Relationship between Particulate Matter Pollution and Acute Coronary Syndrome Incidence
Abstract
:1. Introduction
2. Materials and Methods
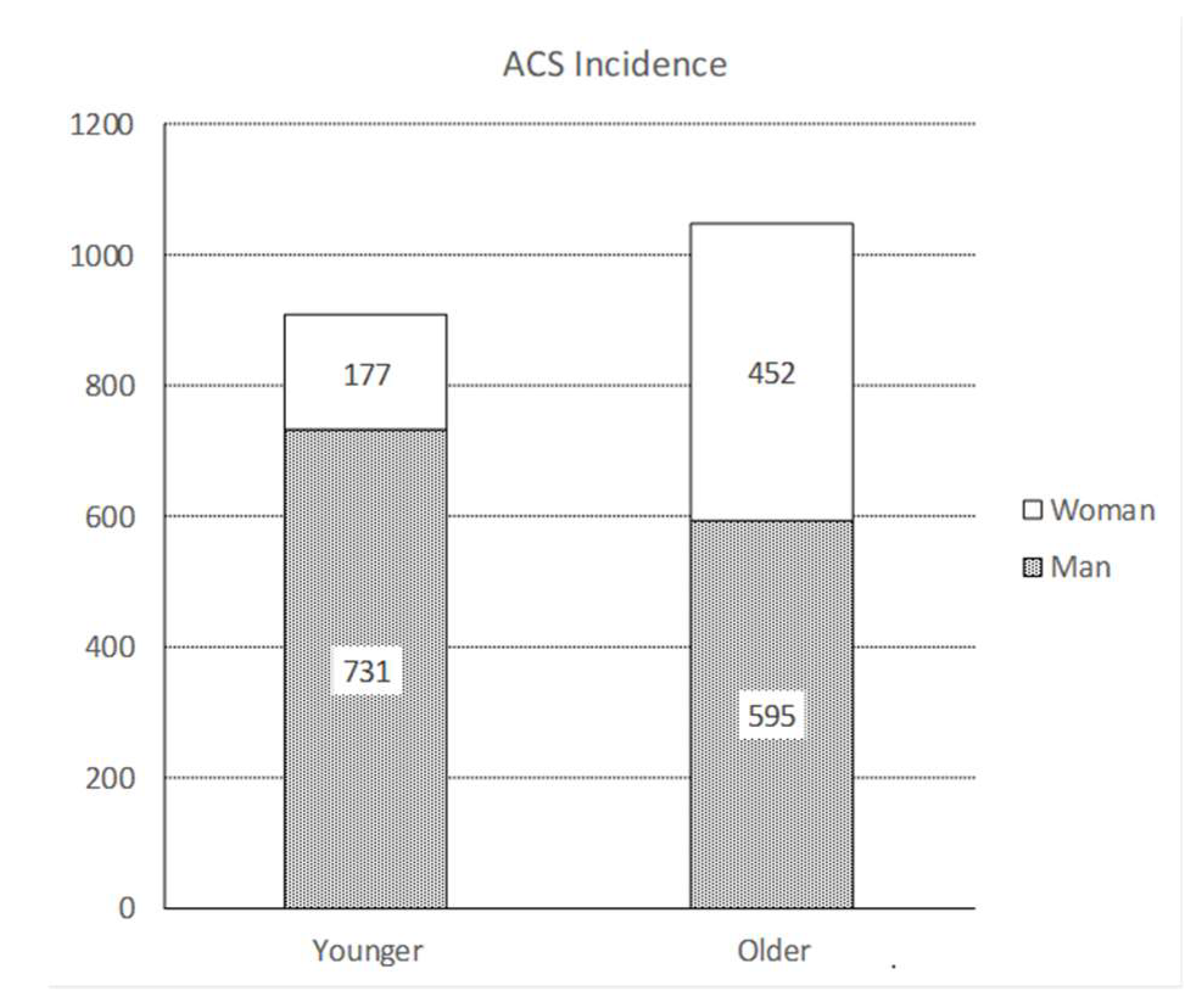
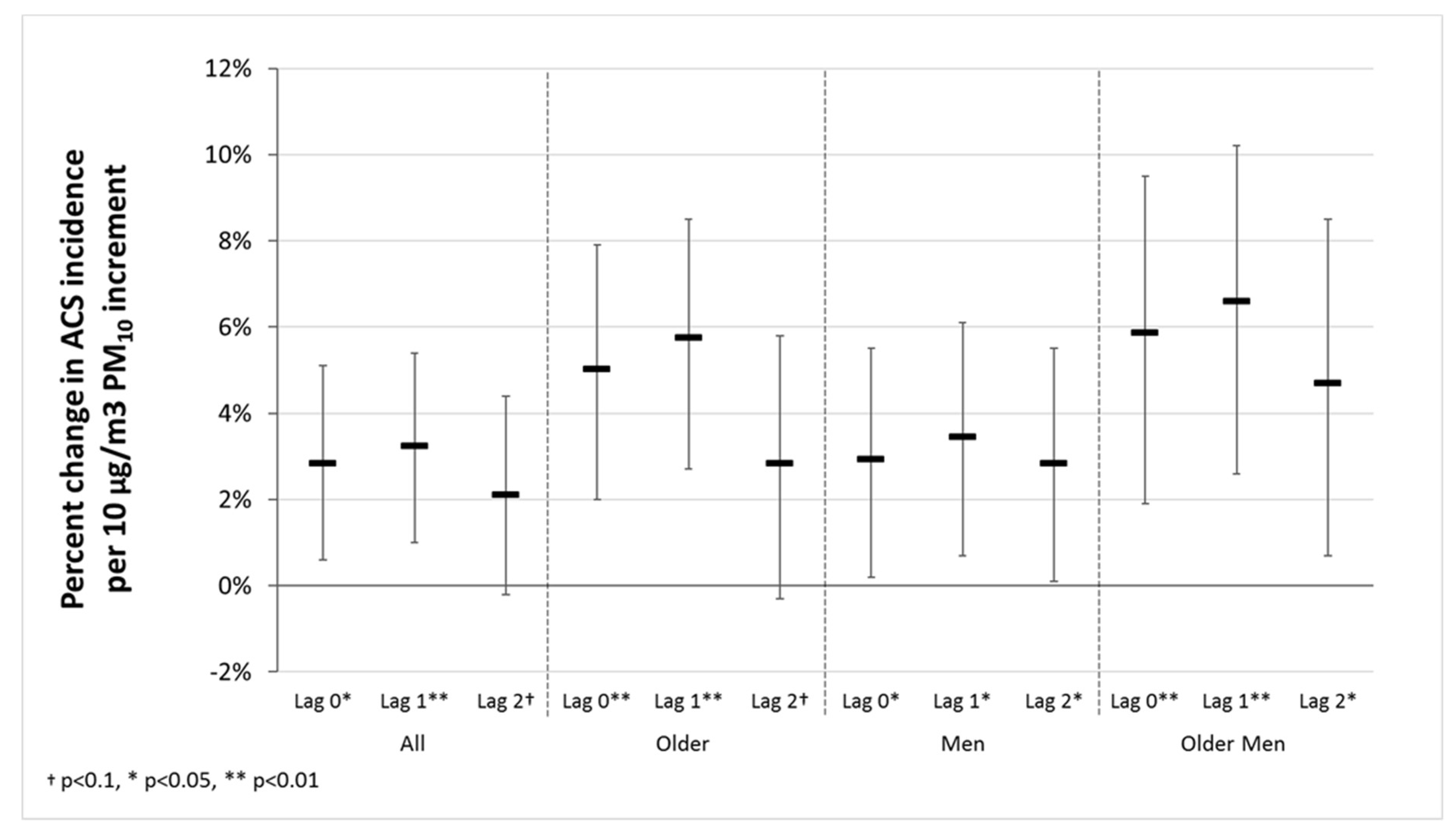
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Elizabeth, W.; Lauren, W.; Kremlin, W.; Prachi, B.; Mike, R.; Nick, T. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- Ito, K.; Mathes, R.; Ross, Z.; Nadas, A.; Thurston, G.; Matte, T. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ. Health Perspect. 2011, 119, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.Q.; Koehoorn, M.; Davies, H.W.; Demers, P.A.; Tamburic, L.; Brauer, M. Long-term exposure to traffic-related air pollution and the risk of coronary heart disease hospitalization and mortality. Environ. Health Perspect. 2011, 119, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.H.; Marra, M.; Ameling, C.B.; Hoek, G.; Beelen, R.; de Hoogh, K.; Breugelmans, O.; Kruize, H.; Janssen, N.A.; Houthuijs, D. Air Pollution and Mortality in Seven Million Adults: The Dutch Environmental Longitudinal Study (DUELS). Environ. Health Perspect. 2015, 123, 697–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Hopke, P.K.; Kane, C.; Utell, M.J.; Chalupa, D.C.; Kumar, P.; Ling, F.; Gardner, B.; Rich, D.Q. Triggering of Myocardial Infarction by Increased Ambient Fine Particle Concentration: Effect Modification by Source Direction. Environ. Res. 2015, 142, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Cendon, S.; Pereira, L.A.; Braga, A.L.; Conceicao, G.M.; Cury, A., Jr.; Romaldini, H.; Lopes, A.C.; Saldiva, P.H. Air pollution effects on myocardial infarction. Rev. Saude Publica 2006, 40, 414–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milojevic, A.; Wilkinson, P.; Armstrong, B.; Bhaskaran, K.; Smeeth, L.; Hajat, S. