Network of Interactions between the Mut Domains of the E2 Protein of Atypical Porcine Pestivirus and Host Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, Antibodies, and Reagents
2.2. Construction of Recombinant Expression Plasmids
2.3. Y2H Screening
2.4. Functional Analysis of Interacting Proteins
2.5. Glutathione S-Transferase Pull-Down Assays
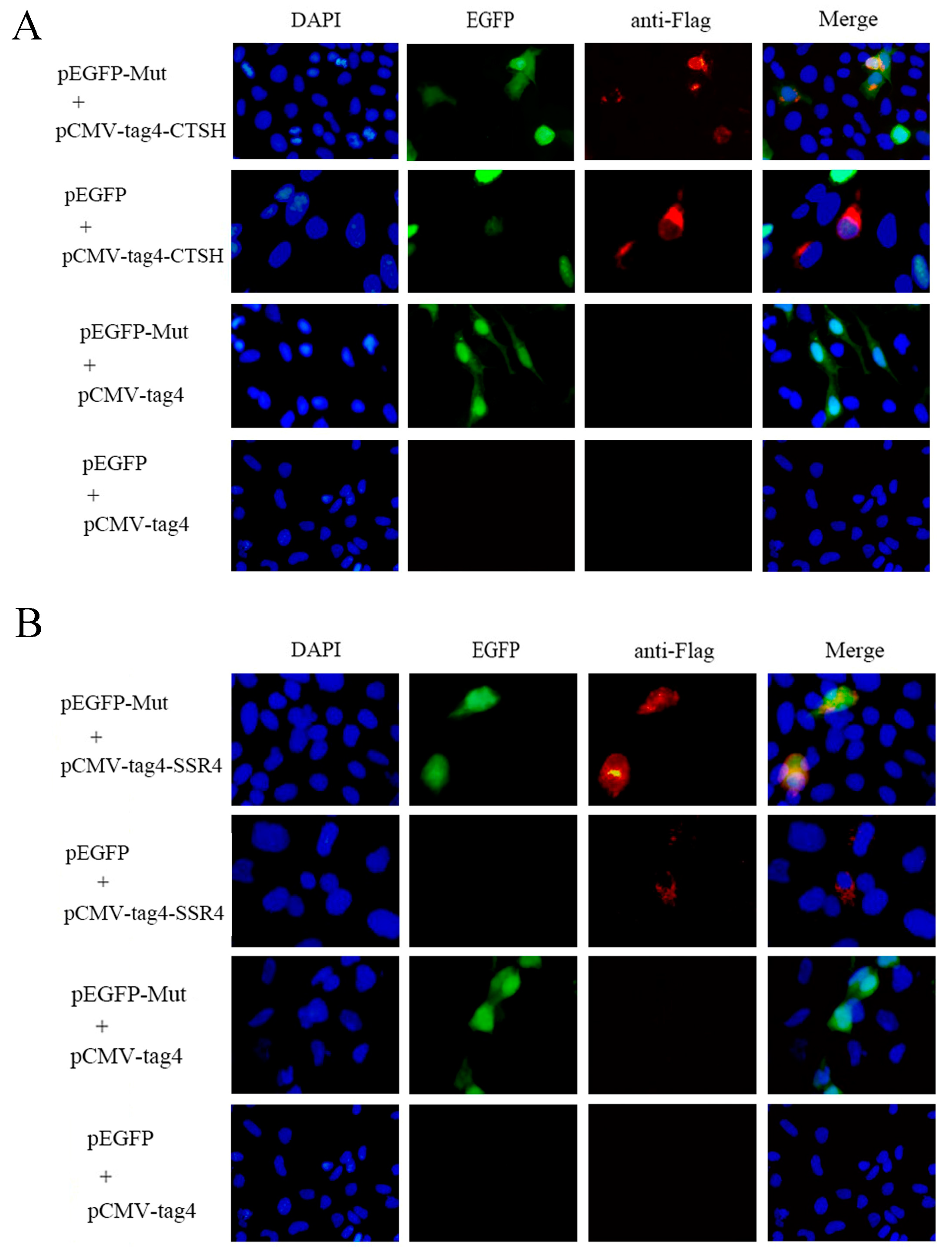
2.6. Co-Localization Analysis in Cells
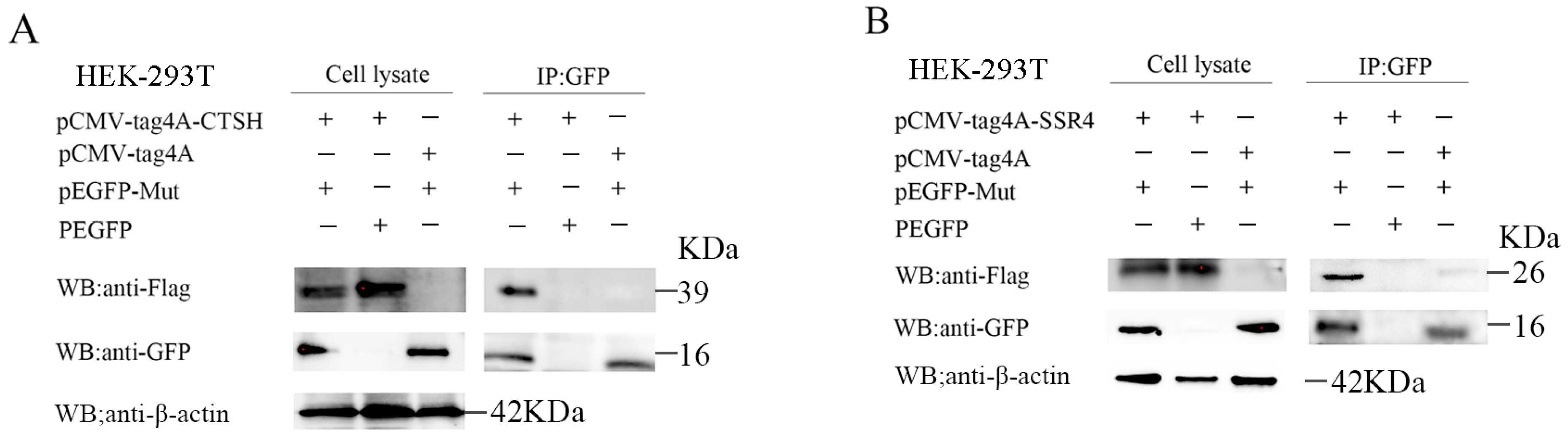
2.7. Co-Immunoprecipitation Assays
2.8. The Effects of Signal Sequence Receptor Subunit 4 on APPV Infection
2.9. Western Blotting Assay
2.10. Quantitative Real-Time Polymerase Chain Reaction
2.11. Statistical Analysis
3. Results
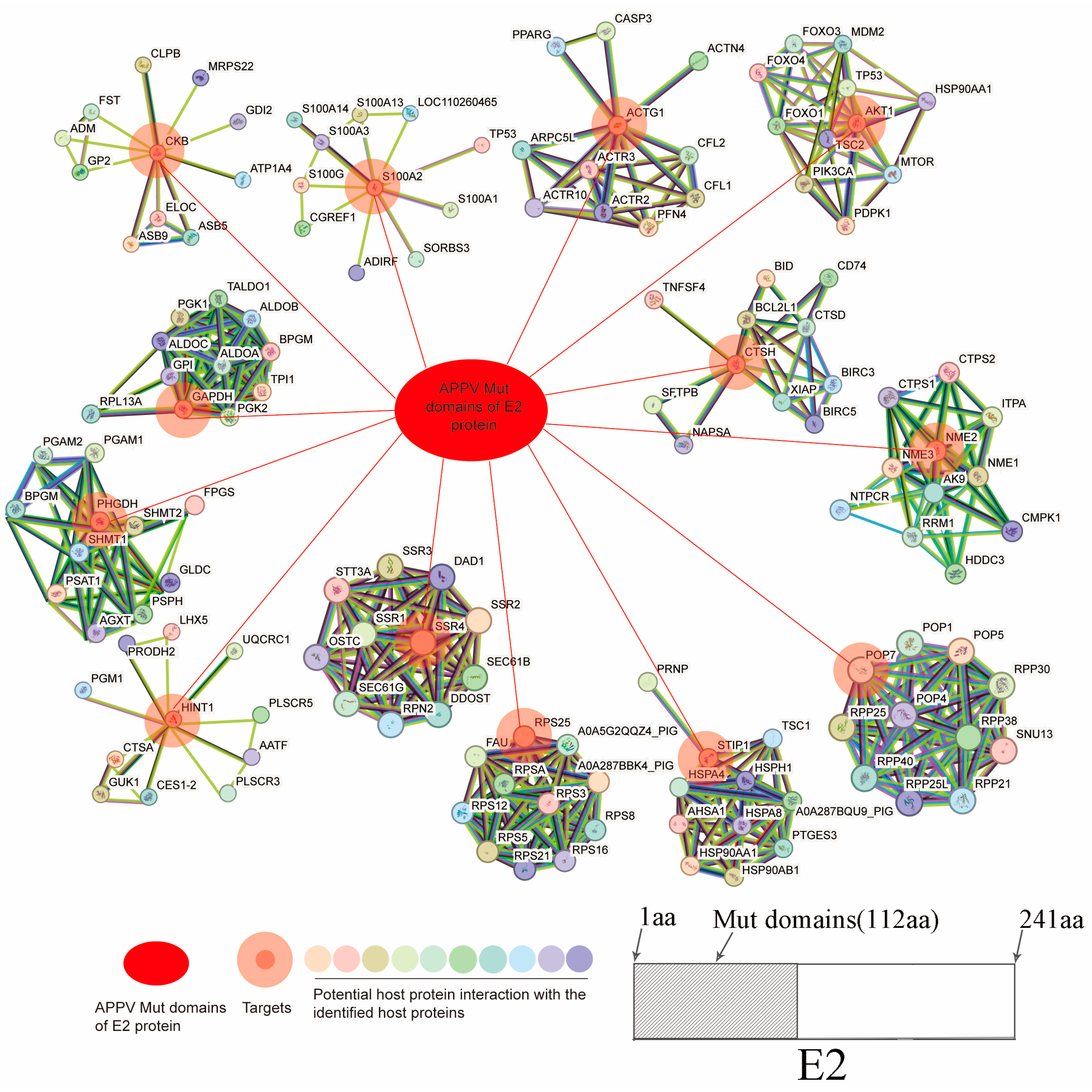
3.1. Screening of the Mut Domains of the E2 Protein of Atypical Porcine Pestivirus Interacting Proteins Using the Y2H System
3.2. Construction and Analysis of the Mut Domains of the E2 Protein–Cellular Protein Interaction Network
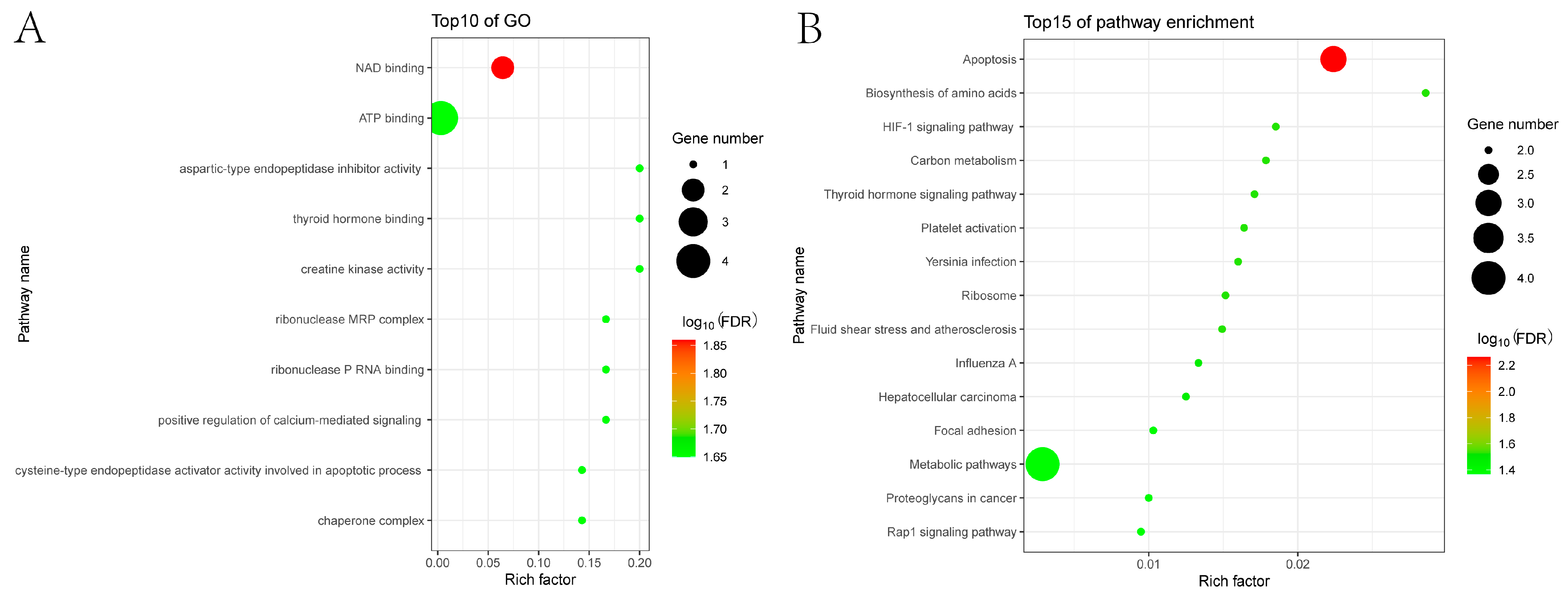
3.3. GO and KEGG Enrichment Analyses
3.4. Mut Domains of the E2 Protein Interact with CTSH and SSR4
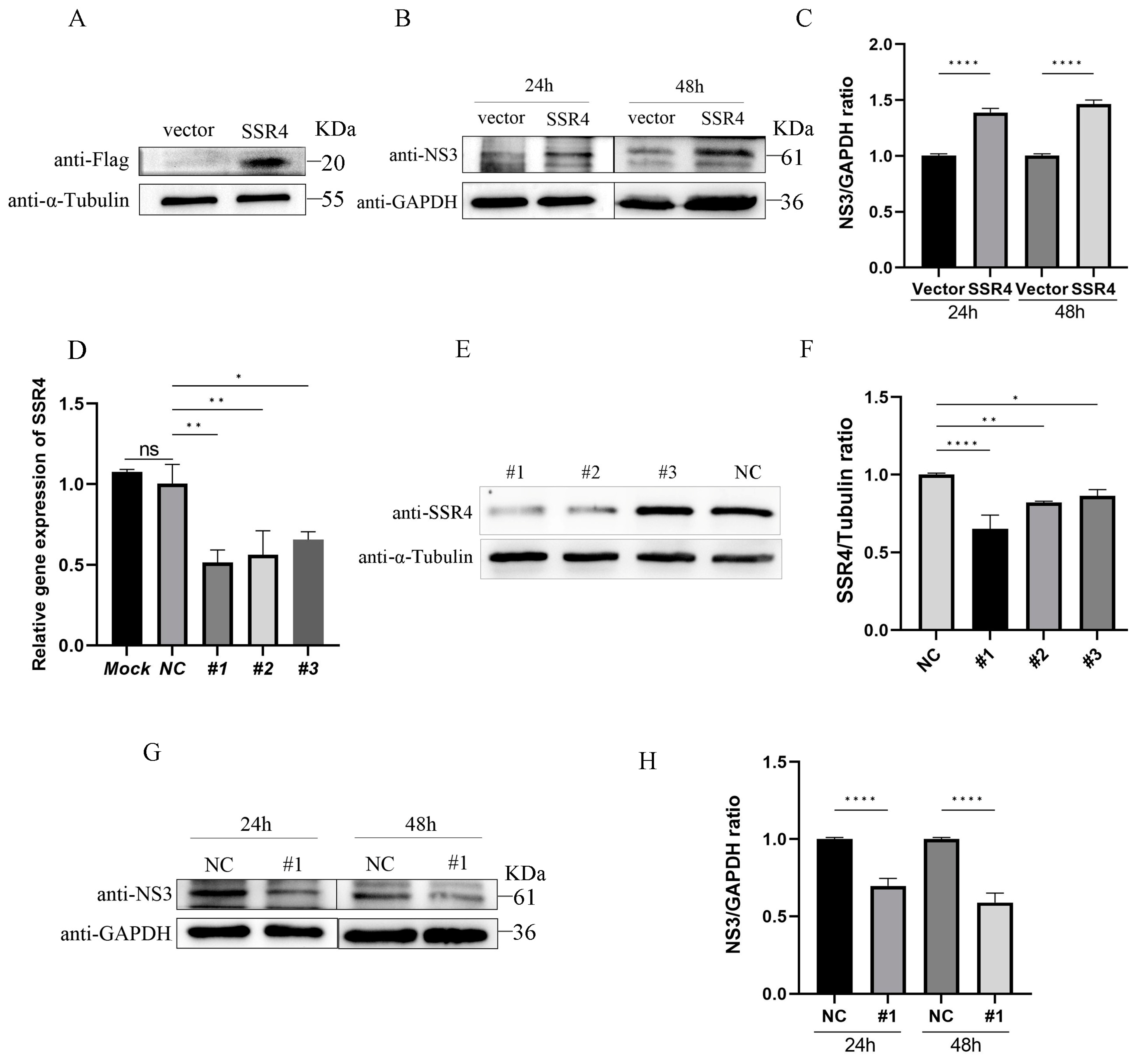
3.5. Effects of SSR4 on the Replication of APPV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stenberg, H.; Jacobson, M.; Malmberg, M. A review of congenital tremor type A-II in piglets. Anim. Health Res. Rev. 2020, 21, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Hause, B.M.; Collin, E.A.; Peddireddi, L.; Yuan, F.F.; Chen, Z.H.; Hesse, R.A.; Gauger, P.C.; Clement, T.; Fang, Y.; Anderson, G. Discovery of a novel putative atypical porcine pestivirus in pigs in the USA. J. Gen. Virol. 2015, 96, 2994–2998. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Hansmann, F.; Baechlein, C.; Fischer, N.; Alawi, M.; Grundhoff, A.; Derking, S.; Tenhündfeld, J.; Pfankuche, V.M.; Herder, V.; et al. Presence of atypical porcine pestivirus (APPV) genomes in newborn piglets correlates with congenital tremor. Sci. Rep. 2016, 6, 27735. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, S.; Canturri, A.; Pérez-Simó, M.; Bohórquez, J.A.; Rosell, R.; Cabezón, O.; Segalés, J.; Domingo, M.; Ganges, L. First report of the novel atypical porcine pestivirus in Spain and a retrospective study. Transbound. Emerg. Dis. 2017, 64, 1645–1649. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.; Stalder, H.; Sidler, X.; Renzullo, S.; Gurtner, C.; Grahofer, A.; Schweizer, M. Long-Term Circulation of Atypical Porcine Pestivirus (APPV) within Switzerland. Viruses 2019, 11, 653. [Google Scholar] [CrossRef]
