Quantitative Trait Loci Mapping of Heading Date in Wheat under Phosphorus Stress Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Plot Design
2.2. Phenotypic Evaluation and Data Analysis
2.3. Genetic Linkage Map Construction and QTL Mapping
2.4. Identification of Candidate Genes
3. Results
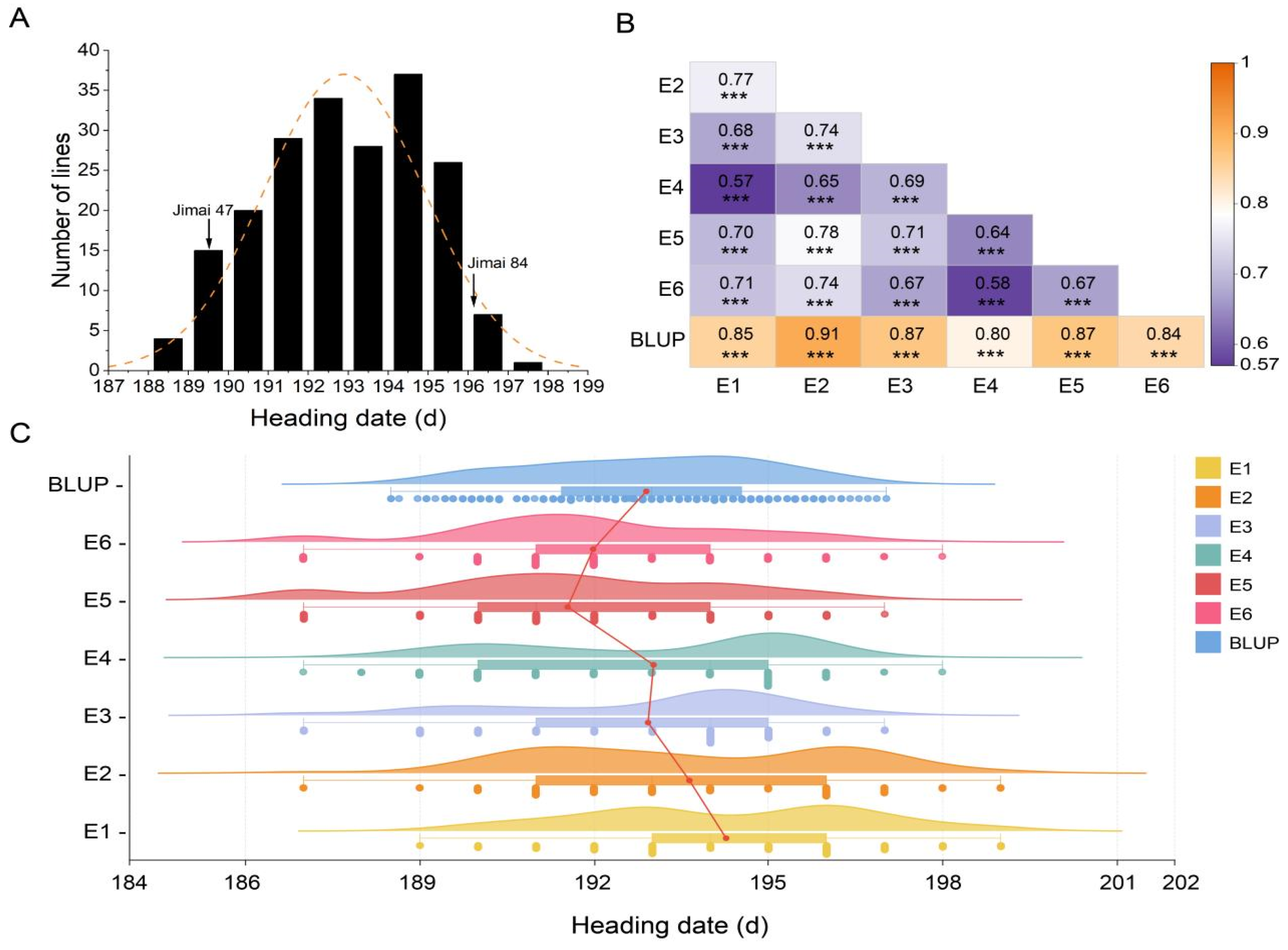
3.1. Phenotypic Evaluation
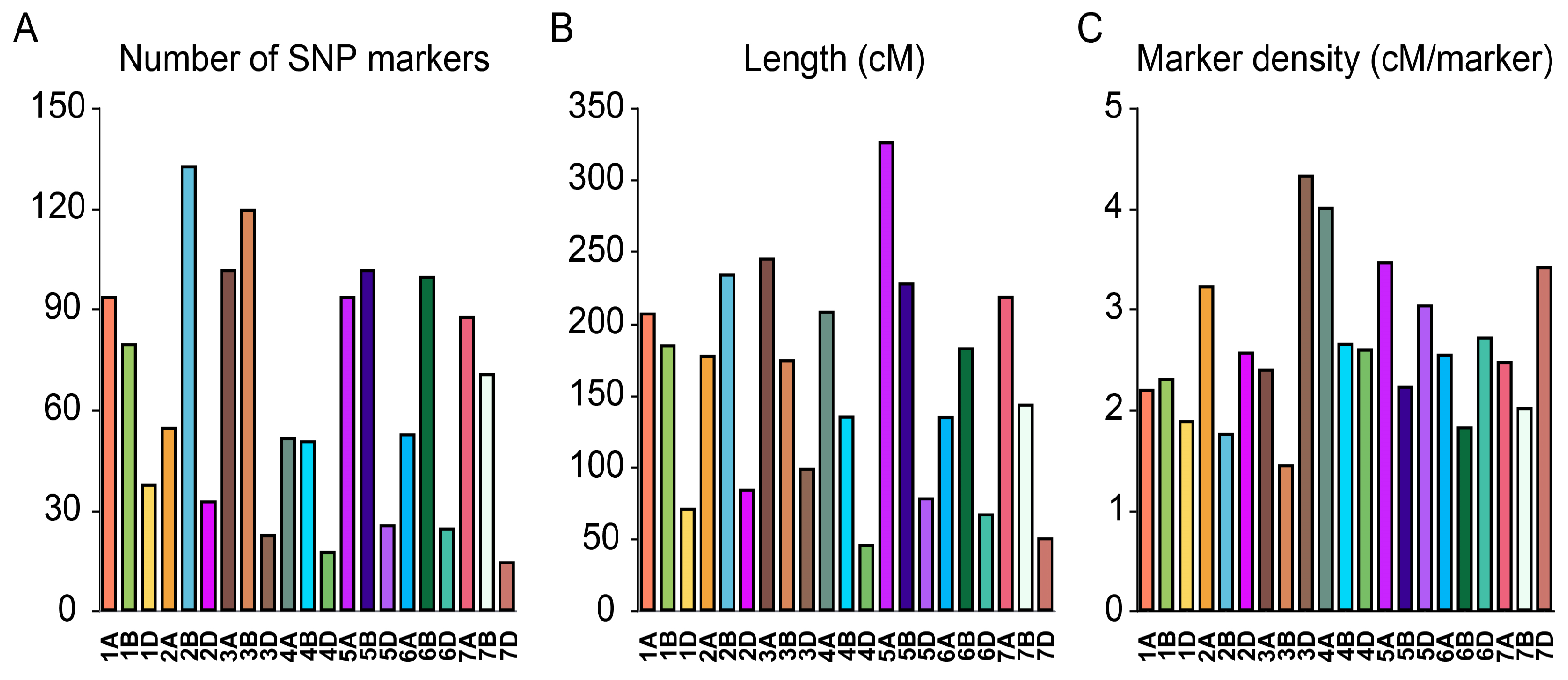
3.2. Genetic Linkage Map Construction
3.3. QTL Mapping for Heading Date under Different Phosphorus Conditions
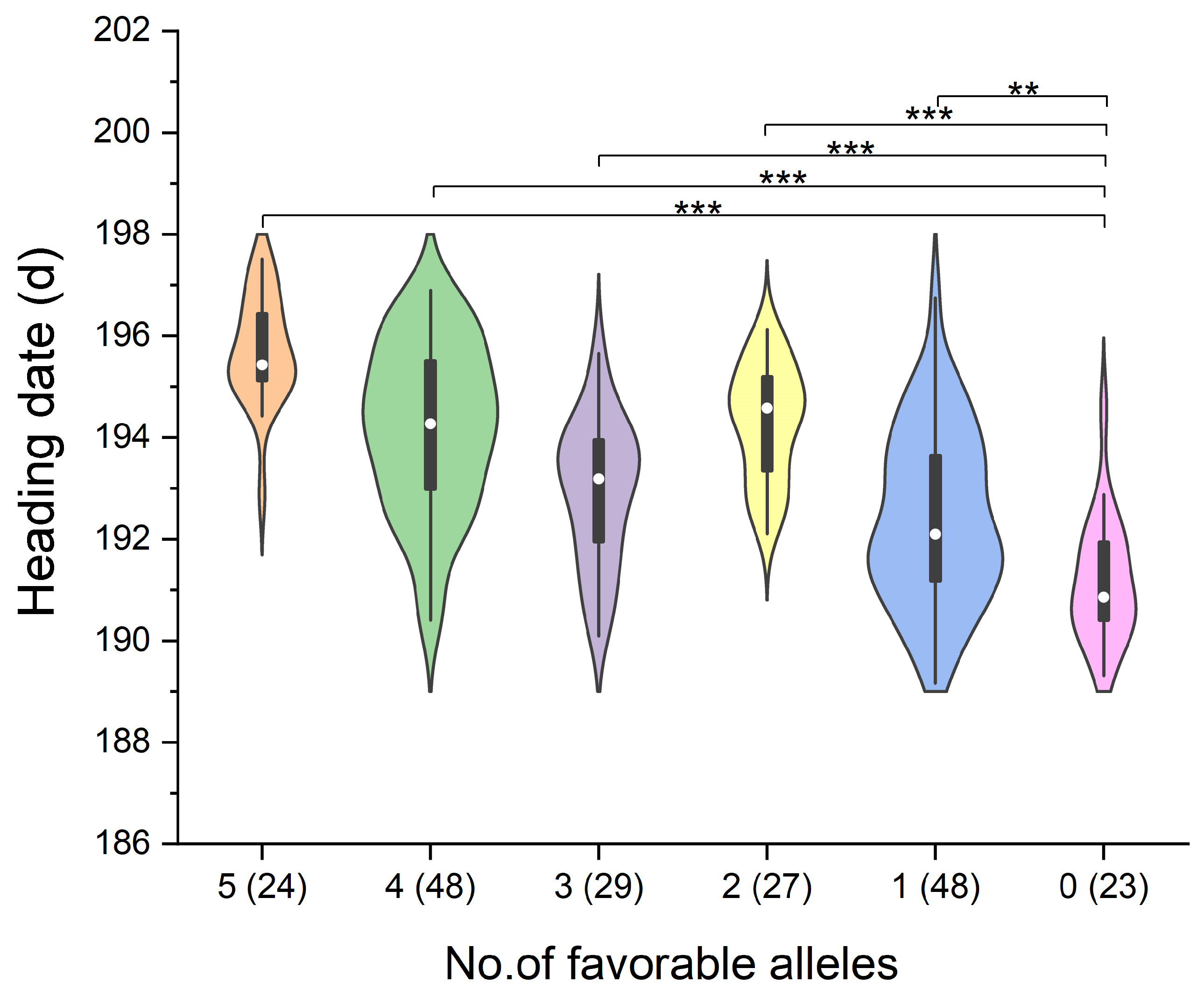
3.4. Additive Effects Analysis of the Five Major QTLs
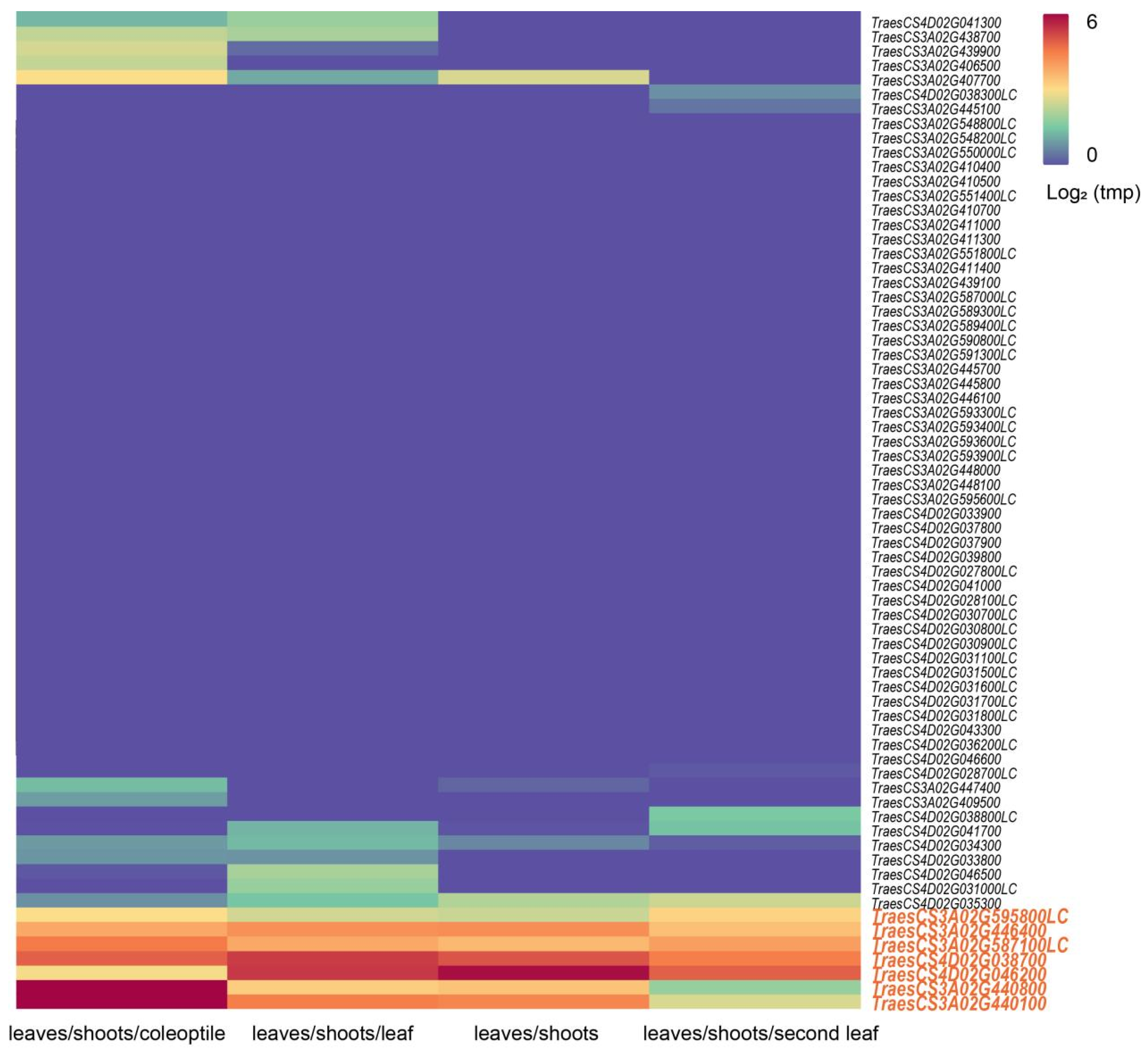
3.5. Prediction and Analysis of Candidate Genes for Qhd.saw-3A.8, Qhd.saw-3A.9, and Qhd.saw-4D.3
4. Discussion
4.1. The Impact of Phosphorus Stress on Wheat Heading Date
4.2. Comparison of Stable and Major QTLs with Previous Studies
4.3. Candidate Gene Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubcovsky, J.; Dvorak, J. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 2007, 316, 1862–1866. [Google Scholar] [CrossRef]
- Cao, S.H.; Xu, D.G.; Hanif, M.; Xia, X.C.; He, Z.H. Genetic architecture underpinning yield component traits in wheat. Theor. Appl. Genet. 2020, 133, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, X.; Zhang, G.; Jiang, P.; Chen, W.; Hao, Y.; Ma, X.; Xu, S.; Jia, J.; Kong, L.; et al. QTL mapping for yield-related traits in wheat based on four RIL populations. Theor. Appl. Genet. 2020, 133, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Snape, J.W.; Butterworth, K.; Whitechurch, E.; Worland, A.J. Waiting for fine times: Genetics of flowering time in wheat. Euphytica 2001, 119, 185–190. [Google Scholar] [CrossRef]
- Jung, C.; Mueller, A.E. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009, 14, 563–573. [Google Scholar] [CrossRef]
- Benaouda, S.; Dadshani, S.; Koua, P.; Leon, J.; Balivora, A. Identification of QTLs for wheat heading time across multiple environments. Theor. Appl. Genet. 2022, 135, 2833–2848. [Google Scholar] [CrossRef]
- Ma, C.; Liu, L.; Liu, T.; Jia, Y.; Jiang, Q.; Bai, H.; Ma, S.; Li, S.; Wang, Z. QTL mapping for important agronomic traits using a Wheat55K SNP array-based genetic map in tetraploid wheat. Plants 2023, 12, 847. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, L.; Zhang, X.; Lv, G.; Pan, Y.; Chen, F. Gene regulatory network and abundant genetic variation play critical roles in heading stage of polyploidy wheat. BMC Plant Biol. 2019, 19, 6. [Google Scholar] [CrossRef]
- Distelfeld, A.; Li, C.; Dubcovsky, J. Regulation of flowering in temperate cereals. Curr. Opin. Plant Biol. 2009, 12, 178–184. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, H.; Guo, H.; Zhou, C.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Liu, L. Identification of the vernalization gene VRN-B1 responsible for heading date variation by QTL mapping using a RIL population in wheat. BMC Plant Biol. 2020, 20, 331. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Tranquilli, G.; Helguera, M.; Fahima, T.; Dubcovsky, J. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 2003, 100, 6263–6268. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.L.; Loukoianov, A.; Blechl, A.; Tranquilli, G.; Ramakrishna, W.; Sanmiguel, P.; Bennetzen, J.L.; Echenique, V.; Dubcovsky, J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 2004, 303, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Fu, D.; Li, C.; Blechl, A.; Tranquilli, G.; Bonafede, M.; Sanchez, A.; Valarik, M.; Yasuda, S.; Dubcovsky, J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 2006, 103, 19581–19586. [Google Scholar] [CrossRef] [PubMed]