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: Case-crossover analysis of the MINAP database, hospital admissions and mortality. Heart 2014, 100, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Lanki, T.; Pekkanen, J.; Aalto, P.; Elosua, R.; Berglind, N.; D’Ippoliti, D.; Kulmala, M.; Nyberg, F.; Peters, A.; Picciotto, S.; et al. Associations of traffic related air pollutants with hospitalisation for first acute myocardial infarction: The HEAPSS study. Occup. Environ. Med. 2006, 63, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef] [PubMed]
- Torbicki, A.; Kastrati, A.; Vahanian, A.; Auricchio, A.; Hoes, A.; Merkely, B.; Popescu, B.A.; Deaton, C.; Vrints, C.J.M.; Funck-Brentano, C.; et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 2999–3054. [Google Scholar] [CrossRef] [Green Version]
- Fanaroff, A.C.; Rymer, J.A.; Goldstein, S.A. Acute Coronary Syndrome. JAMA 2015, 314, 1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglind, N.; Bellander, T.; Forastiere, F.; von Klot, S.; Aalto, P.; Elosua, R.; Kulmala, M.; Lanki, T.; Lowel, H.; Peters, A.; et al. Ambient air pollution and daily mortality among survivors of myocardial infarction. Epidemiology 2009, 20, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Fischer, P.; Marra, M.; Ameling, C.; Cassee, F.R. Short-term effects of PM2.5, PM10 and PM2.5–10 on daily mortality in the Netherlands. Sci. Total Environ. 2013, 463, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.; Zanobetti, A.; Schwartz, J. The lag structure between particulate air pollution and respiratory and cardiovascular deaths in 10 US cities. J. Occup. Environ. Med. 2001, 43, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Bateson, T.F.; Schwartz, J. Who is sensitive to the effects of particulate air pollution on mortality? A case-crossover analysis of effect modifiers. Epidemiology 2004, 15, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Mannucci, P.M. Short-term effects of air pollution on cardiovascular diseases: Outcomes and mechanisms. J. Thrombos. Haemost. 2007, 5, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; He, M.; Zhu, W. Acute effects of outdoor air pollution on emergency department visits due to five clinical subtypes of coronary heart diseases in Shanghai, China. J. Epidemiol. 