.; Stenzinger, A.; Weichert, W.; Keilholz, U.; Budach, V. Next-Generation Sequencing: Hype and Hope for Development of Personalized Radiation Therapy? Radiat. Oncol. 2015, 10, 183. [Google Scholar] [CrossRef]
- Giannuzzi, D.; Marconato, L.; Elgendy, R.; Ferraresso, S.; Scarselli, E.; Fariselli, P.; Nicosia, A.; Pegolo, S.; Leoni, G.; Laganga, P.; et al. Longitudinal Transcriptomic and Genetic Landscape of Radiotherapy Response in Canine Melanoma. Vet. Comp. Oncol. 2019, 17, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Tivey, A.; Church, M.; Rothwell, D.; Dive, C.; Cook, N. Circulating Tumour DNA—Looking beyond the Blood. Nat. Rev. Clin. Oncol. 2022, 19, 600–612. [Google Scholar] [CrossRef]
- Yuan, D.; Zhu, K.; Dang, C.; Zheng, Y.; Yan, R.; Shi, L.; Li, K. NS5ATP9 mRNA Levels in Peripheral Blood Mononuclear Cells Predict Prognosis in Patients with Gastric Cancer. Med. Oncol. 2014, 31, 106. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Park, Y.S.; Lee, H.W.; Park, T.Y.; Lee, J.K.; Heo, E.Y. Peripheral Lymphocyte Count as a Surrogate Marker of Immune Checkpoint Inhibitor Therapy Outcomes in Patients with Non-Small-Cell Lung Cancer. Sci. Rep. 2022, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Bergin, I.L.; Smedley, R.C.; Esplin, D.G.; Spangler, W.L.; Kiupel, M. Prognostic Evaluation of Ki67 Threshold Value in Canine Oral Melanoma. Vet. Pathol. 2011, 48, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Smedley, R.C.; Spangler, W.L.; Esplin, D.G.; Kitchell, B.E.; Bergman, P.J.; Ho, H.Y.; Bergin, I.L.; Kiupel, M. Prognostic Markers for Canine Melanocytic Neoplasms: A Comparative Review of the Literature and Goals for Future Investigation. Vet. Pathol. 2011, 48, 54–72. [Google Scholar] [CrossRef]
- Spangler, W.L.; Kass, P.H. The Histologic and Epidemiologic Bases for Prognostic Considerations in Canine Melanocytic Neoplasia. Vet. Pathol. 2006, 43, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Booth, C.M. Defining Clinically Important Overall Survival Thresholds: Lessons from Quality of Life. Nat. Rev. Clin. Oncol. 2022, 19, 613–614. [Google Scholar] [CrossRef]
- Andrews, S.; Krueger, F.; Seconds-Pichon, A.; Biggins, F.; Wingett, S.F. A Quality Control Tool for High Throughput Sequence Data. Babraham Bioinformatics; Babraham Institute: Babraham, UK, 2015. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-Optimal Probabilistic RNA-Seq Quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential Analyses for RNA-Seq: Transcript-Level Estimates Improve Gene-Level Inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef]
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping Identifiers for the Integration of Genomic Datasets with the R/Bioconductor Package BiomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Fan, T.M.; Selting, K.A. Exploring the Potential Utility of Pet Dogs with Cancer for Studying Radiation-Induced Immunogenic Cell Death Strategies. Front. Oncol. 2019, 8, 680. [Google Scholar] [CrossRef]
- Polton, G.; Borrego, J.F.; Clemente-Vicario, F.; Clifford, C.A.; Jagielski, D.; Kessler, M.; Kobayashi, T.; Lanore, D.; Queiroga, F.L.; Rowe, A.T.; et al. Melanoma of the Dog and Cat: Consensus and Guidelines. Front. Vet. Sci. 2024, 11, 1359426. [Google Scholar] [CrossRef]
- Hoopes, P.J.; Wagner, R.J.; Duval, K.; Kang, K.; Gladstone, D.J.; Moodie, K.L.; Crary-Burney, M.; Ariaspulido, H.; Veliz, F.A.; Steinmetz, N.F.; et al. Treatment of Canine Oral Melanoma with Nanotechnology-Based Immunotherapy and Radiation. Mol. Pharm. 2018, 15, 3717–3722. [Google Scholar] [CrossRef]
