Identification of Key Proteins Related to Cashmere Fiber Diameter by Integrated Proteomics and Bioinformatic Analyses in the Alpas and Alxa Goat Breeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Measurement of Important Economic Characteristics
2.3. Protein Extraction and Digestion
2.4. Label-Free LC/MS Quantitative and Qualitative and Profiling
2.5. Bioinformatics Analysis
2.6. Western Blotting
2.7. Co-Immunoprecipitation (Co-IP)
3. Results
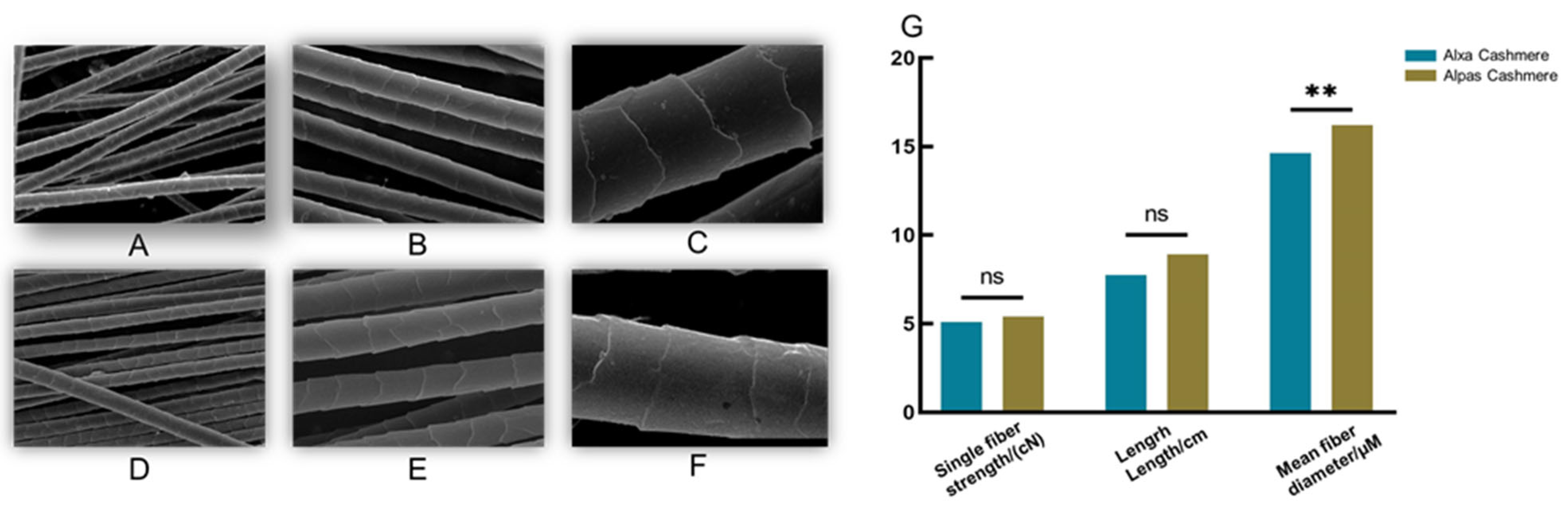
3.1. Performance of Fiber Quality Traits
3.2. Proteome Analysis during Fiber Development
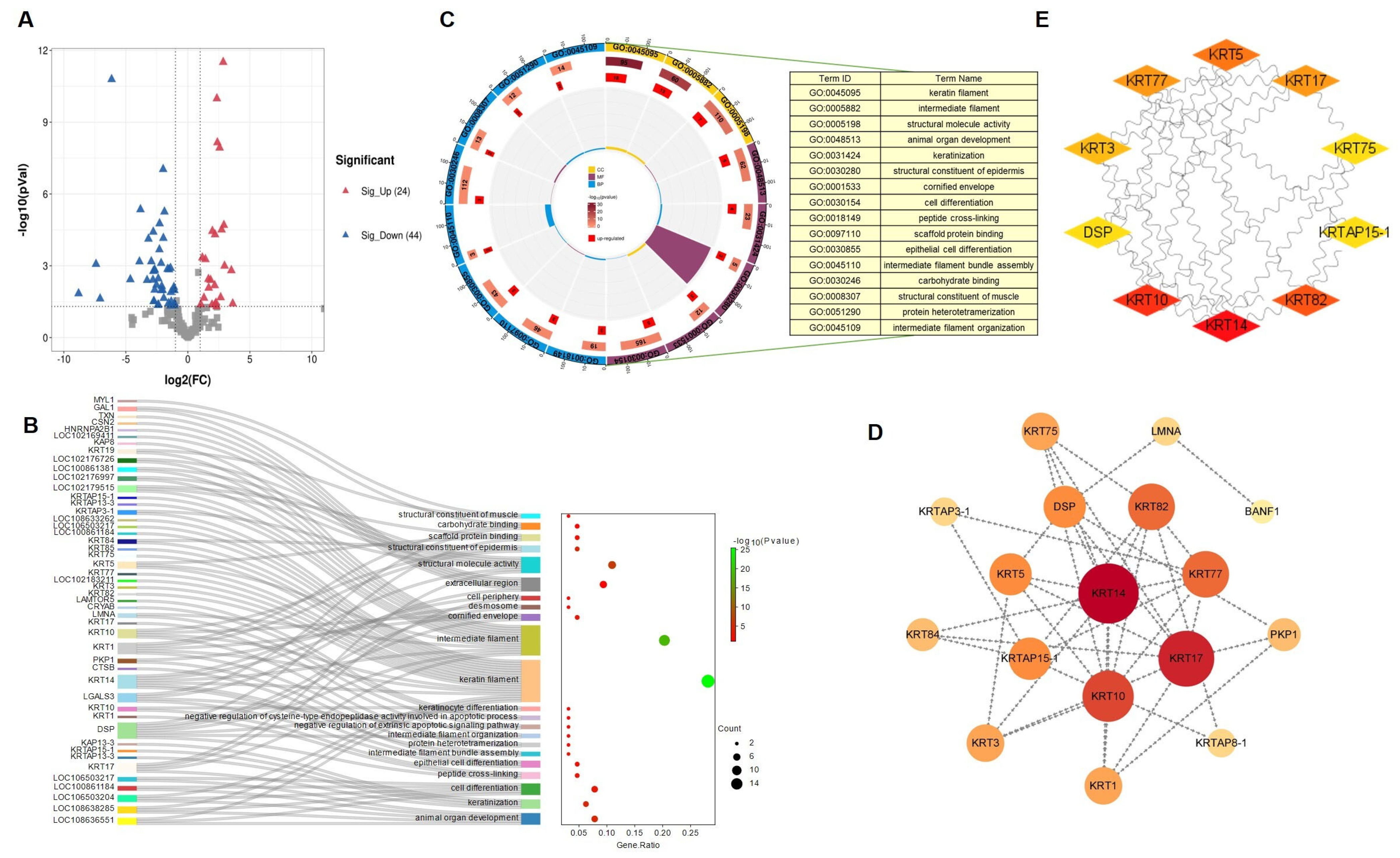
3.3. Proteome Analysis of Differentially Expressed Proteins during Fiber Development
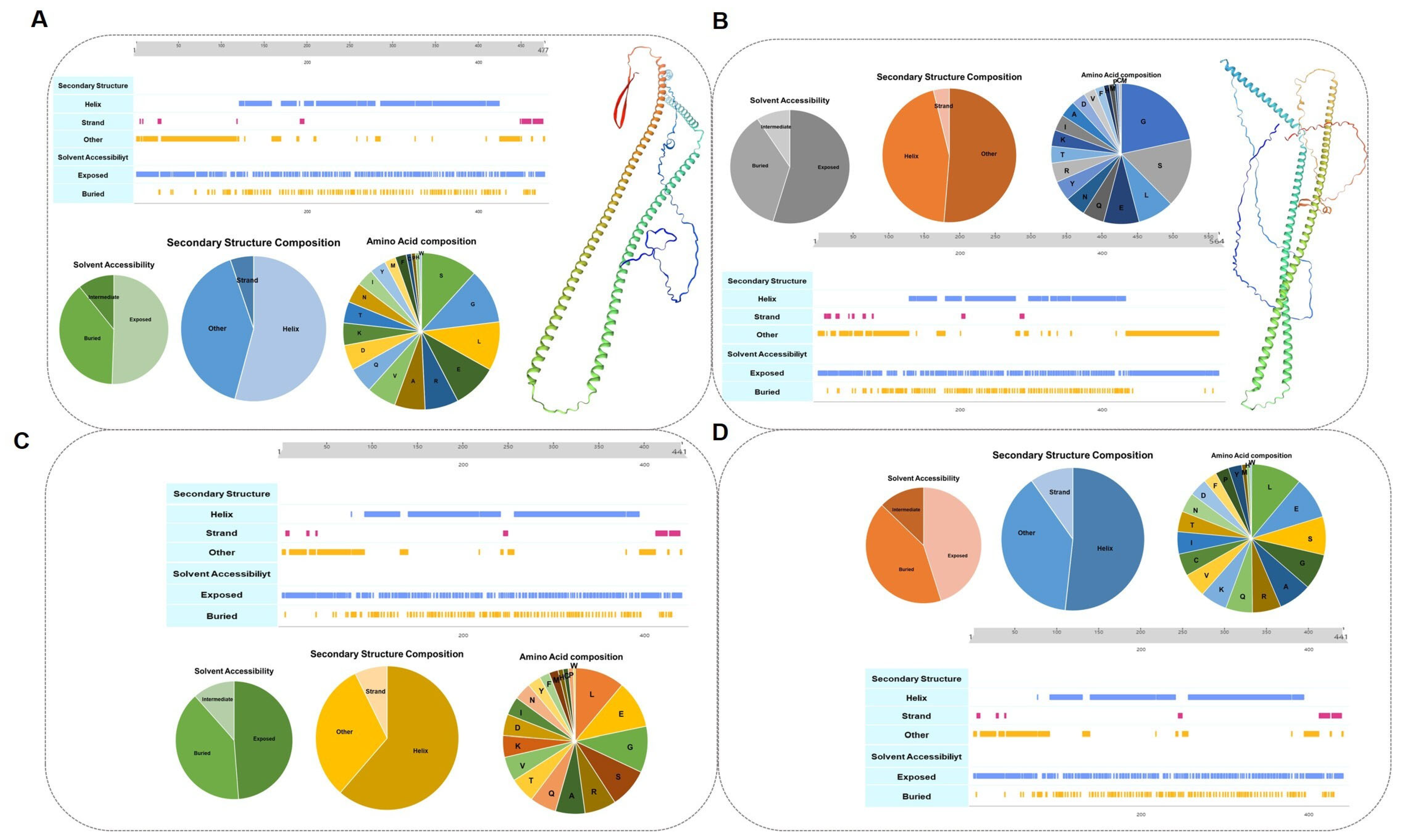
3.4. Key Protein Structure Domain Analysis
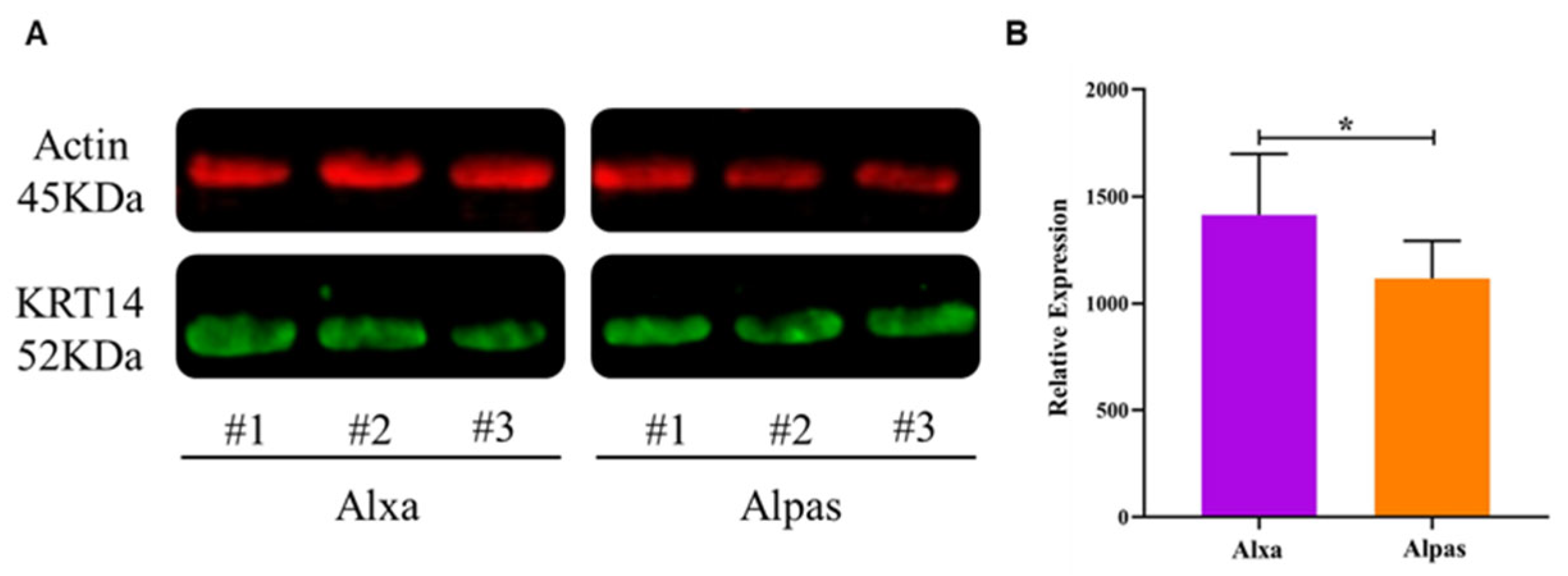
3.5. Western Blot Analysis of KRT14 Expression
3.6. KRT14 Interacts with Other Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Westhuysen, J.M. Marketing goat fibres. Small Rumin. Res. 2005, 60, 215–218. [Google Scholar] [CrossRef]
- Lupton, C.J.; McColl, A.; Stobart, R.H. Fiber characteristics of the Huacaya Alpaca. Small Rumin. Res. 2006, 64, 211–224. [Google Scholar] [CrossRef]
- Quispe, E.C.; Ramos, H.; Mayhua, P.; Alfonso, L. Fibre characteristics of vicuña (Vicugna vicugna mensalis). Small Rumin. Res. 2010, 93, 64–66. [Google Scholar] [CrossRef]
- Duan, C.; Xu, J.; Sun, C.; Jia, Z.; Zhang, W. Effects of melatonin implantation on cashmere yield, fibre characteristics, duration of cashmere growth as well as growth and reproductive performance of Inner Mongolian cashmere goats. J. Anim. Sci. Biotechnol. 2015, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Yokohama, M.; Wada, K.; Fujita, M.; Kotani, M.; Nagura, Y.; Kanno, M.; Nomura, K.; Amano, T.; Kikkawa, Y. Expression analysis of the type I keratin protein keratin 33A in goat coat hair. Anim. Sci. J. 2011, 82, 773–781. [Google Scholar] [CrossRef]