- Kippes, N.; Debernardi, J.M.; Vasquez-Gross, H.A.; Akpinar, B.A.; Budak, H.; Kato, K.; Chao, S.; Akhunov, E.; Dubcovsky, J. Identification of the VERNALIZATION 4 gene reveals the origin of spring growth habit in ancient wheats from South Asia. Proc. Natl. Acad. Sci. USA 2015, 112, 5401–5410. [Google Scholar] [CrossRef] [PubMed]
- Strejcková, B.; Milec, Z.; Holusová, K.; Cápal, P.; Vojtková, T.; Cegan, R.; Safár, J. In-depth sequence analysis of bread wheat VRN1 genes. Int. J. Mol. Sci. 2021, 22, 12284. [Google Scholar] [CrossRef]
- Beales, J.; Turner, A.; Griffiths, S.; Snape, J.W.; Laurie, D.A. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 115, 721–733. [Google Scholar] [CrossRef]
- Mohler, V.; Lukman, R.; Ortiz-Islas, S.; William, M. Genetic and physical mapping of photoperiod insensitive gene Ppd-B1 in common wheat. Euphytica 2004, 138, 33–40. [Google Scholar] [CrossRef]
- Wilhelm, E.P.; Turner, A.S.; Laurie, D.A. Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum Desf.). Theor. Appl. Genet. 2009, 118, 285–294. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Giura, A.; Roeder, M.S.; Boerner, A. A new gene controlling the flowering response to photoperiod in wheat. Euphytica 2009, 165, 579–585. [Google Scholar] [CrossRef]
- Lewis, S.; Faricelli, M.E.; Appendino, M.L.; Valarik, M.; Dubcovsky, J. The chromosome region including the earliness per se locus Eps-Am1 affects the duration of early developmental phases and spikelet number in diploid wheat. J. Exp. Bot. 2008, 59, 3595–3607. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Tranquilli, G.; Lewis, S.; Kippes, N.; Dubcovsky, J. Genetic and physical mapping of the earliness per se locus Eps-Am1 in Triticum monococcum identifies EARLY FLOWERING 3 (ELF3) as a candidate gene. Funct. Integr. Genom. 2016, 16, 365–382. [Google Scholar] [CrossRef]
- Basavaraddi, P.A.; Savin, R.; Bencivenga, S.; Griffiths, S.; Slafer, G.A. Phenology and floret development as affected by the interaction between Eps-7D and Ppd-D1. Plants 2021, 10, 533. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, R.; Wang, M.; Li, L.; Yan, L.; Wang, Z.; Zhu, J.; Chen, X.; Zhao, A.; Su, Z.; et al. Identification and characterization of QTL for spike morphological traits, plant height and heading date derived from the D genome of natural and resynthetic allohexaploid wheat. Theor. Appl. Genet. 2022, 135, 389–403. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Hocking, P.J.; Simpson, R.J.; George, T.S. Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci. 2009, 60, 124–143. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, Q.; Chen, X.; Cui, Z.; Yue, S.; Zhang, F.; Roemheld, V. Alternative cropping systems for sustainable water and nitrogen use in the North China Plain. Agric. Ecosyst. Environ. 2012, 146, 93–102. [Google Scholar] [CrossRef]
