Air Pollution-Related Brain Metal Dyshomeostasis as a Potential Risk Factor for Neurodevelopmental Disorders and Neurodegenerative Diseases
Abstract
:1. Epidemiological Studies Increasingly Associate Air Pollution with Neurodevelopmental Disorders and Neurodegenerative Diseases
2. What Component(s) of Air Pollution Contribute to This Neurotoxicity?
3. Metals as a Source of AP-Related Neurotoxicity
4. Evidence for the Involvement of Metals in NDDs and NDGDs
5. Could AP-Related Brain Metals Contribute to Brain Metal Dyshomeostasis?
6. Shared Features of Neurodevelopmental Disorders and Neurodegenerative Diseases
7. Current Evidence That AP-Related Metals and Trace Elements Can Reproduce Shared Characteristics of NDDs and NDGDs
8. Conclusions and Future Research Needs
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Sunyer, J.; Dadvand, P. Pre-natal brain development as a target for urban air pollution. Basic Clin. Pharmacol. Toxicol. 2019, 125, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.C.; Wang, X.; Wellenius, G.A.; Serre, M.L.; Driscoll, I.; Casanova, R.; McArdle, J.J.; Manson, J.E.; Chui, H.C.; Espeland, M.A. Ambient air pollution and neurotoxicity on brain structure: Evidence from women’s health initiative memory study. Ann. Neurol. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Pajot, M.C.; Ofner, M.; Do, M.T.; Lavigne, E.; Villeneuve, P.J. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: A review and meta-analysis. Environ. Res. 2016, 151, 763–776. [Google Scholar] [CrossRef]
- Annavarapu, R.N.; Kathi, S. Cognitive disorders in children associated with urban vehicular emissions. Environ. Pollut. 2016, 208, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Kalkbrenner, A.E.; Windham, G.C.; Serre, M.L.; Akita, Y.; Wang, X.; Hoffman, K.; Thayer, B.P.; Daniels, J.L. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology 2015, 26, 30–42. [Google Scholar] [CrossRef]
- Volk, H.E.; Lurmann, F.; Penfold, B.; Hertz-Picciotto, I.; McConnell, R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 2013, 70, 71–77. [Google Scholar] [CrossRef]
- Volk, H.E.; Hertz-Picciotto, I.; Delwiche, L.; Lurmann, F.; McConnell, R. Residential proximity to freeways and autism in the CHARGE study. Environ. Health Perspect. 2011, 119, 873–877. [Google Scholar] [CrossRef]
- Fluegge, K. Does environmental exposure to the greenhouse gas, N2O, contribute to etiological factors in neurodevelopmental disorders? A mini-review of the evidence. Environ. Toxicol. Pharmacol. 2016, 47, 6–18. [Google Scholar] [CrossRef]
- Becerra, T.A.; Wilhelm, M.; Olsen, J.; Cockburn, M.; Ritz, B. Ambient air pollution and autism in Los Angeles county, California. Environ. Health Perspect. 2013, 121, 380–386. [Google Scholar] [CrossRef]
- Dickerson, A.S.; Rahbar, M.H.; Bakian, A.V.; Bilder, D.A.; Harrington, R.A.; Pettygrove, S.; Kirby, R.S.; Durkin, M.S.; Han, I.; Moye, L.A., 3rd; et al. Autism spectrum disorder prevalence and associations with air concentrations of lead, mercury, and arsenic. Environ. Monit. Assess. 2016, 188, 407. [Google Scholar] [CrossRef]