2014, 24, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Nuvolone, D.; Balzi, D.; Chini, M.; Scala, D.; Giovannini, F.; Barchielli, A. Short-term association between ambient air pollution and risk of hospitalization for acute myocardial infarction: Results of the cardiovascular risk and air pollution in Tuscany (RISCAT) study. Am. J. Epidemiol 2011, 174, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Beelen, R.; Stafoggia, M.; Raaschou-Nielsen, O.; Andersen, Z.J.; Hoffmann, B.; Fischer, P.; Houthuijs, D.; Nieuwenhuijsen, M.; Weinmayr, G.; et al. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: Results from the ESCAPE and TRANSPHORM projects. Environ. Int. 2014, 66, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Xu, D.; Cheng, Y.; Dong, S.; Guo, C.; Jiang, X.; Zheng, X. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ. Res. 2015, 136, 196–204. [Google Scholar] [CrossRef] [PubMed]
- von Klot, S.; Peters, A.; Aalto, P.; Bellander, T.; Berglind, N.; D'Ippoliti, D.; Elosua, R.; Hormann, A.; Kulmala, M.; Lanki, T.; et al. Ambient air pollution is associated with increased risk of hospital cardiac readmissions of myocardial infarction survivors in five European cities. Circulation 2005, 112, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.K.; Tager, I.B.; Lurmann, F.; Segal, M.; Quesenberry, C.P., Jr.; Lugg, M.M.; Shan, J.; Van Den Eeden, S.K. Air pollution and hospital admissions for ischemic heart disease in persons with congestive heart failure or arrhythmia. Environ. Health Perspect. 2002, 110, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Mustafic, H.; Jabre, P.; Caussin, C.; Murad, M.H.; Escolano, S.; Tafflet, M.; Perier, M.C.; Marijon, E.; Vernerey, D.; Empana, J.P.; et al. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA 2012, 307, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Bard, D.; Kihal, W.; Schillinger, C.; Fermanian, C.; Segala, C.; Glorion, S.; Arveiler, D.; Weber, C. Traffic-related air pollution and the onset of myocardial infarction: Disclosing benzene as a trigger? A small-area case-crossover study. PLoS ONE 2014, 9, e100307. [Google Scholar] [CrossRef] [PubMed]
