Functional Equivalence of Insulin and IGF-1 in the In Vitro Culture of Chicken Primordial Germ Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. PGC Culture Conditions
2.2. Preparation and Treatment of Small Molecule Compounds
2.3. Cell Counting Kit-8 (CCK-8) Assay
2.4. EdU Proliferation Assay
2.5. RNA Extraction and Real-Time Quantitative PCR (RT–qPCR)
2.6. Detection of Apoptosis by the FITC/PI Double Staining Method
2.7. Reactive Oxygen Species Assay
2.8. Cell Cycle Analysis
2.9. Assay for GSH/GSSG/MDA/Fe2+
2.10. Detection of PGC Migration
2.11. Statistical Analysis
3. Results
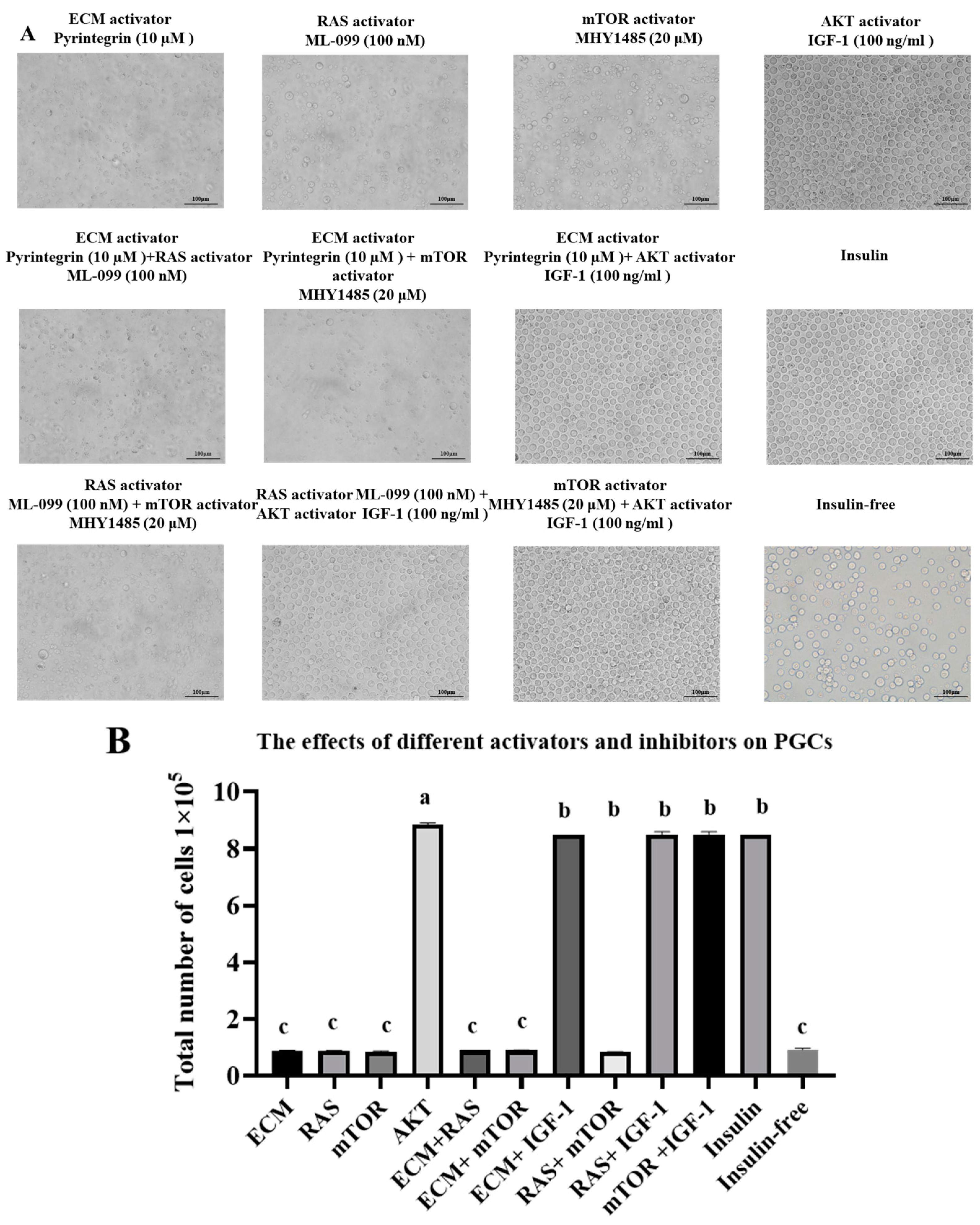
3.1. Phenotypic Characteristics of the Combination of Activators and Inhibitors in an Insulin-Based Culture Medium
3.2. Effect on PGC Morphology and Proliferation of the Addition of Different Activators Without Insulin
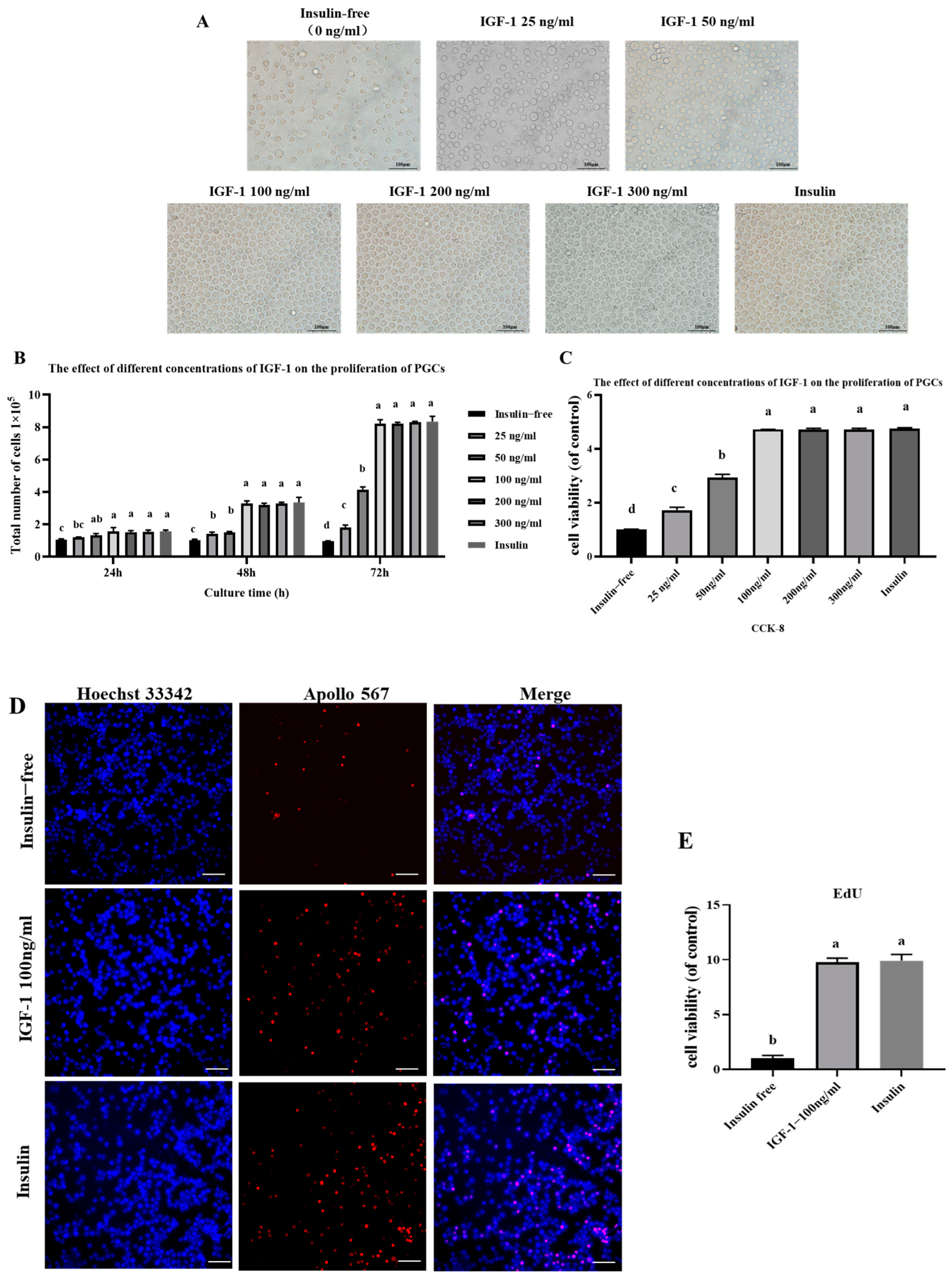
3.3. Effect of IGF-1 on PGC Proliferation
3.4. Effect of IGF-1 on PGC Cell Cycle
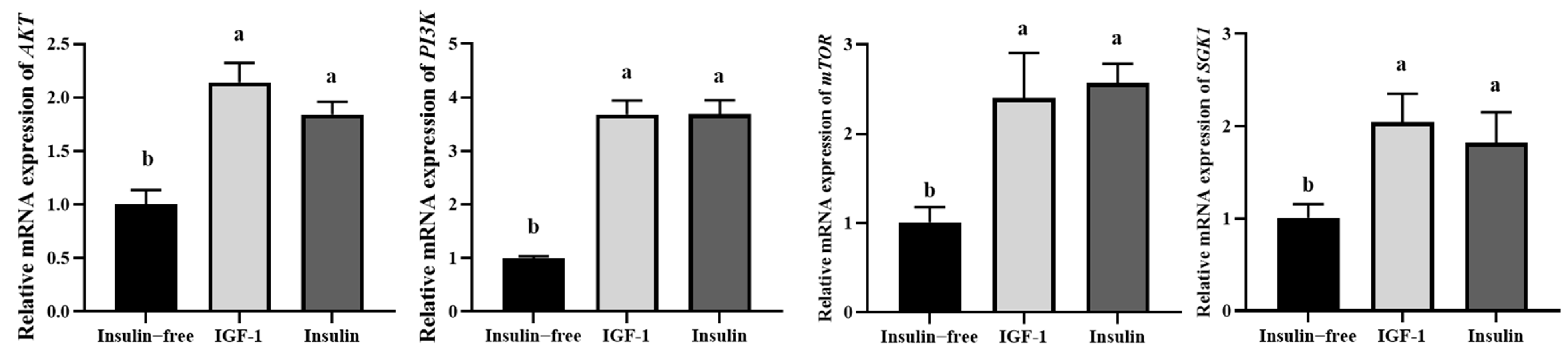
3.5. IGF-1 Can Activate the PI3K-AKT Signaling Pathway
3.6. IGF-1 Can Inhibit PGC Cell Apoptosis
3.7. IGF-1 and Insulin Can Inhibit the Initiation and Progression of Ferroptosis in PGCs
3.8. Effects of IGF-1 and Insulin on Key Gene Expression and Migration in Chicken PGCs
3.9. Effects of IGF-1 and Insulin on the Efficiency of Establishing PGCs Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farzaneh, M.; Khoshnam, S.E.; Nokhbatolfoghahai, M. First scientific record of two cases of partial twinning in the chick embryo, Gallus gallus domesticus. Vet. Rec. Case Rep. 2016, 4, e000353. [Google Scholar] [CrossRef]
- Trefil, P.; Aumann, D.; Koslová, A.; Mucksová, J.; Beneová, B.; Kalina, J.; Wurmser, C.; Fries, R.; Elleder, D.; Schusser, B. Male fertility restored by transplanting primordial germ cells into testes: A new way towards efficient transgenesis in chicken. Sci. Rep. 2017, 7, 14246. [Google Scholar] [CrossRef]
- Han, J.Y.; Park, Y.H. Primordial germ cell-mediated transgenesis and genome editing in birds. J. Anim. Sci. Biotechnol. 2018, 9, 19. [Google Scholar] [CrossRef]