- Arruda, B.L.; Arruda, P.H.; Magstadt, D.R.; Schwartz, K.J.; Dohlman, T.; Schleining, J.A.; Patterson, A.R.; Visek, C.A.; Victoria, J.G. Identification of a Divergent Lineage Porcine Pestivirus in Nursing Piglets with Congenital Tremors and Reproduction of Disease following Experimental Inoculation. PLoS ONE 2016, 11, e0150104. [Google Scholar] [CrossRef] [PubMed]
- de Groof, A.; Deijs, M.; Guelen, L.; van Grinsven, L.; van Os-Galdos, L.; Vogels, W.; Derks, C.; Cruijsen, T.; Geurts, V.; Vrijenhoek, M.; et al. Atypical Porcine Pestivirus: A Possible Cause of Congenital Tremor Type A-II in Newborn Piglets. Viruses 2016, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, H.; Jacobson, M.; Malmberg, M. Detection of atypical porcine pestivirus in Swedish piglets with congenital tremor type A-II. BMC Vet. Res. 2020, 16, 260. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.; Riedel, C.; Högler, S.; Sinn, L.J.; Voglmayr, T.; Wöchtl, B.; Dinhopl, N.; Rebel-Bauder, B.; Weissenböck, H.; Ladinig, A.; et al. Congenital infection with atypical porcine pestivirus (APPV) is associated with disease and viral persistence. Vet. Res. 2017, 48, 1. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, K.; Liu, J.; Ge, S.; Xiao, Y.; Shang, Y.; Ning, Z. Identification of atypical porcine pestivirus infection in swine herds in China. Transbound. Emerg. Dis. 2017, 64, 1020–1023. [Google Scholar] [CrossRef]
- Gatto, I.R.H.; Harmon, K.; Bradner, L.; Silva, P.; Linhares, D.C.L.; Arruda, P.H.; de Oliveira, L.G.; Arruda, B.L. Detection of atypical porcine pestivirus in Brazil in the central nervous system of suckling piglets with congenital tremor. Transbound. Emerg. Dis. 2018, 65, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Dénes, L.; Biksi, I.; Albert, M.; Szeredi, L.; Knapp, D.G.; Szilasi, A.; Bálint, A.; Balka, G. Detection and phylogenetic characterization of atypical porcine pestivirus strains in Hungary. Transbound. Emerg. Dis. 2018, 65, 2039–2042. [Google Scholar] [CrossRef] [PubMed]
- Kasahara-Kamiie, M.; Kagawa, M.; Shiokawa, M.; Sunaga, F.; Fukase, Y.; Aihara, N.; Shiga, T.; Kamiie, J.; Aoki, H.; Nagai, M. Detection and genetic analysis of a novel atypical porcine pestivirus from piglets with congenital tremor in Japan. Transbound. Emerg. Dis. 2022, 69, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Dessureault, F.G.; Choinière, M.; Provost, C.; Gagnon, C.A. First report of atypical porcine pestivirus in piglets with congenital tremor in Canada. Can. Vet. J. La Rev. Vet. Can. 2018, 59, 429–432. [Google Scholar]