- Peter, O.; Andrej, U.; Simona, U.; Tanja, C.; Mateja, G.; Tanja, K.D.; Martina, L.; Marjana, M.; Anton, P.; Boštjan, P.; et al. Kakovost Zraka v Sloveniji v Letu 2014; Ministrstvo za Okolje in Prostor, Agencija Republike Slovenije za Okolje: Ljubljana, Slovenia, 2015.
- Stieb, D.M.; Szyszkowicz, M.; Rowe, B.H.; Leech, J.A. Air pollution and emergency department visits for cardiac and respiratory conditions: A multi-city time-series analysis. Environ. Health 2009, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Hou, Q.; Li, N.; Zha, S. Assessment of human exposure level to PM10 in China. Atmos. Environ. 2013, 70, 376–386. [Google Scholar] [CrossRef]
- Nastos, P.T.; Giaouzaki, K.N.; Kampanis, N.A.; Matzarakis, A. Acute coronary syndromes related to bio-climate in a Mediterranean area. The case of Ierapetra, Crete Island, Greece. Int. J. Environ. Health Res. 2013, 23, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, S.; Jusot, J.-F.; Blanchard, M.; Prouvost, H.; Declercq, C.; Fabre, P.; Pascal, L.; Tertre, A.L.; Wagner, V.; Rivière, S.; et al. Short term effects of air pollution on hospitalizations for cardiovascular diseases in eight French cities: The PSAS program. Sci. Total Environ. 2007, 387, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Middleton, N.; Yiallouros, P.; Kleanthous, S.; Kolokotroni, O.; Schwartz, J.; Dockery, D.W.; Demokritou, P.; Koutrakis, P. A 10-year time-series analysis of respiratory and cardiovascular morbidity in Nicosia, Cyprus: The effect of short-term changes in air pollution and dust storms. Environ. Health 2008, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- IBM. SPSS Software Predictive Analytics Software and Solutions; IBM: Armonk, NY, USA, 2014. [Google Scholar]
- The R Foundation. The R Project for Statisitcal Computing. Available online: http://www.r-project.org/ (accessed on 15 October 2018).