- Wu, G.F.; He, Y. Identification of varieties of cashmere by Vis/NIR spectroscopy technology based on PCA-SVM. Guang Pu Xue Yu Guang Pu Fen Xi = Guang Pu 2009, 29, 1541–1544. [Google Scholar]
- Zhang, B.; Chang, L.; Lan, X.; Asif, N.; Guan, F.; Fu, D.; Li, B.; Yan, C.; Zhang, H.; Zhang, X.; et al. Genome-wide definition of selective sweeps reveals molecular evidence of trait-driven domestication among elite goat (Capra species) breeds for the production of dairy, cashmere, and meat. GigaScience 2018, 7, giy105. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Hickford, J.G.H.; Zhao, M.; Gong, H.; Hao, Z.; Shen, J.; Hu, J.; Liu, X.; Li, S.; et al. Identification of Caprine KRTAP28-1 and Its Effect on Cashmere Fiber Diameter. Genes 2020, 11, 121. [Google Scholar] [CrossRef]
- Rogers, M.A.; Langbein, L.; Praetzel-Wunder, S.; Winter, H.; Schweizer, J. Human hair keratin-associated proteins (KAPs). Int. Rev. Cytol. 2006, 251, 209–263. [Google Scholar] [CrossRef]
- Pan, X.; Hobbs, R.P.; Coulombe, P.A. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell Biol. 2013, 25, 47–56. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Forrest, R.H.; Li, S.; Wang, J.; Dyer, J.M.; Luo, Y.; Hickford, J.G. Wool Keratin-Associated Protein Genes in Sheep-A Review. Genes 2016, 7, 24. [Google Scholar] [CrossRef]
- Giesen, M.; Gruedl, S.; Holtkoetter, O.; Fuhrmann, G.; Koerner, A.; Petersohn, D. Ageing processes influence keratin and KAP expression in human hair follicles. Exp. Dermatol. 2011, 20, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, X.; Yan, H.; Zeng, J.; Ma, S.; Niu, Y.; Zhou, G.; Jiang, Y.; Chen, Y. Comparative Transcriptome Analysis of Fetal Skin Reveals Key Genes Related to Hair Follicle Morphogenesis in Cashmere Goats. PLoS ONE 2016, 11, e0151118. [Google Scholar] [CrossRef] [PubMed]