- Choe, S.; Park, G.N.; Cha, R.M.; Hyun, B.H.; Park, B.K.; An, D.J. Prevalence and Genetic Diversity of Atypical Porcine Pestivirus (APPV) Detected in South Korean Wild Boars. Viruses 2020, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, E.; Salogni, C.; Lelli, D.; Barbieri, I.; Moreno, A.; Alborali, G.L.; Lavazza, A. Molecular Survey and Phylogenetic Analysis of Atypical Porcine Pestivirus (APPV) Identified in Swine and Wild Boar from Northern Italy. Viruses 2019, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Colom-Cadena, A.; Ganges, L.; Muñoz-González, S.; Castillo-Contreras, R.; Bohórquez, J.A.; Rosell, R.; Segalés, J.; Marco, I.; Cabezon, O. Atypical porcine pestivirus in wild boar (Sus scrofa), Spain. Vet. Rec. 2018, 183, 569. [Google Scholar] [CrossRef] [PubMed]
- Cagatay, G.N.; Antos, A.; Meyer, D.; Maistrelli, C.; Keuling, O.; Becher, P.; Postel, A. Frequent infection of wild boar with atypical porcine pestivirus (APPV). Transbound. Emerg. Dis. 2018, 65, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, H.; Leveringhaus, E.; Malmsten, A.; Dalin, A.M.; Postel, A.; Malmberg, M. Atypical porcine pestivirus-A widespread virus in the Swedish wild boar population. Transbound. Emerg. Dis. 2022, 69, 2349–2360. [Google Scholar] [CrossRef]
- Ma, H.L.; Li, W.T.; Zhang, M.J.; Yang, Z.X.; Lin, L.L.; Ghonaim, A.H.; He, Q.G. The Diversity and Spatiotemporally Evolutionary Dynamic of Atypical Porcine Pestivirus in China. Front. Microbiol. 2022, 13, 937918. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Q.; Shan, H.; Cao, Z.; Huang, J. Genome characteristics of atypical porcine pestivirus from abortion cases in Shandong Province, China. Virol. J. 2023, 20, 282. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, X.; Zhang, P.; Wang, L.; Liu, Y.; Zhang, L.; Liang, P.; Song, C. Identification and characterization of atypical porcine pestivirus genomes in newborn piglets with congenital tremor in China. J. Vet. Sci. 2018, 19, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Z.; Ren, X.; Li, H.; Lu, R.; Zhang, Y.; Ning, Z. Viral load and histological distribution of atypical porcine pestivirus in different tissues of naturally infected piglets. Arch. Virol. 2019, 164, 2519–2523. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Han, Z.Y.; Li, J.; Huang, Y.Z.; Yang, J.F.; Ding, H.X.; Zhang, J.Y.; Zhu, M.J.; Zhang, Y.Y.; Liao, J.D.; et al. Atypical Porcine Pestivirus as a Novel Type of Pestivirus in Pigs in China. Front. Microbiol. 2017, 8, 862. [Google Scholar] [CrossRef] [PubMed]