- Yang, B.; Wen, H.; Wang, S.; Zhang, J.; Wang, Y.; Zhang, T.; Yuan, K.; Lu, L.; Liu, Y.; Xue, Q.; et al. Enhancing drought resistance and yield of wheat through inoculation with Streptomyces pactum Act12 in drought field environments. Agronomy 2024, 14, 692. [Google Scholar] [CrossRef]
- Yang, B.; Chen, N.; Dang, Y.; Wang, Y.; Wen, H.; Zheng, J.; Zheng, X.; Zhao, J.; Lu, J.; Qiao, L. Identification and validation of quantitative trait loci for chlorophyll content of flag leaf in wheat under different phosphorus treatments. Front. Plant Sci. 2022, 13, 1019012. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Kuehl, R.O.; Ray, I.M.; Hui, R.; Soleri, D. Evaluation of simple methods for estimating broad-sense heritability in stands of randomly planted genotypes. Crop Sci. 1998, 38, 1125–1129. [Google Scholar] [CrossRef]
- Devi, K.D.; Punyarani, K.; Singh, N.S.; Devi, H.S. An efficient protocol for total DNA extraction from the members of order Zingiberales- suitable for diverse PCR based downstream applications. SpringerPlus 2013, 2, 669. [Google Scholar] [CrossRef]
- Meng, L.; Li, H.H.; Zhang, L.Y.; Wang, J.K. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]
- Mccouch, S.R.; Chen, X.L.; Panaud, O.; Temnykh, S.; Xu, Y.B.; Cho, Y.G.; Huang, N.; Ishii, T.; Blair, M. Microsatellite marker development, mapping and applications in rice genetics and breeding. Plant Mol. Biol. 1997, 35, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fang, H.; Gao, Y.; Fan, G.; Shi, X.; Yu, S.; Ding, S.; Huang, T.; Wang, W.; Song, J. Genome-wide association analysis of time to heading and maturity in bread wheat using 55K microarrays. Front. Plant Sci. 2023, 14, 1296197. [Google Scholar] [CrossRef]
- Mohler, V.; Albrecht, T.; Castell, A.; Diethelm, M.; Schweizer, G.; Hartl, L. Considering causal genes in the genetic dissection of kernel traits in common wheat. J. Appl. Genet. 2016, 57, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Cui, F.; Ji, J.; Zhang, W.; Zhao, X.; Liu, J.; Meng, D.; Tong, Y.; Wang, T.; Li, J. Dissection of pleiotropic QTL regions controlling wheat spike characteristics under different nitrogen treatments using traditional and conditional QTL mapping. Front. Plant Sci. 2019, 10, 187. [Google Scholar] [CrossRef]
- Luo, W.; Ma, J.; Zhou, X.H.; Sun, M.; Kong, X.C.; Wei, Y.M.; Jiang, Y.F.; Qi, P.F.; Jiang, Q.T.; Liu, Y.X.; et al. Identification of quantitative trait loci controlling agronomic traits indicates breeding potential of Tibetan semiwild wheat (Triticum aestivum ssp. tibetanum). Crop Sci. 2016, 56, 2410–2420. [Google Scholar] [CrossRef]
- Rustgi, S.; Shafqat, M.N.; Kumar, N.; Baenziger, P.S.; Ali, M.L.; Dweikat, I.; Campbell, B.T.; Gill, K.S. Genetic dissection of yield and its component traits using high-density composite map of wheat chromosome 3A: Bridging gaps between QTLs and underlying genes. PLoS ONE 2013, 8, e70526. [Google Scholar] [CrossRef]
- Li, F.J.; Wen, W.E.; He, Z.H.; Liu, J.D.; Jin, H.; Cao, S.H.; Geng, H.W.; Yan, J.; Zhang, P.Z.; Wan, Y.X.; et al. Genome-wide linkage mapping of yield-related traits in three Chinese bread wheat populations using high-density SNP markers. Theor. Appl. Genet. 2018, 131, 1903–1924. [Google Scholar] [CrossRef]