- Powell, B.A.; Rogers, G.E. The role of keratin proteins and their genes in the growth, structure and properties of hair. EXS 1997, 78, 59–148. [Google Scholar]
- Hearle, J.W. A critical review of the structural mechanics of wool and hair fibres. Int. J. Biol. Macromol. 2000, 27, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.A.; Steinert, P.M. Intermediate filament structure. Curr. Opin. Cell Biol. 1992, 4, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wildermoth, J.E.; Wallace, O.A.; Gordon, S.W.; Maqbool, N.J.; Maclean, P.H.; Nixon, A.J.; Pearson, A.J. Annotation of sheep keratin intermediate filament genes and their patterns of expression. Exp. Dermatol. 2011, 20, 582–588. [Google Scholar] [CrossRef]
- Purvis, I.W.; Franklin, I.R. Major genes and QTL influencing wool production and quality: A review. Genet. Sel. Evol. 2005, 37, S97–S107. [Google Scholar] [CrossRef]
- Zhao, J.; Qin, H.; Xin, J.; Liu, N.; Han, R.; Perez-Campo, F.M.; Li, H. Discovery of genes and proteins possibly regulating mean wool fibre diameter using cDNA microarray and proteomic approaches. Sci. Rep. 2020, 10, 7726. [Google Scholar] [CrossRef]
- Xie, Y.; Rile, N.; Li, X.; Li, H.; Zhao, M.; Che, T.; Cai, T.; Liu, Z.; Li, J. Analysis of cashmere Goat meat by label-Free Proteomics Shows That myl3 is a potential Molecular Marker of meat toughness. Czech J. Anim. Sci. 2022, 67, 137–146. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Murgiano, L.; D’Alessandro, A.; Egidi, M.G.; Crisà, A.; Prosperini, G.; Timperio, A.M.; Valentini, A.; Zolla, L. Proteomics and transcriptomics investigation on longissimus muscles in Large White and Casertana pig breeds. J. Proteome Res. 2010, 9, 6450–6466. [Google Scholar] [CrossRef] [PubMed]