- Suades-Gonzalez, E.; Gascon, M.; Guxens, M.; Sunyer, J. Air pollution and neuropsychological development: A review of the latest evidence. Endocrinology 2015, 156, 3473–3482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbott, E.O.; Arena, V.C.; Rager, J.R.; Clougherty, J.E.; Michanowicz, D.R.; Sharma, R.K.; Stacy, S.L. Fine particulate matter and the risk of autism spectrum disorder. Environ. Res. 2015, 140, 414–420. [Google Scholar] [CrossRef]
- Raz, R.; Roberts, A.L.; Lyall, K.; Hart, J.E.; Just, A.C.; Laden, F.; Weisskopf, M.G. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: A nested case–control analysis within the Nurses’ Health Study II cohort. Environ. Health Perspect. 2015, 123, 264–270. [Google Scholar] [CrossRef]
- Jung, C.R.; Lin, Y.T.; Hwang, B.F. Air pollution and newly diagnostic autism spectrum disorders: A population-based cohort study in Taiwan. PLoS ONE 2013, 8, e75510. [Google Scholar] [CrossRef] [Green Version]
- Roberts, A.L.; Lyall, K.; Hart, J.E.; Laden, F.; Just, A.C.; Bobb, J.F.; Koenen, K.C.; Ascherio, A.; Weisskopf, M.G. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ. Health Perspect. 2013, 121, 978–984. [Google Scholar] [CrossRef] [Green Version]
- Pagalan, L.; Bickford, C.; Weikum, W.; Lanphear, B.; Brauer, M.; Lanphear, N.; Hanley, G.E.; Oberlander, T.F.; Winters, M. Association of prenatal exposure to air pollution with autism spectrum disorder. JAMA Pediatrics 2018. [Google Scholar] [CrossRef] [PubMed]
- Mintz, M. Evolution in the understanding of autism spectrum disorder: Historical perspective. Indian J. Pediatr. 2017, 84, 44–52. [Google Scholar] [CrossRef]
- Goldson, E. Advances in autism-2016. Adv. Pediatr. 2016, 63, 333–355. [Google Scholar] [CrossRef]
- van Rees, G.F.; Lago, S.G.; Cox, D.A.; Tomasik, J.; Rustogi, N.; Weigelt, K.; Ozcan, S.; Cooper, J.; Drexhage, H.; Leweke, F.M.; et al. Evidence of microglial activation following exposure to serum from first-onset drug-naive schizophrenia patients. Brain Behav. Immun. 2018, 67, 364–373. [Google Scholar] [CrossRef]
- Merchán-Naranjo, J.; Boada, L.; del Rey-Mejías, Á.; Mayoral, M.; Llorente, C.; Arango, C.; Parellada, M. Executive function is affected in autism spectrum disorder, but does not correlate with intelligence. Rev. Psiquiatr. Salud Ment. 2016, 9, 39–50. [Google Scholar] [CrossRef]
- Carter Leno, V.; Chandler, S.; White, P.; Pickles, A.; Baird, G.; Hobson, C.; Smith, A.B.; Charman, T.; Rubia, K.; Simonoff, E. Testing the specificity of executive functioning impairments in adolescents with ADHD, ODD/CD and ASD. Eur. Child Adolesc. Psychiatry 2018, 27, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzefovsky, F.; Allison, C.; Smith, P.; Baron-Cohen, S. Brief Report: The go/no-go task online: Inhibitory control deficits in autism in a large sample. J. Autism Dev. Disord. 2016, 46, 2774–2779. [Google Scholar] [CrossRef] [Green Version]
- Allred, E.N.; Dammann, O.; Fichorova, R.N.; Hooper, S.R.; Hunter, S.J.; Joseph, R.M.; Kuban, K.; Leviton, A.; O’Shea, T.M.; Scott, M.N.; et al. Systemic inflammation during the first postnatal month and the risk of attention deficit hyperactivity disorder characteristics among 10 year-old children born extremely preterm. J. Neuroimmune Pharmacol. 2017, 12, 531–543. [Google Scholar] [CrossRef]