- Sutton, K.M.; Lahmers, K.K.; Harris, S.P.; Wijesena, H.R.; Mote, B.E.; Kachman, S.D.; Borza, T.; Ciobanu, D.C. Detection of atypical porcine pestivirus genome in newborn piglets affected by congenital tremor and high preweaning mortality1. J. Anim. Sci. 2019, 97, 4093–4100. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.Q.; Lefkowitz, E.J.; Mushegian, A.R.; Adams, M.J.; Dutilh, B.E.; Gorbalenya, A.E.; Harrach, B.; Harrison, R.L.; Junglen, S.; Knowles, N.J.; et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2018). Arch. Virol. 2018, 163, 2601–2631. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Beer, M.; Wernike, K. New Leaves in the Growing Tree of Pestiviruses. Adv. Virus Res. 2017, 99, 139–160. [Google Scholar] [CrossRef]
- Bashashati, M.; Chung, D.H.; Fallah Mehrabadi, M.H.; Lee, D.H. Evolution of H9N2 avian influenza viruses in Iran, 2017–2019. Transbound Emerg. Dis. 2021, 68, 3405–3414. [Google Scholar] [CrossRef]
- Zhang, H.W.; Wen, W.; Hao, G.X.; Chen, H.C.; Qian, P.; Li, X.M. A Subunit Vaccine Based on E2 Protein of Atypical Porcine Pestivirus Induces Th2-type Immune Response in Mice. Viruses 2018, 10, 673. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Kong, L.-F.; Zhang, X.-M.; Zheng, H.-L.; Lin, M.-X.; Chen, H.-T.; Yang, Y.-A. Construction of a chimeric virus based on atypical classical swine fever virus Yunnan strain with Shimen strain. Chin. J. Prev. Vet. Med. 2012, 34. [Google Scholar] [CrossRef]
- Perrin-Cocon, L.; Diaz, O.; Jacquemin, C.; Barthel, V.; Ogire, E.; Ramière, C.; André, P.; Lotteau, V.; Vidalain, P.O. The current landscape of coronavirus-host protein-protein interactions. J. Transl. Med. 2020, 18, 319. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.B.; Li, X.T.; Lv, X.Y.; Liu, K.; Ren, J.Q.; Zhai, J.B.; Song, Y. Current progress on innate immune evasion mediated by N. protein of pestiviruses. Front. Immunol. 2023, 14, 1136051. [Google Scholar] [CrossRef]
- Chen, X.N.; Chen, X.J.; Liang, Y.F.; Xu, S.J.; Weng, Z.J.; Gao, Q.; Huang, Z.; Zhang, G.H.; Gong, L. Interaction network of African swine fever virus structural protein p30 with host proteins. Front. Microbiol. 2022, 13, 971888. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.D.; Zhang, M.R.; Liu, C.C.; Zhu, M.J.; Zhang, Z.L.; Wu, K.K.; Li, Z.Y.; Li, W.H.; Fan, S.Q.; Ju, C.M.; et al. The Network of Interactions Between Classical Swine Fever Virus Nonstructural Protein p7 and Host Proteins. Front. Microbiol. 