- Engeland, K. Cell Cycle Regulation: P53-P21-RB Signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Xu, H.E.; Luo, Z.; Zhou, J.; Zhou, Y.; Liu, M. Expression and Significance of DDX43 in Lung Adenocarcinoma. Pak. J. Pharm. Sci. 2017, 30, 1491–1496. [Google Scholar]
- Amer, N.N.; Khairat, R.; Hammad, A.M.; Kamel, M.M. DDX43 mRNA Expression and Protein Levels in Relation to Clinicopathological Profile of Breast Cancer. PLoS ONE 2023, 18, e0284455. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fatah, T.M.A.; McArdle, S.E.B.; Johnson, C.; Moseley, P.M.; Ball, G.R.; Pockley, A.G.; Ellis, I.O.; Rees, R.C.; Chan, S.Y.T. HAGE (DDX43) Is a Biomarker for Poor Prognosis and a Predictor of Chemotherapy Response in Breast Cancer. Br. J. Cancer 2014, 110, 2450–2461. [Google Scholar] [CrossRef]
- Wang, H.; Liu, W.; Liu, L.; Wu, C.; Wu, W.; Zheng, J.; Zhang, M.; Chen, X.; Zhou, B.; Gao, Z.; et al. Overexpression of Centromere Protein K (CENP-K) Gene in Hepatocellular Carcinoma Promote Cell Proliferation by Activating AKT/TP53 Signal Pathway. Oncotarget 2017, 8, 73994–74005. [Google Scholar] [CrossRef]
- Lee, Y.C.; Huang, C.C.; Lin, D.Y.; Chang, W.C.; Lee, K.H. Overexpression of Centromere Protein K (CENPK) in Ovarian Cancer Is Correlated with Poor Patient Survival and Associated with Predictive and Prognostic Relevance. PeerJ 2015, 3, e1386. [Google Scholar] [CrossRef]
- Si, M.; Lang, J. The Roles of Metallothioneins in Carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Faller, W.J.; Rafferty, M.; Hegarty, S.; Gremel, G.; Ryan, D.; Fraga, M.F.; Esteller, M.; Dervan, P.A.; Gallagher, W.M. Metallothionein 1E Is Methylated in Malignant Melanoma and Increases Sensitivity to Cisplatin-Induced Apoptosis. Melanoma Res. 2010, 20, 392–400. [Google Scholar] [CrossRef]
- Tóth, E.; Vékey, K.; Ozohanics, O.; Jeko, A.; Dominczyk, I.; Widlak, P.; Drahos, L. Changes of Protein Glycosylation in the Course of Radiotherapy. J. Pharm. Biomed. Anal. 2016, 118, 380–386. [Google Scholar] [CrossRef]
- Jaillet, C.; Morelle, W.; Slomianny, M.C.; Paget, V.; Tarlet, G.; Buard, V.; Selbonne, S.; Caffin, F.; Rannou, E.; Martinez, P.; et al. Radiation-Induced Changes in the Glycome of Endothelial Cells with Functional Consequences. Sci. Rep. 2017, 7, 5290. [Google Scholar] [CrossRef]
- Jeon, S.H.; Song, C.; Eom, K.Y.; Kim, I.A.; Kim, J.S. Modulation of CD8+ T Cell Responses by Radiotherapy—Current Evidence and Rationale for Combination with Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2023, 24, 16691. [Google Scholar] [CrossRef]
- Ma, Y.; Adjemian, S.; Mattarollo, S.R.; Yamazaki, T.; Aymeric, L.; Yang, H.; Portela Catani, J.P.; Hannani, D.; Duret, H.; Steegh, K.; et al. Anticancer Chemotherapy-Induced Intratumoral Recruitment and Differentiation of Antigen-Presenting Cells. Immunity 2013, 38, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Pitt, J.M.; Li, Q.; Yang, H. The Renaissance of Anti-neoplastic Immunity from Tumor Cell Demise. Immunol. Rev. 2017, 280, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Mahuron, K.M.; Moreau, J.M.; Glasgow, J.E.; Boda, D.P.; Pauli, M.L.; Gouirand, V.; Panjabi, L.; Grewal, R.; Luber, J.M.; Mathur, A.N.; et al. Layilin Augments Integrin Activation to Promote Antitumor Immunity. J. Exp. Med. 2020, 217, e20192080. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ge, Y.; Niu, K.; Li, Y.; Qi, L.W.; Zhu, H.; Ma, G. MLXIPL Associated with Tumor-Infiltrating CD8+ T Cells Is Involved in Poor Prostate Cancer Prognosis. Front. Immunol. 2024, 15, 1364329. [Google Scholar] [CrossRef] [PubMed]