- Addison, C.K.; Mason, R.E.; Brown-Guedira, G.; Guedira, M.; Hao, Y.; Miller, R.G.; Subramanian, N.; Lozada, D.N.; Acuna, A.; Arguello, M.N.; et al. QTL and major genes influencing grain yield potential in soft red winter wheat adapted to the southern United States. Euphytica 2016, 209, 665–677. [Google Scholar] [CrossRef]
- Lee, H.S.; Jung, J.-U.; Kang, C.-S.; Heo, H.-Y.; Park, C.S. Mapping of QTL for yield and its related traits in a doubled haploid population of Korean wheat. Plant Biotechnol. Rep. 2014, 8, 443–454. [Google Scholar] [CrossRef]
- Cuthbert, J.L.; Somers, D.J.; Brule-Babel, A.L.; Brown, P.D.; Crow, G.H. Molecular mapping of quantitative trait loci for yield and yield components in spring wheat (Triticum aestivum L.). Theor. Appl. Genet. 2008, 117, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.; Simmonds, J.; Leverington, M.; Wang, Y.; Fish, L.; Sayers, L.; Alibert, L.; Orford, S.; Wingen, L.; Herry, L.; et al. Meta-QTL analysis of the genetic control of ear emergence in elite European winter wheat germplasm. Theor. Appl. Genet. 2009, 119, 383–395. [Google Scholar] [CrossRef] [PubMed]
- El-Feki, W.M.; Byrne, P.F.; Reid, S.D.; Haley, S.D. Mapping quantitative trait loci for agronomic traits in winter wheat under different soil moisture levels. Agronomy 2018, 8, 133. [Google Scholar] [CrossRef]
- Sherman, J.D.; Martin, J.M.; Blake, N.K.; Lanning, S.P.; Talbert, L.E. Genetic basis of agronomic differences between a modern and a historical spring wheat cultivar. Crop Sci. 2014, 54, 1–13. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, X.; Chai, L.; Wang, Z.; Du, D.; Wang, Z.; Bian, R.; Zhao, A.; Xin, M.; Guo, W.; et al. Pleiotropic QTL influencing spikelet number and heading date in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2020, 133, 1825–1838. [Google Scholar] [CrossRef]
- Nezhad, N.M.; Kamali, M.R.J.; McIntyre, C.L.; Fakheri, B.A.; Omidi, M.; Masoudi, B. Mapping QTLs with main and epistatic effect on Seri “M82×Babax” wheat population under salt stress. Euphytica 2019, 215, 130. [Google Scholar] [CrossRef]
- Peret, B.; Desnos, T.; Jost, R.; Kanno, S.; Berkowitz, O.; Nussaume, L. Root architecture responses: In search of phosphate. Plant Physiol. 2014, 166, 1713–1723. [Google Scholar] [CrossRef]
- Lopez-Arredondo, D.L.; Leyva-Gonzalez, M.A.; Gonzalez-Morales, S.I.; Lopez-Bucio, J.; Herrera-Estrella, L. Phosphate nutrition: Improving low-phosphate tolerance in crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Raghothama, K.G.; Karthikeyan, A.S. Phosphate acquisition. Plant Soil 2005, 274, 37–49. [Google Scholar] [CrossRef]
- Lambers, H.; Finnegan, P.M.; Jost, R.; Plaxton, W.C.; Shane, M.W.; Stitt, M. Phosphorus nutrition in Proteaceae and beyond. Nat. Plants 2015, 1, 15109. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- Rouached, H.; Arpat, A.B.; Poirier, Y. Regulation of phosphate starvation responses in plants: Signaling players and cross-talks. Mol. Plant. 