- Woodcock, M.E.; Gheyas, A.A.; Mason, A.S.; Nandi, S.; Mcgrew, M.J. Reviving rare chicken breeds using genetically engineered sterility in surrogate host birds. Proc. Natl. Acad. Sci. USA 2019, 116, 201906316. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.; Glover, J.D.; Woodcock, M.; Brzeszczynska, J.; Taylor, L.; Sherman, A.; Kaiser, P.; Mcgrew, M.J. FGF, Insulin, and SMAD Signaling Cooperate for Avian Primordial Germ Cell Self-Renewal. Stem Cell Rep. 2015, 5, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Liu, C.C.; Van Ingelgom, A.J.; Martens, Y.A.; Linares, C.; Knight, J.A.; Painter, M.M.; Sullivan, P.M.; Bu, G. Apolipoprotein E4 Impairs Neuronal Insulin Signaling by Trapping Insulin Receptor in the Endosomes. Neuron 2017, 96, 115–129.e115. [Google Scholar] [CrossRef] [PubMed]
- Alyas, J.; Rafiq, A.; Amir, H.; Khan, S.; Sultana, T.; Ali, A.; Hameed, A.; Ahmad, I.; Kazmi, A.; Sajid, T. Human Insulin: History, Recent Advances, and Expression Systems for Mass Production. Biomed. Res. Ther. 2021, 89, 4540–4561. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, J.; Han, S.; Liu, J.; Holzbeierlein, J.; Thrasher, J.B.; Li, B. Suppression of glycogen synthase kinase 3 activity reduces tumor growth of prostate cancer in vivo. Prostate 2011, 71, 835–845. [Google Scholar] [CrossRef]
- Atif, F.; Yousuf, S.; Stein, D.G. Anti-tumor effects of progesterone in human glioblastoma multiforme: Role of PI3K/Akt/mTOR signaling. J. Steroid Biochem. Mol. Biol. 2015, 146, 62–73. [Google Scholar] [CrossRef]
- Boreddy, S.R.; Pramanik, K.C.; Srivastava, S.K. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin. Cancer Res. 2011, 17, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V. Control of reproductive processes by growth hormone: Extra- and intracellular mechanisms. Vet. J. 2005, 170, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.J.; Wu, J.; Ren, W.; Liu, G.; Wu, G.; Peng, Y.; Zheng, D.; Jin, K.; Zuo, Q.; Li, G.; et al. Aflatoxin B1 impairs the growth and development of chicken PGCs through oxidative stress and mitochondrial dysfunction. Ecotoxicol. Environ. Saf. 2025, 290, 117727. [Google Scholar] [CrossRef]
- Liu, G.; Ren, W.; Jin, K.; Zheng, D.; Zuo, Q.; Zhang, Y.; Chen, G.; Li, B.; Niu, Y. PGC-mediated conservation strategies for germplasm resources of Rugao Yellow Chicken and Shouguang Chicken in China1. J. Integr. Agric. 2024, 2095–3119. [Google Scholar] [CrossRef]