- Lippi, G.; Franchini, M.; Montagnana, M.; Filippozzi, L.; Favaloro, E.J.; Guidi, G.C. Relationship between 24-h air pollution, emergency department admission and diagnosis of acute coronary syndrome. J. Thromb Thrombolys. 2010, 29, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Belleudi, V.; Faustini, A.; Stafoggia, M.; Cattani, G.; Marconi, A.; Perucci, C.A.; Forastiere, F. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology 2010, 21, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Colais, P.; Faustini, A.; Stafoggia, M.; Berti, G.; Bisanti, L.; Cadum, E.; Cernigliaro, A.; Mallone, S.; Pacelli, B.; Serinelli, M.; et al. Particulate air pollution and hospital admissions for cardiac diseases in potentially sensitive subgroups. Epidemiology 2012, 23, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; De Palma, G.; Manerba, A.; Goldoni, M.; Triggiani, M.; Apostoli, P.; Dei Cas, L.; Nodari, S. Risk of Cardiovascular Hospitalizations from Exposure to Coarse Particulate Matter (PM10) Below the European Union Safety Threshold. Am. J. Cardiol. 2016, 117, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Qorbani, M.; Yunesian, M.; Fotouhi, A.; Zeraati, H.; Sadeghian, S. Effect of air pollution on onset of acute coronary syndrome in susceptible subgroups. East. Mediterr. Health J. 2012, 18, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Schwartz, J. The effect of particulate air pollution on emergency admissions for myocardial infarction: A multicity case-crossover analysis. Environ. Health Perspect. 2005, 113, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Hajat, S.; Armstrong, B.; Haines, A.; Herrett, E.; Wilkinson, P.; Smeeth, L. The effects of hourly differences in air pollution on the risk of myocardial infarction: Case crossover analysis of the MINAP database. BMJ 2011, 343, d5531. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Dockery, D.W.; Muller, J.E.; Mittleman, M.A. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 2001, 103, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Hajat, S.; Haines, A.; Herrett, E.; Wilkinson, P.; Smeeth, L. Effects of ambient temperature on the incidence of myocardial infarction. Heart 2009, 95, 1760–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, X.; Li, Z.; Scott, E.M.; Li, X.; Tang, M. Short-term effects of atmospheric particulate matter on myocardial infarction: A cumulative meta-analysis. Environ. Sci. Pollut. Res. Int. 2016, 23, 6139–6148. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Guida, A.; Tufano, A.; Coppola, A. Air pollution, vascular disease and thrombosis: Linking clinical data and pathogenic mechanisms. J. Thromb. Haemost. 2012, 10, 2438–2451. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhu, X.; Yao, C.; Hou, L.; Zhang, J.; Cao, J.; Wang, A. Short-term exposure to particulate air pollution and risk of myocardial infarction: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2015, 22, 14651–14662. [Google Scholar] [CrossRef] [PubMed]
- Godleski, J.J.; Verrier, R.L.; Koutrakis, P.; Catalano, P.; Coull, B.; Reinisch, U.; Lovett, E.G.; Lawrence, J.; Murthy, G.G.; Wolfson, J.M.; et al. Mechanisms of morbidity and mortality from exposure to ambient air particles. Res. Rep. Health Eff. Inst. 2000, 91, 5–88. [Google Scholar]
- Pope, C.A., 3rd; Verrier, R.L.; Lovett, E.G.; Larson, A.C.; Raizenne, M.E.; Kanner, R.E.; Schwartz, J.; Villegas, G.M.; Gold, D.R.; Dockery, D.W. Heart rate variability associated with particulate air pollution. Am. Heart J. 1999, 138, 890–899. [Google Scholar] [CrossRef]