2010, 3, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.J.; Lin, S.I. Signaling network in sensing phosphate availability in plants. Annu. Rev. Plant Biol. 2011, 62, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, F.; Yan, X.; Zhang, X.; Dong, Z.; Cui, D.; Chen, F. Genome-wide association study for 13 agronomic traits reveals distribution of superior alleles in bread wheat from the Yellow and Huai Valley of China. Plant Biotechnol. J. 2017, 15, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jia, L.J.; Lu, L.H.; Qin, D.D.; Zhang, J.P.; Guan, P.F.; Ni, Z.F.; Yao, Y.Y.; Sun, Q.X.; Peng, H.R. Mapping QTLs of yield-related traits using RIL population derived from common wheat and Tibetan semi-wild wheat. Theor. Appl. Genet. 2014, 127, 2415–2432. [Google Scholar] [CrossRef]
- Dixon, L.E.; Greenwood, J.R.; Bencivenga, S.; Zhang, P.; Cockram, J.; Mellers, G.; Ramm, K.; Cavanagh, C.; Swain, S.M.; Boden, S.A. TEOSINTE BRANCHED1 regulates inflorescence architecture and development in bread wheat (Triticum aestivum). Plant Cell 2018, 30, 563–581. [Google Scholar] [CrossRef]
- Gao, S.; Song, T.; Han, J.; He, M.; Zhang, Q.; Zhu, Y.; Zhu, Z. A calcium-dependent lipid binding protein, OsANN10, is a negative regulator of osmotic stress tolerance in rice. Plant Sci. 2020, 293, 110420. [Google Scholar] [CrossRef]
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef]
- Ni, W.M.; Xie, D.X.; Hobbie, L.; Feng, B.M.; Zhao, D.Z.; Akkara, J.; Ma, H. Regulation of flower development in Arabidopsis by SCF complexes. Plant Physiol. 2004, 134, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Baute, J.; Polyn, S.; De Block, J.; Blomme, J.; Van Lijsebettens, M.; Inze, D. F-box protein FBX92 affects leaf size in Arabidopsis thaliana. Plant Cell Physiol. 2017, 58, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Risseeuw, E.P.; Daskalchuk, T.E.; Banks, T.W.; Liu, E.; Cotelesage, J.; Hellmann, H.; Estelle, M.; Somers, D.E.; Crosby, W.L. Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J. 2003, 34, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liu, X.; Xu, K.; Zhang, B. Genome-wide characterization, evolution and expression profiling of UDP-glycosyltransferase family in pomelo (Citrus grandis) fruit. BMC Plant Biol. 2020, 20, 459. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Cui, H.; Yang, W.; Yu, B.; Zhang, C.; Wen, J.; Kang, J.; Wang, Z.; Yang, Q. The UDP-glycosyltransferase MtUGT84A1 regulates anthocyanin accumulation and plant growth via JA signaling in Medicago truncatula. Environ. Exp. Bot. 2022, 201, 104972. [Google Scholar] [CrossRef]
- Busse-Wicher, M.; Wicher, K.B.; Kusche-Gullberg, M. The extostosin family: Proteins with many functions. Matrix Biol. 2014, 35, 25–33. [Google Scholar] [CrossRef]
- Sasaki, T.; Matsumoto, T.; Yamamoto, K.; Sakata, K.; Baba, T.; Katayose, Y.; Wu, J.Z.; Niimura, Y.; Cheng, Z.K.; Nagamura, Y.; et al. The genome sequence and structure of rice chromosome 1. Nature 2002, 420, 312–316. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Zhang, L. Zinc finger-homeodomain transcriptional factors (ZF-HDs) in wheat (Triticum aestivum L.): Identification, evolution, expression analysis and response to abiotic stresses. Plants 2021, 10, 593. [Google Scholar] [CrossRef]
- Griffiths, S.; Dunford, R.P.; Coupland, G.; Laurie, D.A. The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol. 2003, 131, 1855–1867. [Google Scholar] [CrossRef]