- Liu, X.; Ye, L.; Ding, Y.; Gong, W.; Qian, H.; Jin, K.; Niu, Y.; Zuo, Q.; Song, J.; Han, W.; et al. Role of PI3K/AKT signaling pathway involved in self-renewing and maintaining biological properties of chicken primordial germ cells. Poult. Sci. 2024, 103, 104140. [Google Scholar] [CrossRef]
- Han, C.; Wei, S.; He, F.; Liu, D.; Wan, H.; Liu, H.; Li, L.; Xu, H.; Du, X.; Xu, F. The Regulation of Lipid Deposition by Insulin in Goose Liver Cells Is Mediated by the PI3K-AKT-mTOR Signaling Pathway. PLoS ONE 2015, 10, e0098759. [Google Scholar] [CrossRef]
- Pereira, R.I.; Draznin, B. Inhibition of the phosphatidylinositol 3′-kinase signaling pathway leads to decreased insulin-stimulated adiponectin secretion from 3T3-L1 adipocytes. Metabolism 2005, 54, 1636–1643. [Google Scholar] [CrossRef]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The extracellular matrix and insulin resistance. Trends Endocrinol. Metab. 2015, 26, 357–366. [Google Scholar] [CrossRef]
- Hocking, D.C. Therapeutic Applications of Extracellular Matrix. Adv. Wound Care (New Rochelle) 2015, 4, 441–443. [Google Scholar] [CrossRef]
- Macri, L.; Clark, R.A.; Silverstein, D. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 1366–1381. [Google Scholar] [CrossRef]
- Maquart, F.X.; Monboisse, J.C. Extracellular matrix and wound healing. Pathol. Biol. 2014, 62, 91–95. [Google Scholar] [CrossRef]
- McCormick, F. Signal transduction. How receptors turn Ras on. Nature 1993, 363, 15–16. [Google Scholar] [CrossRef]
- Skolnik, E.Y.; Batzer, A.; Li, N.; Lee, C.H.; Lowenstein, E.; Mohammadi, M.; Margolis, B.; Schlessinger, J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science 1993, 260, 1953–1955. [Google Scholar] [CrossRef]
- Mor, A.; Philips, M.R. Compartmentalized Ras/MAPK signaling. Annu. Rev. Immunol. 2006, 24, 771–800. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.F. Ras proteins: Different signals from different locations. Nat. Rev. Mol. Cell Biol. 2003, 4, 373–384. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Inoki, K.; Münzberg, H.; Opland, D.; Faouzi, M.; Villanueva, E.C.; Ikenoue, T.; Kwiatkowski, D.; MacDougald, O.A.; Myers, M.G., Jr.; et al. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 2009, 9, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Brown, M.S.; Goldstein, J.L. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3441–3446. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.; Cong, S.; Zhang, F. Insulin-Like Growth Factor-1 Promotes Human Uterine Leiomyoma Cell Proliferation via PI3K/AKT/mTOR Pathway. Cells Tissues Organs 2023, 212, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Sakowski, S.A.; Feldman, E.L. Insulin-Like Growth Factors in the Peripheral Nervous System. Endocrinology 2008, 149, 5963–5971. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.S.; Sable, R.B.; Kothari, R.M. Role of insulin-like growth factors (IGFs), their receptors and genetic regulation in the chondrogenesis and growth of the mandibular condylar cartilage. J. Cell. Physiol. 2012, 227, 1796–1804. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Shen, Y.; Yan, Z.Q.; Zhang, P.; Yao, Q.P.; Shen, B.R.; Gao, L.Z.; Qi, Y.X.; Jiang, Z.L. Endothelial Insulin-Like Growth Factor-1 Modulates Proliferation and Phenotype of Smooth Muscle Cells Induced by Low Shear Stress. Ann. Biomed. Eng. 2014, 42, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, J. The combined effect of azithromycin and insulin-like growth factor-1 on cultured human meibomian gland epithelial cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5596–5601. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Sun, S.; Qu, L. Insulin-like growth factor-1 promotes the proliferation and odontoblastic differentiation of human dental pulp cells under high glucose conditions. Int. J. Mol. Med. 2017, 40, 1253–1260. [Google Scholar] [CrossRef]
- Pandini, G.; Conte, E.; Medico, E.; Sciacca, L.; Vigneri, R.; Belfiore, A. IGF-II binding to insulin receptor isoform A induces a partially different gene expression profile from insulin binding. Ann. N. Y. Acad. Sci. 2004, 1028, 450–456. [Google Scholar] [CrossRef]
- Bunter, K.; Hermesch, S.; Luxford, B.; Lahti, K.; Sutcliffe, E. IGF-1 concentration measured in juvenile pigs provides information for breeding programs: A mini review. In Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, France, 19–23 August 2002; pp. 19–23. [Google Scholar]