- Bovo, S.; Di Luca, A.; Galimberti, G.; Dall’Olio, S.; Fontanesi, L. A comparative analysis of label-free liquid chromatography-mass spectrometry liver proteomic profiles highlights metabolic differences between pig breeds. PLoS ONE 2018, 13, e0199649. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. S4), S11. [Google Scholar] [CrossRef] [PubMed]
- Bernhofer, M.; Dallago, C.; Karl, T.; Satagopam, V.; Heinzinger, M.; Littmann, M.; Olenyi, T.; Qiu, J.; Schütze, K.; Yachdav, G.; et al. PredictProtein—Predicting Protein Structure and Function for 29 Years. Nucleic Acids Res. 2021, 49, W535–W540. [Google Scholar] [CrossRef]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Sheng, S.D.; Hui, T.Y.; Yue, C.; Sun, J.M.; Guo, D.; Guo, S.L.; Li, B.J.; Xue, H.L.; Wang, Z.Y.; et al. An Integrated Analysis of Cashmere Fineness lncRNAs in Cashmere Goats. Genes 2019, 10, 266. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, H.; Luo, Y.; Wang, J.; Hu, J.; Liu, X.; Li, S.; Hao, Z.; Jin, X.; Song, Y.; et al. Variation in the Caprine Keratin-Associated Protein 27-1 Gene is Associated with Cashmere Fiber Diameter. Genes 2020, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gong, H.; Zhou, H.; Wang, J.; Liu, X.; Li, S.; Luo, Y.; Hickford, J.G.H. Variation in the ovine keratin-associated protein 15-1 gene affects wool yield. J. Agric. Sci. 2018, 156, 922–928. [Google Scholar] [CrossRef]
- Wang, Y.N.; Chang, W.C. Induction of disease-associated keratin 16 gene expression by epidermal growth factor is regulated through cooperation of transcription factors Sp1 and c-Jun. J. Biol. Chem. 2003, 278, 45848–45857. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Liang, H.; Guo, D.D.; Bi, Z.W.; Yuan, J.L.; Jin, Y.; Huan, L.; Guo, X.D.; Cang, M.; Liu, D.J. The Overexpression of Tbeta4 in the Hair Follicle Tissue of Alpas Cashmere Goats Increases Cashmere Yield and Promotes Hair Follicle Development. Animals 2019, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, G.; Zhang, R.; Guo, J.; Li, C.; Martin, G.; Chen, Y.; Wang, X. Comparative proteomic analyses using iTRAQ-labeling provides insights into fiber diversity in sheep and goats. J. Proteom. 