- Decousset, L.; Griffiths, S.; Dunford, R.P.; Pratchett, N.; Laurie, D.A. Development of STS markers closely linked to the Ppd-H1 photoperiod response gene of barley (Hordeum vulgare L.). Theor. Appl. Genet. 2000, 101, 1202–1206. [Google Scholar] [CrossRef]
| Parent | DH Population | |||||||
|---|---|---|---|---|---|---|---|---|
| Jinmai 47 | Jinmai 84 | Mean | SD | Range | Kurtosis | Skewness | H2 | |
| E1 | 190.33 ** | 196.67 | 194.27 | 2.41 | 189–199 | −0.86 | −0.06 | 0.8171 |
| E2 | 189.33 ** | 198.67 | 193.66 | 2.68 | 188–199 | −0.93 | −0.04 | |
| E3 | 189.67 ** | 196.67 | 192.93 | 2.48 | 187–197 | −0.42 | −0.73 | |
| E4 | 189.33 ** | 195.67 | 193.02 | 2.58 | 187–198 | −1.27 | −0.40 | |
| E5 | 188.33 ** | 194.67 | 191.62 | 2.54 | 187–197 | −0.61 | −0.11 | |
| E6 | 188.67 ** | 195.67 | 192.04 | 2.38 | 187–198 | −0.09 | −0.04 | |
| BLUP | 189.53 ** | 196.09 | 192.90 | 2.01 | 188.50–197.03 | −0.82 | −0.17 | |
| QTL | Environment | LOD | Add. | R2 | Left Marker | Right Marker | Interval (cM) | Physical Interval (Mb) |
|---|---|---|---|---|---|---|---|---|
| Qhd.saw-1B.1 | E2\E5\BLUP | 2.66–3.21 | −0.52–−0.32 | 2.64–3.82 | 1B_654087630 | 1B_643455303 | 55.9–69.9 | 654.087–643.455 |
| Qhd.saw-1B.2 | E2 | 2.65 | −0.48 | 3.17 | 1B_454990828 | 1B_378500287 | 169.3–177.7 | 454.990–378.500 |
| Qhd.saw-2B.1 | E6 | 2.61 | 0.45 | 3.91 | 2B_122765594 | 2B_95067270 | 102.4–108.5 | 122.765–95.067 |
| Qhd.saw-2B.2 | E6 | 2.88 | 0.47 | 4.29 | 2B_72594141 | 2B_67981922 | 119.1–123.6 | 72.594–67.981 |
| Qhd.saw-3A.1 | E4\E5 | 3.06–4.81 | −0.63–−0.55 | 4.45–6.74 | 3A_33447270 | 3A_60938981 | 77.7–86.9 | 33.447–60.938 |
| Qhd.saw-3A.2 | E5 | 4.79 | −0.63 | 6.70 | 3A_69702468 | 3A_106442355 | 89.5–93.0 | 69.702–106.442 |
| Qhd.saw-3A.3 | E5 | 6.55 | −0.73 | 8.99 | 3A_179770248 | 3A_207453847 | 98.5–101.0 | 179.770–207.453 |
| Qhd.saw-3A.4 | E5 | 7.55 | −0.78 | 10.25 | 3A_484578779 | 3A_477796895 | 108.5–109.9 | 484.578–477.796 |
| Qhd.saw-3A.5 | E4 | 4.13 | −0.63 | 5.94 | 3A_492185407 | 3A_525825022 | 112.4–115.4 | 492.185–525.825 |
| Qhd.saw-3A.6 | E4\E5 | 3.84–9.24 | −0.91–−0.61 | 5.55–13.88 | 3A_534326783 | 3A_597825929 | 123.5–131.4 | 534.326–597.825 |
| Qhd.saw-3A.7 | E2\E4\E5\BLUP | 5.37–12.81 | −0.92–−0.71 | 7.61–14.65 | 3A_609429465 | 3A_623405134 | 135.7–139.5 | 609.429–623.405 |
| Qhd.saw-3A.8 | E1\E2\E3\E4\E5\E6\BLUP | 5.51–14.03 | −0.94–−0.72 | 7.80–15.83 | 3A_650835555 | 3A_655885348 | 142.0–149.4 | 650.835–655.885 |
| Qhd.saw-3A.9 | E1\E2\E4\E5\E6\BLUP | 5.72–12.85 | −0.92–−0.69 | 8.40–14.97 | 3A_681755353 | 3A_688622043 | 152.8–157.0 | 681.755–688.622 |
| Qhd.saw-3A.10 | E5 | 8.40 | −0.89 | 13.49 | 3A_688693273 | 3A_701009953 | 160.7–167.6 | 688.693–701.009 |
| Qhd.saw-3B.1 | BLUP | 2.57 | 0.31 | 2.39 | 3B_585846704 | 3B_592694374 | 98.9–110.5 | 585.846–592.694 |
| Qhd.saw-3B.2 | E1 | 2.67 | 0.44 | 3.18 | 3B_592694374 | 3B_508486802 | 110.5–119.8 | 592.694–508.486 |
| Qhd.saw-4A.1 | E3\E6 | 3.48–4.09 | 0.51–0.54 | 4.56–5.65 | 4A_700964251 | 4A_644613024 | 39.1–58.9 | 700.964–644.613 |
| Qhd.saw-4A.2 | E3\E6 | 3.71–4.47 | 0.54–0.57 | 5.63–5.67 | 4A_644613024 | 4A_672508553 | 58.9–73.9 | 644.613–672.508 |