- Reiss, K.; Cheng, W.; Pierzchalski, P.; Kodali, S.; Li, B.; Wang, S.; Liu, Y.; Anversa, P. Insulin-like growth factor-1 receptor and its ligand regulate the reentry of adult ventricular myocytes into the cell cycle. Exp. Cell Res. 1997, 235, 198–209. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, N.; Xu, H.; Zhou, X.; Wang, J.; Fang, X.; Zhang, Y.; Li, Y.; Yang, J.; Wang, X. Regulation of Hippo-YAP signaling by insulin-like growth factor-1 receptor in the tumorigenesis of diffuse large B-cell lymphoma. J. Hematol. Oncol. 2020, 13, 77. [Google Scholar] [CrossRef]
- Chirivella, L.; Kirstein, M.; Ferrón, S.R.; Domingo-Muelas, A.; Durupt, F.C.; Acosta-Umanzor, C.; Cano-Jaimez, M.; Pérez-Sánchez, F.; Barbacid, M.; Ortega, S.; et al. Cyclin-Dependent Kinase 4 Regulates Adult Neural Stem Cell Proliferation and Differentiation in Response to Insulin. Stem Cells 2017, 35, 2403–2416. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, N.K. Up-regulation of cyclinD1 and Bcl2A1 by insulin is involved in osteoclast proliferation. Life Sci. 2014, 114, 57–61. [Google Scholar] [CrossRef]
- Lewis, D.A.; Travers, J.B.; Somani, A.K.; Spandau, D.F. The IGF-1/IGF-1R signaling axis in the skin: A new role for the dermis in aging-associated skin cancer. Oncogene 2010, 29, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Papaconstantinou, J. Insulin/IGF-1 and ROS signaling pathway cross-talk in aging and longevity determination. Mol. Cell. Endocrinol. 2009, 299, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kajstura, J.; Fiordaliso, F.; Andreoli, A.M.; Li, B.; Chimenti, S.; Medow, M.S.; Limana, F.; Nadal-Ginard, B.; Leri, A.; Anversa, P. IGF-1 Overexpression Inhibits the Development of Diabetic Cardiomyopathy and Angiotensin II–Mediated Oxidative Stress. Diabetes 2001, 50, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.W.; Sullivan, K.A.; Windebank, A.J.; Herrmann, D.N.; Feldman, E.L. Neurons Undergo Apoptosis in Animal and Cell Culture Models of Diabetes. Neurobiol. Dis. 1999, 6, 347–363. [Google Scholar] [CrossRef]