- Le, P.T.; Ha, N.; Tran, N.K.; Newman, A.G.; Esselen, K.M.; Dalrymple, J.L.; Schmelz, E.M.; Bhandoola, A.; Xue, H.H.; Singh, P.B.; et al. Targeting Cbx3/HP1γ Induces LEF-1 and IL-21R to Promote Tumor-Infiltrating CD8 T-Cell Persistence. Front. Immunol. 2021, 12, 738958. [Google Scholar] [CrossRef] [PubMed]
- Meiser, P.; Knolle, M.A.; Hirschberger, A.; de Almeida, G.P.; Bayerl, F.; Lacher, S.; Pedde, A.M.; Flommersfeld, S.; Hönninger, J.; Stark, L.; et al. A Distinct Stimulatory CDC1 Subpopulation Amplifies CD8+ T Cell Responses in Tumors for Protective Anti-Cancer Immunity. Cancer Cell 2023, 41, 1498–1515. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical Role for CD103+/CD141+ Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Ventero, M.P.; Fuentes-Baile, M.; Quereda, C.; Perez-Valeciano, E.; Alenda, C.; Garcia-Morales, P.; Esposito, D.; Dorado, P.; Barbera, V.M.; Saceda, M. Radiotherapy Resistance Acquisition in Glioblastoma. Role of SOCS1 and SOCS3. PLoS ONE 2019, 14, e0212581. [Google Scholar] [CrossRef]
- de Totero, D.; Capaia, M.; Fabbi, M.; Croce, M.; Meazza, R.; Cutrona, G.; Zupo, S.; Loiacono, F.; Truini, M.; Ferrarini, M.; et al. Heterogeneous Expression and Function of IL-21R and Susceptibility to IL-21-Mediated Apoptosis in Follicular Lymphoma Cells. Exp. Hematol. 2010, 38, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.; Lee, W.K. KLF10 as a Tumor Suppressor Gene and Its TGF-β Signaling. Cancers 2018, 10, 161. [Google Scholar] [CrossRef]
- Chang, V.H.S.; Tsai, Y.C.; Tsai, Y.L.; Peng, S.L.; Chen, S.L.; Chang, T.M.; Yu, W.C.Y.; Ch’ang, H.J. Krüpple-like Factor 10 Regulates Radio-Sensitivity of Pancreatic Cancer via UV Radiation Resistance-Associated Gene. Radiother. Oncol. 2017, 122, 476–484. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, S.C.; Kim, S.J.; Park, C.H.; Jeung, H.C.; Kim, Y.B.; Ahn, J.B.; Chung, H.C.; Rha, S.Y. Identification of a Radiosensitivity Signature Using Integrative Metaanalysis of Published Microarray Data for NCI-60 Cancer Cells. BMC Genom. 2012, 13, 348. [Google Scholar] [CrossRef]
- Fuse, C.; Ishida, Y.; Hikita, T.; Asai, T.; Oku, N. Junctional Adhesion Molecule-C Promotes Metastatic Potential of HT1080 Human Fibrosarcoma. J. Biol. Chem. 2007, 282, 8276–8283. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.F.; Orlova, V.V.; Xie, C.; Kaul, S.; Schneider, D.; Lonsdorf, A.S.; Fahrleitner, M.; Choi, E.Y.; Dutoit, V.; Pellegrini, M.; et al. A Novel Function of Junctional Adhesion Molecule-C in Mediating Melanoma Cell Metastasis. Cancer Res. 2011, 71, 4096–4105. [Google Scholar] [CrossRef] [PubMed]
- Ghislin, S.; Obino, D.; Middendorp, S.; Boggetto, N.; Alcaide-Loridan, C.; Deshayes, F. Junctional Adhesion Molecules Are Required for Melanoma Cell Lines Transendothelial Migration in Vitro. Pigment. Cell Melanoma Res. 2011, 24, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Wang, J.; Wang, J.; Wang, Y.; Ji, B.; Xu, Y.; He, J.; Zhang, L.; Zhang, L.; Ding, B.; et al. JAM3: A Prognostic Biomarker for Bladder Cancer via Epithelial–Mesenchymal Transition Regulation. Biomol. Biomed. 2024, 24, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, M.L.; Frontera, V.; Bardin, F.; Thomassin, J.; Chetaille, B.; Adams, S.; Adams, R.H.; Aurrand-Lions, M. The Junctional Adhesion Molecule-B Regulates JAM-C-dependent Melanoma Cell Metastasis. FEBS Lett. 2012, 586, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Nurzat, Y.; Su, W.; Min, P.; Li, K.; Xu, H.; Zhang, Y. Identification of Therapeutic Targets and Prognostic Biomarkers Among Integrin Subunits in the Skin Cutaneous Melanoma Microenvironment. Front. Oncol. 2021, 11, 751875. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal Cells in the Tumor Microenvironment: Accomplices of Tumor Progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Fujimura, T. Stromal Factors as a Target for Immunotherapy in Melanoma and Non-Melanoma Skin Cancers. Int. J. Mol. Sci. 2022, 23, 4044. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.B.; Bozlar, M.; De Valk, A.F.; Greten, F.R. Cancer-Associated Fibroblasts in Inflammation and Antitumor Immunity. Clin. Cancer Res. 2023, 29, 1009–1016. [Google Scholar] [CrossRef]