2020, 11, 597893. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; You, R.; Kang, Y.; Xie, P.; Zhu, W.; Sun, M.; Gao, P.; Li, Y.; Ren, T. Host immune responses of pigeons infected with Newcastle disease viruses isolated from pigeons. Microb. Pathog. 2019, 127, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qu, S.; Liang, Z.; Liu, Y.; Chen, H.; Ma, S.; Liu, X. Cathepsin H Knockdown Reverses Radioresistance of Hepatocellular Carcinoma via Metabolic Switch Followed by Apoptosis. Int. J. Mol. Sci. 2023, 24, 5257. [Google Scholar] [CrossRef]
- Phoomak, C.; Cui, W.; Hayman, T.J.; Yu, S.H.; Zhao, P.; Wells, L.; Steet, R.; Contessa, J.N. The translocon-associated protein (TRAP) complex regulates quality control of N-linked glycosylation during ER stress. Sci. Adv. 2021, 7, eabc6364. [Google Scholar] [CrossRef] [PubMed]
- Mehrbod, P.; Ande, S.R.; Alizadeh, J.; Rahimizadeh, S.; Shariati, A.; Malek, H.; Hashemi, M.; Glover, K.K.M.; Sher, A.A.; Coombs, K.M.; et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 2019, 10, 376–413. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.P.; Liang, L.B.; Zhao, Y.H.; Wen, X.; Kong, F.D.; Wang, G.W.; Shi, W.J.; Li, Q.B.; Jiang, L.; et al. Interaction between influenza virus PA protein and host protein SSR4. Chin. J. Prev. Vet. Med. 2021, 43, 1029–1033. [Google Scholar]
- Pan, S.N.; Mou, C.X.; Chen, Z.H. An emerging novel virus: Atypical porcine pestivirus (APPV). Rev. Med. Virol. 2019, 29, e2018. [Google Scholar] [CrossRef]
- Liang, Y.Y. Pathogenicity and virulence of influenza. Virulence 2023, 14, 2223057. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J. Pathogenesis of Hypervirulent Fowl Adenovirus Serotype 4: The Contributions of Viral and Host Factors. Viruses-Basel 2019, 11, 741. [Google Scholar] [CrossRef]
- Borca, M.V.; Vuono, E.A.; Ramirez-Medina, E.; Azzinaro, P.; Berggren, K.A.; Singer, M.; Rai, A.; Pruitt, S.; Silva, E.B.; Velazquez-Salinas, L.; et al. Structural Glycoprotein E2 of Classical Swine Fever Virus Interacts with Host Protein Dynactin Subunit 6 (DCTN6) during the Virus Infectious Cycle. J. Virol. 2020, 94, e01642-19. [Google Scholar] [CrossRef]
- Vuono, E.A.; Ramirez-Medina, E.; Velazquez-Salinas, L.; Berggren, K.; Rai, A.; Pruitt, S.; Espinoza, N.; Gladue, D.P.; Borca, M.V. Structural Glycoprotein E2 of Classical Swine Fever Virus Critically Interacts with Host Protein Torsin-1A during the Virus Infectious Cycle. J. Virol. 2021, 95, e00314-21. [Google Scholar] [CrossRef]