2018, 172, 82–88. [Google Scholar] [CrossRef]
- Aubin-Horth, N.; Renn, S.C. Genomic reaction norms: Using integrative biology to understand molecular mechanisms of phenotypic plasticity. Mol. Ecol. 2009, 18, 3763–3780. [Google Scholar] [CrossRef]
- Wang, L.; Cai, B.; Zhou, S.; Zhu, H.; Qu, L.; Wang, X.; Chen, Y. RNA-seq reveals transcriptome changes in goats following myostatin gene knockout. PLoS ONE 2017, 12, e0187966. [Google Scholar] [CrossRef]
- Galarneau, L.; Loranger, A.; Gilbert, S.; Marceau, N. Keratins modulate hepatic cell adhesion, size and G1/S transition. Exp. Cell Res. 2007, 313, 179–194. [Google Scholar] [CrossRef]
- McGowan, K.M.; Coulombe, P.A. Keratin 17 expression in the hard epithelial context of the hair and nail, and its relevance for the pachyonychia congenita phenotype. J. Investig. Dermatol. 2000, 114, 1101–1107. [Google Scholar] [CrossRef]
- Chen, Y.; Bao, Z.; Liu, M.; Li, J.; Dai, Y.; Wang, F.; Zhang, X.; Zhai, P.; Zhao, B.; Wu, X. Promoter Methylation Changes in KRT17: A Novel Epigenetic Marker for Wool Production in Angora Rabbit. Int. J. Mol. Sci. 2022, 23, 6077. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, H.; Fujimoto, A.; Farooq, M.; Ito, M.; Shimomura, Y. Characterization of the human hair shaft cuticle-specific keratin-associated protein 10 family. J. Investig. Dermatol. 2013, 133, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, J.; Magin, T.M. Hyperproliferation, induction of c-Myc and 14-3-3sigma, but no cell fragility in keratin-10-null mice. J. Cell Sci. 2002, 115, 2639–2650. [Google Scholar] [CrossRef]