- Gustafsson, H.; Sderdahl, T.; Jnsson, G.; Bratteng, J.O.; Forsby, A. Insulin-like growth factor type 1 prevents hyperglycemia-induced uncoupling protein 3 down-regulation and oxidative stress. J. Neurosci. Res. 2010, 77, 285–291. [Google Scholar] [CrossRef]
- Baregamian, N.; Song, J.; Jeschke, M.G.; Evers, B.M.; Chung, D.H. IGF-1 Protects Intestinal Epithelial Cells from Oxidative Stress-Induced Apoptosis. J. Surg. Res. 2006, 136, 31–37. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, J.; Liu, Q.; Cai, J.; Zheng, Y.; Zhang, Y.; Yu, D.; Liu, H.; Zhang, Z. IGF1 Knockdown Hinders Myocardial Development through Energy Metabolism Dysfunction Caused by ROS-Dependent FOXO Activation in the Chicken Heart. Oxid. Med. Cell Longev. 2019, 2019, 7838754. [Google Scholar] [CrossRef]
- Jenkins, N.L.; James, S.A.; Salim, A.; Sumardy, F.; Speed, T.P.; Conrad, M.; Richardson, D.R.; Bush, A.I.; McColl, G. Changes in ferrous iron and glutathione promote ferroptosis and frailty in aging Caenorhabditis elegans. eLife 2020, 9, e56580. [Google Scholar] [CrossRef]
- Kang, K.A.; Wang, Z.H.; Zhang, R.; Piao, M.J.; Kim, K.C.; Kang, S.S.; Kim, Y.W.; Lee, J.; Park, D.; Hyun, J.W. Myricetin Protects Cells against Oxidative Stress-Induced Apoptosis via Regulation of PI3K/Akt and MAPK Signaling Pathways. Int. J. Mol. Sci. 2010, 11, 4348–4360. [Google Scholar] [CrossRef]
- Higuchi, M.; Honda, T.; Proske, R.J.; Yeh, E.T. Regulation of reactive oxygen species-induced apoptosis and necrosis by caspase 3-like proteases. Oncogene 1998, 17, 2753–2760. [Google Scholar] [CrossRef]
- Liao, W.; Chen, X.; Zhang, S.; Chen, J.; Liu, C.; Yu, K.; Zhang, Y.; Chen, M.; Chen, F.; Shen, M.; et al. Megakaryocytic IGF1 coordinates activation and ferroptosis to safeguard hematopoietic stem cell regeneration after radiation injury. Cell Commun. Signal. 2024, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Song, Y.H.; Geng, Y.J.; Lin, Q.X.; Shan, Z.X.; Lin, S.G.; Li, Y. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem. Biophys. Res. Commun. 2008, 376, 548–552. [Google Scholar] [CrossRef]