- Vuono, E.A.; Ramirez-Medina, E.; Azzinaro, P.; Berggren, K.A.; Rai, A.; Pruitt, S.; Silva, E.; Velazquez-Salinas, L.; Borca, M.V.; Gladue, D.P. SERTA Domain Containing Protein 1 (SERTAD1) Interacts with Classical Swine Fever Virus Structural Glycoprotein E2, Which Is Involved in Virus Virulence in Swine. Viruses 2020, 12, 421. [Google Scholar] [CrossRef] [PubMed]
- Vuono, E.A.; Ramirez-Medina, E.; Berggren, K.; Rai, A.; Pruitt, S.; Silva, E.; Velazquez-Salinas, L.; Gladue, D.P.; Borca, M.V. Swine Host Protein Coiled-Coil Domain-Containing 115 (CCDC115) Interacts with Classical Swine Fever Virus Structural Glycoprotein E2 during Virus Replication. Viruses 2020, 12, 388. [Google Scholar] [CrossRef]
- Wang, J.H.; Chen, S.C.; Liao, Y.J.; Zhang, E.Y.; Feng, S.; Yu, S.X.; Li, L.F.; He, W.R.; Li, Y.F.; Luo, Y.Z.; et al. Mitogen-Activated Protein Kinase Kinase 2, a Novel E2-Interacting Protein, Promotes the Growth of Classical Swine Fever Virus via Attenuation of the JAK-STAT Signaling Pathway. J. Virol. 2016, 90, 10271–10283, Erratum in 2017, 91, e01523-17. [Google Scholar] [CrossRef] [PubMed]
- Vuono, E.A.; Ramirez-Medina, E.; Holinka, L.G.; Baker-Branstetter, R.; Borca, M.V.; Gladue, D.P. Interaction of Structural Glycoprotein E2 of Classical Swine Fever Virus with Protein Phosphatase 1 Catalytic Subunit beta (PPP1CB). Viruses 2019, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, Z.X.; Guo, H.C.; Qu, H.; Zhang, Y.; Tu, C.C. Annexin 2 is a host protein binding to classical swine fever virus E2 glycoprotein and promoting viral growth in PK-15 cells. Virus Res. 2015, 201, 16–23. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.H.; He, W.R.; Feng, S.; Li, Y.F.; Wang, X.; Liao, Y.J.; Qin, H.Y.; Li, L.F.; Dong, H.; et al. Thioredoxin 2 Is a Novel E2-Interacting Protein That Inhibits the Replication of Classical Swine Fever Virus. J. Virol. 2015, 89, 8510–8524. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Cotter, P.A.; Kumamoto, C.A. Microbial pathogenesis: Mechanisms of infectious disease. Cell Host Microbe 2007, 2, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.M.; Mao, Q.; Yi, L.; Zhao, M.Q.; Chen, J.D. Apoptosis, Autophagy, and Pyroptosis: Immune Escape Strategies for Persistent Infection and Pathogenesis of Classical Swine Fever Virus. Pathogens 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Guo, K.; Kang, K.; Zhang, Y.; He, L.; Wang, J. Classical swine fever virus NS2 protein promotes interleukin-8 expression and inhibits MG132-induced apoptosis. Virus Genes 2011, 42, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Johns, H.L.; Doceul, V.; Everett, H.; Crooke, H.; Charleston, B.; Seago, J. The classical swine fever virus N-terminal protease N(pro) binds to cellular HAX-1. J. Gen. Virol. 