- Adar, S.D.; Gold, D.R.; Coull, B.A.; Schwartz, J.; Stone, P.H.; Suh, H. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology 2007, 18, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Pajnic, M.; Drasler, B.; Sustar, V.; Krek, J.L.; Stukelj, R.; Simundic, M.; Kononenko, V.; Makovec, D.; Hagerstrand, H.; Drobne, D.; et al. Effect of carbon black nanomaterial on biological membranes revealed by shape of human erythrocytes, platelets and phospholipid vesicles. J. Nanobiotechnol. 2015, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Ishii, H.; Hogg, J.C.; Shih, C.H.; Yatera, K.; Vincent, R.; van Eeden, S.F. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am. J. Respir. Crit. Care Med. 2004, 170, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Zanobetti, A.; Martinelli, I.; Grillo, P.; Hou, L.; Giacomini, S.; Bonzini, M.; Lanzani, G.; Mannucci, P.M.; Bertazzi, P.A.; et al. Effects of exposure to air pollution on blood coagulation. J. Thromb. Haemost. 2007, 5, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermylen, J.; Nemmar, A.; Nemery, B.; Hoylaerts, M.F. Ambient air pollution and acute myocardial infarction. J. Thromb. Haemost. 2005, 3, 1955–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, A.G.; Williams, G.M.; Schwartz, J.; Best, T.L.; Neller, A.H.; Petroeschevsky, A.L.; Simpson, R.W. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ. Health Perspect. 2006, 114, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.D.; Stanek, L.W.; Luben, T.J.; Johns, D.O.; Buckley, B.J.; Brown, J.S.; Ross, M. Particulate matter-induced health effects: Who is susceptible? Environ. Health Perspect. 2011, 119, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Pun, V.C.; Yu, I.T.-S.; Ho, K.-F.; Qiu, H.; Sun, Z.; Tian, L. Differential Effects of Source-Specific Particulate Matter on Emergency Hospitalizations for Ischemic Heart Disease in Hong Kong. Environ. Health Perspect. 2014, 122, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemmar, A.; Nemery, B.; Hoet, P.H.; Van Rooijen, N.; Hoylaerts, M.F. Silica particles enhance peripheral thrombosis: Key role of lung macrophage-neutrophil cross-talk. Am. J. Respir. Crit. Care Med. 2005, 171, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
| Parameter | β | Std. Error | 95% Confidence Interval | Hypothesis Test | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | Wald Chi-Square | df | Sig. | |||
| All 1 | 0.028 | 0.0113 | 0.006 | 0.051 | 6.286 | 1 | 0.012 * |
| ≤65 years 2 | 0.002 | 0.0174 | −0.032 | 0.036 | 0.011 | 1 | 0.915 |
| ˃65 years 3 | 0.049 | 0.0149 | 0.020 | 0.079 | 11.060 | 1 | 0.001 ** |
| Parameter | β | Std. Error | 95% Confidence Interval | Hypothesis Test | |||
|---|---|---|---|---|---|---|---|
| Lower | Upper | Wald Chi-Square | df | Sig. | |||
| Man 1 | 0.029 | 0.0137 | 0.002 | 0.055 | 4.315 | 1 | 0.038 * |
| Women 2 | 0.028 | 0.0199 | −0.011 | 0.067 | 1.972 | 1 | 0.160 |
| man ≤ 65 3 | 0.003 | 0.0193 | −0.035 | 0.041 | 0.020 | 1 | 0.888 |
| man ˃ 65 4 | 0.057 | 0.0195 | 0.019 | 0.095 | 8.665 | 1 | 0.003 ** |
| women ≤ 65 5 | 0.002 | 0.0396 | −0.079 | 0.076 | 0.002 | 1 | 0.964 |
| women ˃ 65 6 | 0.039 | 0.0231 | −0.006 | 0.084 | 2.836 | 1 | 0.092 |
| Parameter | Lag 0 | Lag 1 | Lag 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | Sig. | Change | β | Sig. | Change | β | Sig. | Change | |
| all 1 | 0.028 | 0.012 * | 2.8% | 0.032 | 0.004 ** | 3.3% | 0.021 | 0.068 † | 2.1% |
| ˃65 years 2 | 0.049 | 0.001 ** | 5% | 0.056 | 0.000 ** | 5.8% | 0.028 | 0.077 † | 2.8% |
| man 3 | 0.029 | 0.038 * | 2.9% | 0.034 | 0.014 * | 3.5% | 0.028 | 0.046 * | 2.8% |
| man ˃ 65 years 4 | 0.057 | 0.003 ** | 5.9% | 0.064 | 0.001 ** | 6.6% | 0.046 | 0.021 * | 4.7% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravljen, M.; Hovelja, T.; Vavpotič, D. Relationship between Particulate Matter Pollution and Acute Coronary Syndrome Incidence. Atmosphere 2019, 10, 103. https://doi.org/10.3390/atmos10030103
Ravljen M, Hovelja T, Vavpotič D. Relationship between Particulate Matter Pollution and Acute Coronary Syndrome Incidence. Atmosphere. 2019; 10(3):103. https://doi.org/10.3390/atmos10030103
Chicago/Turabian StyleRavljen, Mirjam, Tomaž Hovelja, and Damjan Vavpotič. 2019. "Relationship between Particulate Matter Pollution and Acute Coronary Syndrome Incidence" Atmosphere 10, no. 3: 103. https://doi.org/10.3390/atmos10030103
APA StyleRavljen, M., Hovelja, T., & Vavpotič, D. (2019). Relationship between Particulate Matter Pollution and Acute Coronary Syndrome Incidence. Atmosphere, 10(3), 103. https://doi.org/10.3390/atmos10030103