- Koehn, H.; Plowman, J.E.; Morton, J.D.; Dyer, J.M. Identification and quantitation of major structural proteins from enriched cuticle fractions of wools of different breeds. N. Z. J. Agric. Res. 2015, 58, 463–471. [Google Scholar] [CrossRef]
- Fratini, A.; Powell, B.C.; Rogers, G.E. Sequence, expression, and evolutionary conservation of a gene encoding a glycine/tyrosine-rich keratin-associated protein of hair. J. Biol. Chem. 1993, 268, 4511–4518. [Google Scholar] [CrossRef]
- Higgins, C.A.; Chen, J.C.; Cerise, J.E.; Jahoda, C.A.; Christiano, A.M. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19679–19688. [Google Scholar] [CrossRef]
- Katz, M.D.; Cai, L.Q.; Zhu, Y.S.; Herrera, C.; DeFillo-Ricart, M.; Shackleton, C.H.; Imperato-McGinley, J. The biochemical and phenotypic characterization of females homozygous for 5 alpha-reductase-2 deficiency. J. Clin. Endocrinol. Metab. 1995, 80, 3160–3167. [Google Scholar] [CrossRef]
- Thornton, M.J.; Taylor, A.H.; Mulligan, K.; Al-Azzawi, F.; Lyon, C.C.; O’Driscoll, J.; Messenger, A.G. The distribution of estrogen receptor beta is distinct to that of estrogen receptor alpha and the androgen receptor in human skin and the pilosebaceous unit. J. Investig. Dermatol. Symp. Proc. 2003, 8, 100–103. [Google Scholar] [CrossRef]
- Sherlock, R.G.; Harris, P.M.; Lee, J.; Wickham, G.A.; Woods, J.L.; McCutcheon, S.N. Intake and long-term cysteine supplementation change wool characteristics of Romney sheep. Aust. J. Agric. Res. 2001, 52, 29–36. [Google Scholar] [CrossRef]
- Reis, P.; Schinckel, P. Nitrogen utilization and wool production by sheep. Aust. J. Agric. Res. 1961, 12, 335–352. [Google Scholar] [CrossRef]
- Reis, P.; Schinckel, P. The Growth and Composition of Wool II. The Effect of Casein, Gelatin, and Sulphur-Containing Amino Acids Given Per Abomasum. Aust. J. Biol. Sci. 1964, 17, 532–547. [Google Scholar] [CrossRef]