- Luo, L.; Lu, A.M.; Wang, Y.; Hong, A.; Qin, Z.H. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp. Gerontol. 2013, 48, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Hutter, R.; Sauter, B.V.; Reis, E.D.; Roque, M.; Badimon, J.J. Decreased reendothelialization and increased neointima formation with endostatin overexpression in a mouse model of arterial injury. Circulation 2003, 107, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Conti, E.; Carrozza, C.; Capoluongo, E.; Volpe, M.; Crea, F.; Zuppi, C.; Andreotti, F. Insulin-Like Growth Factor-1 as a Vascular Protective Factor. Circulation 2004, 110, 2260–2265. [Google Scholar] [CrossRef]
- Li, Y.; Higashi, Y.; Itabe, H.; Song, Y.H.; Delafontaine, P. Insulin-Like Growth Factor-1 Receptor Activation Inhibits Oxidized LDL-Induced Cytochrome C Release and Apoptosis via the Phosphatidylinositol 3 Kinase/Akt Signaling Pathway. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2178–2184. [Google Scholar] [CrossRef]
- Roostaee, Z.; Mehranjani, M.S.; Cheraghi, E. Fluoxetine Mitigates Human Sperm Quality by Disrupting the Antioxidant Defense System and Altering the Expression of Apoptosis-Related Genes: An In Vitro Study. Reprod. Sci. 2025, 32, 326–342. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wu, J.; Peng, Y.; Liu, G.; Jin, K.; Niu, Y.; Song, J.; Han, W.; Chen, G.; Li, B.; et al. Functional Equivalence of Insulin and IGF-1 in the In Vitro Culture of Chicken Primordial Germ Cells. Genes 2025, 16, 481. https://doi.org/10.3390/genes16050481
Liu X, Wu J, Peng Y, Liu G, Jin K, Niu Y, Song J, Han W, Chen G, Li B, et al. Functional Equivalence of Insulin and IGF-1 in the In Vitro Culture of Chicken Primordial Germ Cells. Genes. 2025; 16(5):481. https://doi.org/10.3390/genes16050481
Chicago/Turabian StyleLiu, Xin, Jun Wu, Yixiu Peng, Guangzheng Liu, Kai Jin, Yingjie Niu, Jiuzhou Song, Wei Han, Guohong Chen, Bichun Li, and et al. 2025. "Functional Equivalence of Insulin and IGF-1 in the In Vitro Culture of Chicken Primordial Germ Cells" Genes 16, no. 5: 481. https://doi.org/10.3390/genes16050481
APA StyleLiu, X., Wu, J., Peng, Y., Liu, G., Jin, K., Niu, Y., Song, J., Han, W., Chen, G., Li, B., & Zuo, Q. (2025). Functional Equivalence of Insulin and IGF-1 in the In Vitro Culture of Chicken Primordial Germ Cells. Genes, 16(5), 481. https://doi.org/10.3390/genes16050481