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lövrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and Functionally Distinct Subclasses of Breast Cancer-Associated Fibroblasts Revealed by Single Cell RNA Sequencing. Nat. Commun. 2018, 9, 5150. [Google Scholar] [CrossRef]
- Yang, X.; Wu, K.; Li, S.; Hu, L.; Han, J.; Zhu, D.; Tian, X.; Liu, W.; Tian, Z.; Zhong, L.; et al. MFAP5 and TNNC1: Potential Markers for Predicting Occult Cervical Lymphatic Metastasis and Prognosis in Early Stage Tongue Cancer. Oncotarget 2017, 8, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, P.; Zhang, Q.; Chen, W.; Liu, X.; Zheng, W. MFAP5 Promotes Basal-like Breast Cancer Progression by Activating the EMT Program. Cell Biosci. 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, K.A.; Zembala-Nożynska, E.; Syrkis, J.P.; Cortez, A.J.; Kupryjańczyk, J.; Lisowska, K.M. Microfibril Associated Protein 5 (MFAP5) Is Related to Survival of Ovarian Cancer Patients but Not Useful as a Prognostic Biomarker. Int. J. Mol. Sci. 2022, 23, 15994. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.S.; Yeung, T.L.; Yip, K.P.; Pradeep, S.; Balasubramanian, L.; Liu, J.; Wong, K.K.; Mangala, L.S.; Armaiz-Pena, G.N.; Lopez-Berestein, G.; et al. Calcium-Dependent FAK/CREB/TNNC1 Signalling Mediates the Effect of Stromal MFAP5 on Ovarian Cancer Metastatic Potential. Nat. Commun. 2014, 5, 5092. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Y.; Sawyers, A.; Yao, H.; Rahmatpanah, F.; Xia, X.Q.; Xu, Q.; Pio, R.; Turan, T.; Koziol, J.A.; et al. Diagnosis of Prostate Cancer Using Differentially Expressed Genes in Stroma. Cancer Res. 2011, 71, 2476–2487. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Westerhoff, M.; Hornick, J.L.; Krausz, T.; Antic, T.; Xiao, S.Y.; Hart, J. Loss of Microfibril-Associated Protein 5 (MFAP5) Expression in Colon Cancer Stroma. Virchows Arch. 2020, 476, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, L.; Hemmerich, A.; Ferguson, N.L.; Guy, C.D.; McCall, S.J.; Cardona, D.M.; Westerhoff, M.; Pai, R.K.; Xiao, S.Y.; et al. Reduced MFAP5 Expression in Stroma of Gallbladder Adenocarcinoma and Its Potential Diagnostic Utility. Virchows Arch. 2021, 478, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.C.; Han, Z.Q.; Guo, M.X.; Zhao, S.; Guo, Y.H.; Meng, B.; Sun, Y.; Chen, L. Decreased Expression of SVEP1 Is Closely Related to a Cancer Stem Cell-like Phenotype and Poor Prognosis in Hepatocellular Carcinoma. Neoplasma 2022, 69, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, D.; Yi, X.; Qi, L.; Tian, X.; Sun, B.; Dong, Q.; Han, Z.; Li, Q.; Song, T.; et al. The Novel MiR-1269b-Regulated Protein SVEP1 Induces Hepatocellular Carcinoma Proliferation and Metastasis Likely through the PI3K/Akt Pathway. Cell Death Dis. 2020, 11, 320. [Google Scholar] [CrossRef]
- Chen, L.; He, Y.; Han, Z.; Gong, W.; Tian, X.; Guo, L.; Guo, H.; Song, T.; Chen, L. The Impact of Decreased Expression of SVEP1 on Abnormal Neovascularization and Poor Prognosis in Patients with Intrahepatic Cholangiocarcinoma. Front. Genet. 2023, 13, 1127753. [Google Scholar] [CrossRef]