| Number | Accession | Protein Name | Gene Name |
|---|---|---|---|
| 1 | A0A452EME4 | Keratin 14 | KRT14 |
| 2 | A0A452FDX9 | IF rod domain-containing protein | LOC108633262 |
| 3 | A0A452FPA3 | KIAA1217 | KIAA1217 |
| 4 | A0A452E4U1 | Keratin 17 | KRT17 |
| 5 | A0A452FUI2 | IF rod domain-containing protein | KRT18 |
| 6 | A0A452G8R7 | Myosin heavy chain 2 | MYH2 |
| 7 | A0A452EZ21 | 26S proteasome non-ATPase regulatory subunit 1 | PSMD1 |
| 8 | A0A452G5G4 | Acid-sensing ion channel subunit 1 | ASIC1 |
| 9 | A0A452G188 | Proline-rich 11 | PRR11 |
| 10 | A0A5D6VZD1 | ABC-F family ATP-binding cassette domain-containing protein | FZ040_10900 |
| 11 | A0A452G6J7 | Obscurin, cytoskeletal calmodulin, and titin-interacting RhoGEF | OBSCN |
| 12 | A0A452FZK5 | LDL receptor-related protein 4 | LRP4 |
| 13 | A0A452ERA9 | Outer dynein arm-docking complex subunit 4 | ODAD4 |
| 14 | A0A5D6WAU5 | Leukotoxin LktA family filamentous adhesin | FZ040_03715 |
| 15 | A0A452E1K6 | G protein-coupled receptor 68 | GPR68 |
| 16 | A0A5D6WDH4 | DUF4405 domain-containing protein | FZ040_00610 |
| 17 | A0A452FNB0 | Glutamate-rich protein 1 | ERICH1 |
| 18 | A0A452ERJ7 | OMA1 zinc metallopeptidase | OMA1 |
| 19 | A0A452EE60 | SHC adaptor protein 1 | SHC1 |
| 20 | A0A452E7K6 | Serine/arginine repetitive matrix 2 | SRRM2 |
| 21 | A0A452FQE8 | MLLT1 super elongation complex subunit | MLLT1 |
| 22 | A0A452DL58 | FH2 domain-containing protein | N\A |
| 23 | A0A452FQU4 | Lengsin, lens protein with glutamine synthetase domain | LGSN |
| 24 | A0A452G661 | Mucin like 3 | MUCL3 |
| 25 | A0A452FWB4 | Corepressor interacting with RBPJ | CIR1 |
| 26 | A0A452ECY9 | Growth Differentiation Factor 5 | GDF5 |
| 27 | A0A452E2Z0 | Transmembrane protein 65 | TMEM65 |
| 28 | A0A5D6WHQ8 | Precorrin-4 C(11)-methyltransferase | cobM |