| Qhd.saw-4A.3 | BLUP | 5.73 | 0.49 | 6.12 | 4A_672508567 | 4A_672508702 | 79.2–79.8 | 672.508–672.508 |
| Qhd.saw-4A.4 | E1\E2\E5\E6\BLUP | 3.08–8.38 | 0.53–0.84 | 4.73–12.04 | 4A_676486292 | 4A_638262359 | 86.3–96.7 | 676.486–638.262 |
| Qhd.saw-4A.5 | E1\E2\E5\BLUP | 2.84–6.59 | 0.48–0.73 | 4.42–9.13 | 4A_638262359 | 4A_635663690 | 97.8–104.2 | 638.262–635.663 |
| Qhd.saw-4A.6 | E6 | 4.32 | 0.56 | 6.03 | 4A_639942192 | 4A_620278986 | 99.9–113.2 | 639.942–620.278 |
| Qhd.saw-4A.7 | E5 | 2.93 | 0.55 | 4.95 | 4A_604578311 | 4A_572876583 | 156.9–176.9 | 604.578–572.876 |
| Qhd.saw-4B.1 | E1\E3\BLUP | 2.78–4.55 | −0.46–−0.42 | 3.54–4.58 | 4B_494243528 | 4B_570190409 | 50.7–58.9 | 494.243–570.190 |
| Qhd.saw-4B.2 | BLUP | 4.35 | −0.41 | 4.40 | 4B_570190409 | 4B_618027704 | 58.9–65.7 | 570.190–618.027 |
| Qhd.saw-4B.3 | E3 | 2.64 | −0.45 | 3.45 | 4B_621814323 | 4B_642460586 | 67.5–75.5 | 621.814–642.460 |
| Qhd.saw-4D.1 | E4 | 2.52 | −0.69 | 4.22 | 4D_481390025 | 4D_433505595 | 0.0–9.1 | 481.390–433.505 |
| Qhd.saw-4D.2 | E2 | 6.57 | −0.84 | 9.74 | 4D_48697668 | 4D_28547729 | 35.9–38.9 | 48.697–28.547 |
| Qhd.saw-4D.3 | E1\E2\E3\E4\E5\BLUP | 4.46–22.14 | −1.32–−0.97 | 8.35–29.52 | 4D_28547729 | 4D_15772687 | 39.6–46.9 | 28.547–15.772 |
| Qhd.saw-5A.1 | E1 | 2.55 | −0.44 | 3.26 | 5A_462122669 | 5A_467547080 | 190.4–193.4 | 462.122–467.547 |
| Qhd.saw-5A.2 | E1\E3\E6 | 3.12–4.31 | −0.58–−0.48 | 3.96–5.70 | 5A_467547080 | 5A_445264090 | 193.6–209.5 | 467.547–445.264 |
| Qhd.saw-5A.3 | E3 | 3.75 | −0.55 | 5.27 | 5A_445264090 | 5A_445832627 | 211.3–214.4 | 445.264–445.832 |
| Qhd.saw-5A.4 | BLUP | 5.05 | −0.46 | 5.33 | 5A_395317374 | 5A_47016091 | 236.6–245.9 | 395.317–47.016 |
| Qhd.saw-5D.1 | E2\E6\BLUP | 2.77–3.10 | −0.54–−0.32 | 2.58–4.26 | 5D_390409478 | 5D_370312603 | 0.0–7.0 | 390.409–370.312 |
| Qhd.saw-5D.2 | E2\E6\BLUP | 3.24–3.77 | −0.58–−0.35 | 3.20–4.83 | 5D_370312603 | 5D_259452342 | 7.0–20.2 | 370.312–259.452 |
| Qhd.saw-5D.3 | E4\BLUP | 2.81–3.07 | −0.52–−0.34 | 2.90–4.10 | 5D_138877074 | 5D_34236785 | 26.2–33.5 | 138.877–34.236 |
| Qhd.saw-5D.4 | E5 | 3.19 | −0.54 | 4.97 | 5D_47009022 | 5D_21550073 | 31.2–38.4 | 47.009–21.550 |
| Qhd.saw-7B.1 | E2 | 4.62 | −0.66 | 6.07 | 7B_725766535 | 7B_725723913 | 0.0–2.5 | 725.766–725.723 |
| Qhd.saw-7B.2 | E2\BLUP | 4.01–5.27 | −0.70–−0.38 | 3.67–6.86 | 7B_718449045 | 7B_712710871 | 8.0–17.5 | 718.449–712.710 |
| Qhd.saw-3A.7 | Qhd.saw-3A.8 | Qhd.saw-3A.9 | Qhd.saw-4A.4 | Qhd.saw-4D.3 | Sample Size | HD (d) | Difference | Percent (%) |
|---|---|---|---|---|---|---|---|---|
| + | + | + | + | + | 24 | 195.72 ± 1.06 a | 4.58 | 2.40 |
| − | + | − | + | + | 1 | 195.66 ± 0.00 ab | 4.53 | 2.37 |
| − | + | + | + | + | 1 | 195.51 ± 0.00 abc | 4.37 | 2.29 |
| − | − | + | + | + | 1 | 195.20 ± 0.00 abcd | 4.06 | 2.13 |
| + | + | + | − | + | 18 | 195.01 ± 1.52 abcd | 3.88 | 2.03 |
| − | − | + | − | − | 1 | 194.89 ± 0.00 abcde | 3.75 | 1.96 |
| − | − | + | − | + | 5 | 194.33 ± 0.64 bcde | 3.19 | 1.67 |
| − | − | − | + | + | 22 | 194.29 ± 1.23 bcde | 3.15 | 1.65 |
| + | + | − | + | − | 1 | 193.81 ± 0.00 bcdef | 2.67 | 1.40 |
| + | + | + | + | − | 29 | 193.64 ± 1.54 bcdef | 2.50 | 1.31 |
| + | + | + | − | − | 26 | 192.70 ± 1.28 cdef | 1.56 | 0.82 |
| − | − | − | − | + | 16 | 192.50 ± 1.68 def | 1.36 | 0.71 |