- Zhuang, J.; Shen, L.; Li, M.; Sun, J.; Hao, J.; Li, J.; Zhu, Z.; Ge, S.; Zhang, D.; Guo, H.; et al. Cancer-Associated Fibroblast–Derived MiR-146a-5p Generates a Niche That Promotes Bladder Cancer Stemness and Chemoresistance. Cancer Res. 2023, 83, 1611–1627. [Google Scholar] [CrossRef]
- Li, S.; Pritchard, D.M.; Yu, L.G. Regulation and Function of Matrix Metalloproteinase-13 in Cancer Progression and Metastasis. Cancers 2022, 14, 3263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, B.; Li, Y.; Liu, Y.; Zhang, D.; Wang, X.; Gu, Q.; Zhao, J.; Dong, X.; Liu, Z.; et al. Dual Effects of Collagenase-3 on Melanoma: Metastasis Promotion and Disruption of Vasculogenic Mimicry. Oncotarget 2015, 6, 8890–8899. [Google Scholar] [CrossRef] [PubMed]
- Meierjohann, S.; Hufnagel, A.; Wende, E.; Kleinschmidt, M.A.; Wolf, K.; Friedl, P.; Gaubatz, S.; Schartl, M. MMP13 Mediates Cell Cycle Progression in Melanocytes and Melanoma Cells: In Vitro Studies of Migration and Proliferation. Mol. Cancer 2010, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Chang, J.S.; Hsiao, J.R.; Yen, Y.C.; Jiang, S.S.; Liu, S.H.; Chen, Y.L.; Shen, Y.Y.; Chang, J.Y.; Chen, Y.W. Tumour Cell-Derived WNT5B Modulates in Vitro Lymphangiogenesis via Induction of Partial Endothelial-Mesenchymal Transition of Lymphatic Endothelial Cells. Oncogene 2017, 36, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Alve, S.; Gramolelli, S.; Jukonen, J.; Juteau, S.; Pink, A.; Manninen, A.A.; Hänninen, S.; Monto, E.; Lackman, M.H.; Carpén, O.; et al. DLL4/Notch3/WNT5B Axis Mediates Bidirectional Prometastatic Crosstalk between Melanoma and Lymphatic Endothelial Cells. JCI Insight 2024, 9, e171821. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.S.; Murray, G.; Suthon, S.; Davis, L.; Perkins, N.B.; Fletcher, L.; Bozzi, A.; Schreiber, S.L.; Lin, J.; Laxton, S.; et al. WNT5B Drives Osteosarcoma Stemness, Chemoresistance and Metastasis. Clin. Transl. Med. 2024, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Suthon, S.; Perkins, R.S.; Bryja, V.; Miranda-Carboni, G.A.; Krum, S.A. WNT5B in Physiology and Disease. Front. Cell Dev. Biol. 2021, 9, 667581. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.J.; Ying, L.; Shi, K.Q. Expression of the Wnt Ligands Gene Family and Its Relationship to Prognosis in Hepatocellular Carcinoma. Cancer Cell Int. 2019, 19, 34. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Shi, Y.; Xin, H. SOSTDC1 Acts as a Tumor Inhibitor in Acute Myeloid Leukemia by Downregulating the Wnt/Β-catenin Pathway. Environ. Toxicol. 2022, 37, 1934–1943. [Google Scholar] [CrossRef]
- Tong, X.; Zhu, C.; Liu, L.; Huang, M.; Xu, J.; Chen, X.; Zou, J. Role of Sostdc1 in Skeletal Biology and Cancer. Front. Physiol. 2022, 13, 1029646. [Google Scholar] [CrossRef] [PubMed]
- Ambatipudi, S.; Bhosale, P.G.; Heath, E.; Pandey, M.; Kumar, G.; Kane, S.; Patil, A.; Maru, G.B.; Desai, R.S.; Watt, F.M.; et al. Downregulation of Keratin 76 Expression during Oral Carcinogenesis of Human, Hamster and Mouse. PLoS ONE 2013, 8, e70688. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, I.; Neves, J.F.; Carrero, D.; Peng, Q.; Palasz, N.; Liakath-Ali, K.; Lord, G.M.; Morgan, P.R.; Lombardi, G.; Watt, F.M. Immunomodulatory Role of Keratin 76 in Oral and Gastric Cancer. Nat. Commun. 2018, 9, 3437. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, L.; Liu, L.; Ruan, D.; Wang, C. Hypermethylated ITGA8 Facilitate Bladder Cancer Cell Proliferation and Metastasis. Appl. Biochem. Biotechnol. 