2010, 91, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Everett, H.; McFadden, G. Apoptosis: An innate immune response to virus infection. Trends Microbiol. 1999, 7, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.C.; Zhao, M.Q.; Fan, S.Q.; Yuan, J.; Liao, J.D.; He, W.C.; Xu, H.L.; Chen, J.D. Autophagy induces apoptosis and death of T lymphocytes in the spleen of pigs infected with CSFV. Sci. Rep. 2017, 7, 13577. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Ming, S.L.; Zhang, S.; Wang, Q.; Zeng, L.; Zhou, L.Y.; Wang, M.D.; Ma, Y.X.; Han, L.Q.; Zhong, K.; Zhu, H.S.; et al. Inhibition of USP14 influences alphaherpesvirus proliferation by degrading viral VP16 protein via ER stress-triggered selective autophagy. Autophagy 2022, 18, 1801–1821. [Google Scholar] [CrossRef]
- Diao, F.F.; Jiang, C.L.; Sun, Y.Y.; Gao, Y.N.; Bai, J.; Nauwynck, H.; Wang, X.W.; Yang, Y.Q.; Jiang, P.; Liu, X. Porcine reproductive and respiratory syndrome virus infection triggers autophagy via ER stress-induced calcium signaling to facilitate virus replication. PLoS Pathog. 2023, 19, e1011295. [Google Scholar] [CrossRef]
- Zou, D.H.; Xu, J.X.; Duan, X.L.; Xu, X.; Li, P.F.; Cheng, L.X.; Zheng, L.; Li, X.Z.; Zhang, Y.T.; Wang, X.H.; et al. Porcine epidemic diarrhea virus ORF3 protein causes endoplasmic reticulum stress to facilitate autophagy. Vet. Microbiol. 2019, 235, 209–219. [Google Scholar] [CrossRef]

| Name | Forward Primer (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| pGBKT7/pEGFP-Mut | GAATTCATGCTCACCACCACCTGG | GGATCCCCTATTGGGCAGACAAGA |
| pET-GST-Mut | GGATCCATGCTCACCACCTGG | GAATTCCCTATTGGGCAGACAAGA |
| pCMV-tag4A-CTSH | GAATTCATGTGGGCCGTCCTGTC | CTCGAGCACCAGGGGGATCGGG |
| pCMV-tag4A-SSR4 | GAATTCATGGCGGCGCTGG | CTCGAGGGCCTGGATGTGGCTC |
| SSR4 (qRT-PCR) | ATCTCCACAGAGACCGTGTT | GCTGTAGGACTCCTCATCGAAG |
| β-actin (qRT-PCR) | CACCATTGGCAATGAGCGGTTC | AGGTCTTTGCGGATGTCCACGT |
| siSSR4-#1 | GAUCACGCCCUCGUACUAUtt | AUAGUACGAGGGCGUGAUCtt |
| siSSR4-#2 | GGAAGGCUCAGAGAAAUAAtt | UUAWUUCUCUGAGCCUUCCtt |
| siSSR4-#3 | GCAAGAACAGGGUCCAGAACAtt | UGUUCUGGACCCUGUUCUUGCtt |
| Negative control (NC) | UUCUCCGAACGUGUCACGU | AAGAGGCUUGCACAGUGCA |
| Gene | Protein | NCBI Accession | Function |