| − | − | − | + | − | 31 | 192.27 ± 1.69 ef | 1.13 | 0.59 |
| − | − | − | − | − | 23 | 191.14 ± 1.20 f | 0.00 | 0.00 |
| Chr. | Left Marker | Right Marker | Physical Interval (Mb) | Reference |
|---|---|---|---|---|
| 3A | wPt-4669 | wPt-1665 | 279.47–432.91 | Luo et al., 2016 [36] |
| 3A | wPt-3041 | wPt-4868 | 9.14–13.50 | Rustgi et al., 2013 [37] |
| 3A | IWB41929 | - | 714.43 | Li et al., 2018 [38] |
| 3A | IWB8499 | - | 638.45 | Li et al., 2018 [38] |
| 3A | IWB64668 | - | 176.56 | Li et al., 2018 [38] |
| 3A | IWA2738 | - | 13.90 | Addison et al., 2016 [39] |
| 3A | gwm247 | - | 197.43 | Lee et al., 2014 [40] |
| 3A | IWA7159 | IWA4298 | 569.43–649.03 | Mohler et al., 2016 [34] |
| 3A | gwm133 | - | 509.54 | Cuthbert et al., 2008 [41] |
| 3A | mag1166 | wPt-9369 | 14.85 | Fan et al., 2019 [35] |
| 3A | wmc264 | - | 625.79 | Griffiths et al., 2009 [42] |
| 3A | wmc532-wmc50 | - | 68.87 | El-Feki et al., 2018 [43] |
| 4A | barc1158 | - | 619.56 | Sherman et al., 2014 [44] |
| 4A | Jagger_c4331_105 | BS00040647_51 | 606.40–594.66 | Hu et al., 2020 [3] |
| 4A | Kukri_c74409_199 | - | 37.77 | Chen et al., 2020 [45] |
| 4A | ACT/CAG-1 | AAC/CAA-3 | 695.97 | Nezhad et al., 2019 [46] |
| 4A | IWA8209 | IWA2761 | 615.71–626.06 | Mohler et al., 2016 [34] |
| 4A | AX-89555314 | AX-89450319 | 2.95–6.04 | Fan et al., 2019 [35] |
| 4A | AX-95239105 | AX-111711476 | 669.58 | Fan et al., 2019 [35] |
| 4D | wPt-0941 | wPt-2379 | 52.63–196.64 | Sherman et al., 2014 [44] |
| QTL Name | Chr. | Gene | Physical Interval (Mb) | Rice Homologous Gene | Function Description |
|---|---|---|---|---|---|
| Qhd.saw-3A.9 | 3A | TraesCS3A02G595800LC | 688.570216–688.570572 | - | Calcium-dependent lipid-binding (CaLB domain) family protein |
| Qhd.saw-3A.9 | 3A | TraesCS3A02G446400 | 686.910184–686.911554 | - | Kelch repeat-containing F-box protein-like |
| Qhd.saw-3A.9 | 3A | TraesCS3A02G587100LC | 683.081967–683.085806 | - | UDP-glycosyltransferase 84A1 |
| Qhd.saw-3A.9 | 3A | TraesCS3A02G440800 | 683.399747–683.402388 | Os01t0926600-01 | glycosyltransferase family exostosin protein |
| Qhd.saw-3A.9 | 3A | TraesCS3A02G440100 | 683.335397–683.338550 | Os01t0926600-01 | glycosyltransferase family exostosin protein |
| Qhd.saw-4D.3 | 4D | TraesCS4D02G038700 | 17.057650–17.059578 | - | Zinc finger family protein |
| Qhd.saw-4D.3 | 4D | TraesCS4D02G046200 | 22.217295–22.219478 | Os03t0711100-01 | CONSTANS-like zinc finger protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Qiao, L.; Zheng, X.; Zheng, J.; Wu, B.; Li, X.; Zhao, J. Quantitative Trait Loci Mapping of Heading Date in Wheat under Phosphorus Stress Conditions. Genes 2024, 15, 1150. https://doi.org/10.3390/genes15091150
Yang B, Qiao L, Zheng X, Zheng J, Wu B, Li X, Zhao J. Quantitative Trait Loci Mapping of Heading Date in Wheat under Phosphorus Stress Conditions. Genes. 2024; 15(9):1150. https://doi.org/10.3390/genes15091150
Chicago/Turabian StyleYang, Bin, Ling Qiao, Xingwei Zheng, Jun Zheng, Bangbang Wu, Xiaohua Li, and Jiajia Zhao. 2024. "Quantitative Trait Loci Mapping of Heading Date in Wheat under Phosphorus Stress Conditions" Genes 15, no. 9: 1150. https://doi.org/10.3390/genes15091150