2024, 196, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, A.; Lo, P.H.; Chueh, A.C.; Prithviraj, P.; Molania, R.; Davalos-Salas, M.; Anaka, M.; Walkiewicz, M.; Cebon, J.; Behren, A. Transketolase-like 1 Ectopic Expression Is Associated with DNA Hypomethylation and Induces the Warburg Effect in Melanoma Cells. BMC Cancer 2016, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Meng, Q.; Sun, H.; Li, Y.; Li, Y.; Gu, L.; Liu, B.; Zhang, Y.; Zhou, H.; Xu, Z.; et al. The Role of Transketolase in Human Cancer Progression and Therapy. Biomed. Pharmacother. 2022, 154, 113607. [Google Scholar] [CrossRef] [PubMed]

| Ensembl Gene ID | Gene Name | Gene Description | lfc | logCPM | BHp |
|---|---|---|---|---|---|
| ENSCAFG00845016275 | GNGT1 | G protein subunit γ transducin 1 | −1.88 | 5.07 | 0.01 |
| ENSCAFG00845030209 | SLCO2A1 | solute carrier organic anion transporter family member 2A1 | 2.79 | 3.23 | 0.01 |
| ENSCAFG00845011968 | DDX43 | DEAD-box helicase 43 | −6.15 | 0.54 | 0.03 |
| ENSCAFG00845009606 1 | (CDKN1A) 2 | 1.03 | 5.43 | 0.03 | |
| ENSCAFG00845027527 1 | (LOC612587) 2 | 4.63 | 1.32 | 0.03 | |
| ENSCAFG00845009602 | CENPK | centromere protein K | −1.25 | 3.51 | 0.03 |
| ENSCAFG00845003828 | MT1E | metallothionein 1E | 1.05 | 6.40 | 0.03 |
| ENSCAFG00845015023 1 | (C20H3orf14) 2 | −2.13 | 0.85 | 0.03 |
| Ensembl Gene ID | Gene Name | Gene Description | lfc | logCPM | BHp |
|---|---|---|---|---|---|
| ENSCAFG00845008674 | ADAMTS2 | ADAM metallopeptidase with thrombospondin type 1 motif 2 | 3.09 | 1.78 | 0.001 |
| ENSCAFG00845007491 | PROK2 | prokineticin 2 | −1.77 | 6.79 | 0.023 |
| ENSCAFG00845016746 | XKRX | XK related X-linked | −1.35 | −0.59 | 0.003 |
| ENSCAFG00845005455 | SLC28A3 | solute carrier family 28 member 3 | −1.26 | 4.00 | 0.002 |
| ENSCAFG00845026869 | AMIGO2 | adhesion molecule with Ig-like domain 2 | −1.24 | −0.90 | 0.038 |
| ENSCAFG00845001908 | KANK1 | KN motif and ankyrin repeat domains 1 | −1.21 | −0.27 | 0.001 |
| ENSCAFG00845008460 | TNFAIP6 | TNF α-induced protein 6 | −1.17 | 1.25 | 0.008 |
| ENSCAFG00845008349 | CD72 | CD72 molecule | −1.14 | 0.70 | 0.027 |
| ENSCAFG00845016972 | MYBPC2 | myosin binding protein C2 | −1.13 | −0.58 | 0.012 |
| ENSCAFG00845015333 | COL4A4 | collagen type IV α 4 chain | −1.10 | −0.50 | 0.029 |
| ENSCAFG00845028251 | MYO18B | myosin XVIIIB | 1.08 | 0.85 | 0.023 |
| ENSCAFG00845015341 | DLGAP3 | DLG-associated protein 3 | −1.06 | 1.19 | 0.000 |
| ENSCAFG00845008907 | CRIP3 | cysteine-rich protein 3 | −1.05 | −0.22 | 0.018 |
| ENSCAFG00845028917 1 | (LOC119864113) 2 | −1.02 | 0.53 | 0.044 | |
| ENSCAFG00845002458 | LAYN | layilin | 1.02 | 1.32 | 0.030 |
| ENSCAFG00845028881 1 | (LOC119864112) 2 | −1.01 | 7.64 | 0.000 | |
| ENSCAFG00845030285 | STAB1 | stabilin 1 | 1.00 | 2.46 | 0.003 |
| ENSCAFG00845003394 | MLXIPL | MLX interacting protein-like | 0.99 | −0.70 | 0.033 |
| ENSCAFG00845014560 | AASS | aminoadipate–semialdehyde synthase | −0.98 | 3.14 | 0.045 |
| ENSCAFG00845030320 | SLAMF1 | signalling lymphocytic activation molecule family member 1 | −0.98 | 2.49 | 0.008 |
| ENSCAFG00845001649 | NIM1K | NIM1 serine/threonine protein kinase | −0.94 | 0.87 | 0.007 |
| ENSCAFG00845015409 | CABLES1 | Cdk5 and Abl enzyme substrate 1 | −0.93 | 0.42 | 0.012 |
| ENSCAFG00845004274 | IL21R | interleukin 21 receptor | −0.92 | 3.23 | 0.042 |
| ENSCAFG00845006782 | CRISP2 | cysteine-rich secretory protein 3 | −0.91 | 3.11 | 0.006 |
| ENSCAFG00845015621 | PSD3 | Pleckstrin and Sec7 domain containing 3 | −0.90 | 3.88 | 0.000 |
| ENSCAFG00845029249 | LEF1 | lymphoid enhancer binding factor 1 | −0.90 | 5.77 | 0.025 |
| ENSCAFG00845014724 | GCH1 | GTP cyclohydrolase 1 | −0.90 | 2.19 | 0.038 |