|---|---|---|---|
| SSR4 | Translocon-associated protein subunit delta | XM_003135482.5 | Regulates the retention of ER resident proteins |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | XM_021091114.1 | Modulates the organization and assembly of the cytoskeleton and innate immunity |
| HINT1 | Histidine triad nucleotide-binding protein 1 | XM_005661623.3 | Involved in phosphorylation of ribonucleotide |
| CTSH | Cathepsin H | XM_017021951.2 | Involved in proteolysis associated with protein catabolic process |
| STIP1 | Stress-induced phosphoprotein 1 | XM_003353794.4 | Co-chaperone that binds to heat shock proteins 70 and 90 |
| POP7 | Ribonuclease P protein subunit p20 | XM_013995472.2 | Involved in rRNA processing, tRNA 5′-leader removal, and tRNA processing |
| PHGDH | D-3-phosphoglycerate dehydrogenase | XM_011541226.3 | Involved in the early steps of L-serine synthesis in animal cells |
| RPS25 | 40S ribosomal protein S25 | NM_001028.3 | Component of the small ribosomal subunit |
| CKB | Creatine kinase B-type | XM_021081502.1 | Reversibly catalyzes the transfer of phosphate between ATP and various phosphagens |
| AKT1 | RAC-alpha serine/threonine-protein kinase | XM_021081500.1 | Protein serine/threonine kinase activity |
| NME2 | Nucleoside diphosphate kinase B | NM_001044610.2 | Involved in the synthesis of nucleoside triphosphates other than ATP |
| RPS26 | Small ribosomal subunit protein eS26 | NM_001097481.2 | Structural constituent of the ribosome |
| S100A2 | S100 calcium-binding protein A2 | XM_001929556.5 | Calcium ion binding |
| ACTG1 | Actin gamma 1 | XM_003357928.4 | Involved in various types of cell motility and maintenance of the cytoskeleton |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Jiang, G.; He, W.; Tian, X.; Zheng, H.; Xiang, B.; Sun, Y. Network of Interactions between the Mut Domains of the E2 Protein of Atypical Porcine Pestivirus and Host Proteins. Genes 2024, 15, 991. https://doi.org/10.3390/genes15080991
Yang Y, Jiang G, He W, Tian X, Zheng H, Xiang B, Sun Y. Network of Interactions between the Mut Domains of the E2 Protein of Atypical Porcine Pestivirus and Host Proteins. Genes. 2024; 15(8):991. https://doi.org/10.3390/genes15080991
Chicago/Turabian StyleYang, Yuai, Guangfei Jiang, Weiqi He, Xin Tian, Huanli Zheng, Bin Xiang, and Yongke Sun. 2024. "Network of Interactions between the Mut Domains of the E2 Protein of Atypical Porcine Pestivirus and Host Proteins" Genes 15, no. 8: 991. https://doi.org/10.3390/genes15080991