| 29 | A0A452G6A8 | Ceramide synthase 6 | CERS6 |
| 30 | A0A452E5L8 | Neurofibromin 1 | NF1 |
| 31 | A0A452F4W3 | Mov10 like RISC complex RNA helicase 1 | MOV10L1 |
| 32 | A0A452E392 | Protein LSM14 homolog B | LSM14B |
| 33 | A0A452DVQ0 | TOPBP1 interacting checkpoint and replication regulator | TICRR |
| 34 | A0A5D6WBZ4 | S41 family peptidase | FZ040_05185 |
| 35 | A0A452FL24 | Flagellum associated containing coiled-coil domains 1 | FLACC1 |
| 36 | A0A452G137 | Vitamin D-binding protein | GC |
| 37 | S4SAK0 | Pyruvate kinase | pklr |
| 38 | A0A452F3A5 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase | PLCB1 |
| 39 | A0A5D6VXX5 | Succinate--CoA ligase | sucC |
| 40 | A0A452ELG6 | Melan-A | MLANA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Qin, Q.; Wang, Y.; Wang, Z.; Liu, Z. Identification of Key Proteins Related to Cashmere Fiber Diameter by Integrated Proteomics and Bioinformatic Analyses in the Alpas and Alxa Goat Breeds. Genes 2024, 15, 1154. https://doi.org/10.3390/genes15091154
Zhang C, Qin Q, Wang Y, Wang Z, Liu Z. Identification of Key Proteins Related to Cashmere Fiber Diameter by Integrated Proteomics and Bioinformatic Analyses in the Alpas and Alxa Goat Breeds. Genes. 2024; 15(9):1154. https://doi.org/10.3390/genes15091154
Chicago/Turabian StyleZhang, Chongyan, Qing Qin, Yichuan Wang, Zhixin Wang, and Zhihong Liu. 2024. "Identification of Key Proteins Related to Cashmere Fiber Diameter by Integrated Proteomics and Bioinformatic Analyses in the Alpas and Alxa Goat Breeds" Genes 15, no. 9: 1154. https://doi.org/10.3390/genes15091154
APA StyleZhang, C., Qin, Q., Wang, Y., Wang, Z., & Liu, Z. (2024). Identification of Key Proteins Related to Cashmere Fiber Diameter by Integrated Proteomics and Bioinformatic Analyses in the Alpas and Alxa Goat Breeds. Genes, 15(9), 1154. https://doi.org/10.3390/genes15091154