| ENSCAFG00845007930 | CCR7 | C-C motif chemokine receptor 7 | −0.89 | 4.80 | 0.005 |
| ENSCAFG00845000734 | SLC23A1 | marginal zone B and B1 cell-specific protein | 0.86 | 4.00 | 0.000 |
| ENSCAFG00845008805 | SCML4 | Scm polycomb group protein-like 4 | −0.86 | 2.37 | 0.000 |
| ENSCAFG00845028411 | TFDP2 | transcription factor Dp−2 | −0.85 | 6.51 | 0.004 |
| ENSCAFG00845028315 | B3GALNT1 | β-1,3-N-acetylgalactosaminyltransferase 1 (globoside blood group) | 0.84 | 2.31 | 0.025 |
| ENSCAFG00845009720 | TXK | TXK tyrosine kinase | −0.84 | 2.33 | 0.044 |
| ENSCAFG00845021928 | TEX14 | testis-expressed 14, intercellular bridge forming factor | 0.82 | −0.08 | 0.007 |
| ENSCAFG00845005503 | JAM3 | junctional adhesion molecule 3 | −0.81 | 1.22 | 0.045 |
| ENSCAFG00845028387 | TOX | thymocyte selection-associated high-mobility group box | −0.81 | 2.46 | 0.021 |
| ENSCAFG00845017349 | GPR84 | G protein-coupled receptor 84 | 0.80 | 2.69 | 0.038 |
| ENSCAFG00845008004 | KLF10 | KLF transcription factor 10 | 0.79 | 6.22 | 0.016 |
| ENSCAFG00845008786 | P2RY2 | purinergic receptor P2Y2 | 0.78 | 5.28 | 0.002 |
| ENSCAFG00845002694 | G0S2 | G0/G1 switch 2 | 0.78 | 3.73 | 0.011 |
| ENSCAFG00845018861 1 | (DSTN) 2 | destrin, actin depolymerizing factor | −0.75 | 1.83 | 0.000 |
| ENSCAFG00845021223 | AQP3 | aquaporin 3 (Gill blood group) | 0.74 | 4.34 | 0.013 |
| ENSCAFG00845014621 | SOCS3 | suppressor of cytokine signalling 3 | −0.72 | 3.24 | 0.038 |
| ENSCAFG00845018321 | ATP10A | ATPase phospholipid transporting 10A (putative) | −0.72 | 3.17 | 0.002 |
| ENSCAFG00845016371 | RGS10 | regulator of G protein signalling 10 | −0.70 | 4.31 | 0.001 |
| ENSCAFG00845021432 | GNAZ | G protein subunit α z | −0.70 | 2.51 | 0.005 |
| ENSCAFG00845023493 | TSPAN5 | tetraspanin 5 | −0.69 | 3.71 | 0.047 |
| ENSCAFG00845005802 | VASH1 | vasohibin 1 | −0.68 | 2.33 | 0.039 |
| ENSCAFG00845019977 | KCNMB4 | potassium calcium-activated channel subfamily M regulatory β subunit 4 | −0.68 | 1.49 | 0.006 |
| ENSCAFG00845025551 1 | (ATP13A4) 2 | ATPase 13A4 | −0.66 | 2.48 | 0.007 |
| ENSCAFG00845004641 | SPOCK2 | SPARC (osteonectin)-, cwcv-, and kazal-like domains proteoglycan 2 | −0.64 | 6.09 | 0.025 |
| ENSCAFG00845016402 1 | (GNG11) 2 | G protein subunit γ 11 | −0.63 | 5.82 | 0.010 |
| ENSCAFG00845009033 1 | (CCL14) 2 | C-C motif chemokine ligand 14 | −0.63 | 3.34 | 0.017 |
| ENSCAFG00845000866 1 | (C4H1orf198) 2 | chromosome 4 C1orf198 homolog | −0.63 | 3.29 | 0.023 |
| ENSCAFG00845008023 | PSEN2 | presenilin 2 | −0.62 | 3.05 | 0.001 |
| ENSCAFG00845012374 | EHD3 | EH domain containing 3 | −0.60 | 4.48 | 0.031 |
| ENSCAFG00845025918 | MYL9 | myosin light chain 9 | −0.60 | 5.95 | 0.028 |
| ENSCAFG00845012329 | APC2 | APC regulator of WNT signalling pathway 2 | 0.59 | 1.30 | 0.011 |
| ENSCAFG00845026987 | ITGB5 | integrin subunit β 5 | −0.59 | 3.04 | 0.008 |
| Ensembl Gene ID | Gene Name | Gene Description | lfc | logCPM | BHp |
|---|---|---|---|---|---|
| ENSCAFG00845023887 | KRT76 | keratin 76 | 8.59 | 8.08 | 0.002 |
| ENSCAFG00845014426 | ITGA8 | integrin subunit α 8 | 5.64 | 6.34 | 0.002 |
| ENSCAFG00845000761 | MMP13 | matrix metallopeptidase 13 | −4.29 | 6.51 | 0.009 |
| ENSCAFG00845010154 | PI16 | peptidase inhibitor 16 | 8.03 | 5.88 | 0.009 |
| ENSCAFG00845004266 | APOA1 | apolipoprotein A1 | 6.36 | 3.63 | 0.009 |
| ENSCAFG00845029417 | MFAP5 | microfibril-associated protein 5 | 4.52 | 5.26 | 0.014 |
| ENSCAFG00845013295 | SVEP1 | sushi, von Willebrand factor type A, EGF, and pentraxin domain containing 1 | 3.71 | 5.28 | 0.019 |
| ENSCAFG00845023760 | ELF5 | E74-like ETS transcription factor 5 | 4.37 | 2.07 | 0.019 |
| ENSCAFG00845028592 | ANKRD55 | ankyrin repeat domain 55 | 5.82 | 3.03 | 0.022 |
| ENSCAFG00845005444 | CDSN | corneodesmosin | 8.82 | 5.25 | 0.023 |
| ENSCAFG00845005605 | SOSTDC1 | sclerostin domain containing 1 | 4.66 | 3.25 | 0.023 |
| ENSCAFG00845029513 | WNT5B | Wnt family member 5B | −2.74 | 4.04 | 0.026 |
| ENSCAFG00845001685 | UOX | urate oxidase | 3.98 | 3.37 | 0.026 |
| ENSCAFG00845017608 | PLA2G4F | phospholipase A2 group IVF | 3.78 | 0.59 | 0.028 |
| ENSCAFG00845025826 1 | (RPTN) 2 | repetin | 7.33 | 4.39 | 0.028 |
| ENSCAFG00845029411 | GDPD2 | glycerophosphodiester phosphodiesterase domain containing 2 | 4.15 | 2.21 | 0.028 |
| Ensembl Gene ID | Gene Name | Gene Description | lfc | logCPM | BHp |
|---|---|---|---|---|---|
| ENSCAFG00845013314 1 | (LOC111098753) 2 | −7.80 | −0.68 | 0.000004 | |
| ENSCAFG00845027442 | TKTL1 | Transketolase-like 1 | 3.04 | 0.32 | 0.000004 |
| ENSCAFG00845026120 | H4C4 | H4 clustered histone 4 | −7.35 | −1.10 | 0.03 |
| Ensembl Gene ID | Gene Name | Gene Description | r | p |
|---|---|---|---|---|
| ENSCAFG00845023887 | KRT76 | keratin 76 | 0.57 | 0.15 |
| ENSCAFG00845014426 | ITGA8 | integrin subunit α 8 | 0.33 | 0.43 |
| ENSCAFG00845000761 | MMP13 | matrix metallopeptidase 13 | −0.79 | 0.03 |
| ENSCAFG00845010154 | PI16 | peptidase inhibitor 16 | 0.33 | 0.43 |
| ENSCAFG00845004266 | APOA1 | apolipoprotein A1 | 0.52 | 0.20 |
| ENSCAFG00845029417 | MFAP5 | microfibril-associated protein 5 | 0.64 | 0.10 |
| ENSCAFG00845013295 | SVEP1 | sushi, von Willebrand factor type A, EGF, and pentraxin domain containing 1 | 0.98 | 0.0004 |
| ENSCAFG00845023760 | ELF5 | E74-like ETS transcription factor 5 | 0.62 | 0.12 |
| ENSCAFG00845028592 | ANKRD55 | ankyrin repeat domain 55 | 0.45 | 0.27 |
| ENSCAFG00845005444 | CDSN | corneodesmosin | 0.57 | 0.15 |
| ENSCAFG00845005605 | SOSTDC1 | sclerostin domain containing 1 | 0.64 | 0.10 |
| ENSCAFG00845029513 | WNT5B | Wnt family member 5B | −0.86 | 0.01 |
| ENSCAFG00845001685 | UOX | urate oxidase | 0.48 | 0.24 |
| ENSCAFG00845017608 | PLA2G4F | phospholipase A2 group IVF | 0.79 | 0.03 |
| ENSCAFG00845025826 | RPTN | repetin | 0.36 | 0.39 |
| ENSCAFG00845029411 | GDPD2 | glycerophosphodiester phosphodiesterase domain containing 2 | 0.55 | 0.17 |
| ENSCAFG00845022756 | Novel gene 1 | 0.38 | 0.36 | |
| ENSCAFG00845001333 | Novel gene 2 | 0.55 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mucignat, G.; Montanucci, L.; Elgendy, R.; Giantin, M.; Laganga, P.; Pauletto, M.; Mutinelli, F.; Vascellari, M.; Leone, V.F.; Dacasto, M.; et al. A Whole-Transcriptomic Analysis of Canine Oral Melanoma: A Chance to Disclose the Radiotherapy Effect and Outcome-Associated Gene Signature. Genes 2024, 15, 1065. https://doi.org/10.3390/genes15081065
Mucignat G, Montanucci L, Elgendy R, Giantin M, Laganga P, Pauletto M, Mutinelli F, Vascellari M, Leone VF, Dacasto M, et al. A Whole-Transcriptomic Analysis of Canine Oral Melanoma: A Chance to Disclose the Radiotherapy Effect and Outcome-Associated Gene Signature. Genes. 2024; 15(8):1065. https://doi.org/10.3390/genes15081065
Chicago/Turabian StyleMucignat, Greta, Ludovica Montanucci, Ramy Elgendy, Mery Giantin, Paola Laganga, Marianna Pauletto, Franco Mutinelli, Marta Vascellari, Vito Ferdinando Leone, Mauro Dacasto, and et al. 2024. "A Whole-Transcriptomic Analysis of Canine Oral Melanoma: A Chance to Disclose the Radiotherapy Effect and Outcome-Associated Gene Signature" Genes 15, no. 8: 1065. https://doi.org/10.3390/genes15081065