- Alemany, S.; Vilor-Tejedor, N.; Garcia-Esteban, R.; Bustamante, M.; Dadvand, P.; Esnaola, M.; Mortamais, M.; Forns, J.; van Drooge, B.L.; Alvarez-Pedrerol, M.; et al. Traffic-related air pollution, APOEepsilon4 status, and neurodevelopmental outcomes among school children enrolled in the BREATHE project (Catalonia, Spain). Environ. Health Perspect. 2018, 126, 087001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuertes, E.; Standl, M.; Forns, J.; Berdel, D.; Garcia-Aymerich, J.; Markevych, I.; Schulte-Koerne, G.; Sugiri, D.; Schikowski, T.; Tiesler, C.M.; et al. Traffic-related air pollution and hyperactivity/inattention, dyslexia and dyscalculia in adolescents of the German GINIplus and LISAplus birth cohorts. Environ. Int. 2016, 97, 85–92. [Google Scholar] [CrossRef]
- Sentis, A.; Sunyer, J.; Dalmau-Bueno, A.; Andiarena, A.; Ballester, F.; Cirach, M.; Estarlich, M.; Fernandez-Somoano, A.; Ibarluzea, J.; Iniguez, C.; et al. Prenatal and postnatal exposure to NO2 and child attentional function at 4-5years of age. Environ. Int. 2017, 106, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Min, J.Y.; Min, K.B. Exposure to ambient PM10 and NO2 and the incidence of attention-deficit hyperactivity disorder in childhood. Environ. Int. 2017, 99, 221–227. [Google Scholar] [CrossRef]
- Newman, N.C.; Ryan, P.; Lemasters, G.; Levin, L.; Bernstein, D.; Hershey, G.K.; Lockey, J.E.; Villareal, M.; Reponen, T.; Grinshpun, S.; et al. Traffic-related air pollution exposure in the first year of life and behavioral scores at 7 years of age. Environ. Health Perspect. 2013, 121, 731–736. [Google Scholar] [CrossRef]
- Siddique, S.; Banerjee, M.; Ray, M.R.; Lahiri, T. Attention-deficit hyperactivity disorder in children chronically exposed to high level of vehicular pollution. Eur. J. Pediatrics 2011, 170, 923–929. [Google Scholar] [CrossRef]
- Luo, Y.; Weibman, D.; Halperin, J.M.; Li, X. A Review of heterogeneity in attention deficit/hyperactivity disorder (ADHD). Front. Hum. Neurosci. 2019, 13, 42. [Google Scholar] [CrossRef] [Green Version]
- Magnus, W.; Nazir, S.; Anilkumar, A.C.; Shaban, K. Attention Deficit Hyperactivity Disorder (ADHD); StatPearls Publishing LLC.: Treasure Island, FL, USA, 2019. [Google Scholar]
- Tandon, M.; Pergjika, A. Attention deficit hyperactivity disorder in preschool-age children. Child Adolesc. Psychiatr. Clin. N Am. 2017, 26, 523–538. [Google Scholar] [CrossRef]
- Hinshaw, S.P. Attention deficit hyperactivity disorder (ADHD): Controversy, developmental mechanisms, and multiple levels of analysis. Annu. Rev. Clin. Psychol. 2018, 14, 291–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, N.M.; King, S.L.; Hilton, D.C.; Rondon, A.T.; Jarrett, M.A. Social functioning in youth with attention-deficit/hyperactivity disorder and sluggish cognitive tempo. Yale J. Biol. Med. 2019, 92, 29–35. [Google Scholar]
- Dean, L. Schizophrenia. In Medical Genetics Summaries; Pratt, V., McLeod, H., Rubinstein, W., Dean, L., Kattman, B., Malheiro, A., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Newbury, J.B.; Arseneault, L.; Beevers, S.; Kitwiroon, N.; Roberts, S.; Pariante, C.M.; Kelly, F.J.; Fisher, H.L. Association of air pollution exposure with psychotic experiences during adolescence. JAMA Psychiatry 2019, 76, 614–623. [Google Scholar] [CrossRef]
- Duan, J.; Luo, X.L.; Chu, W.W.; Gao, J.J.; Xu, Z.H.; Zhang, Y.W.; Cheng, Q.; Bai, L.J.; Wei, Q.N.; Su, H. Time series analysis on the effect of ambient fine particulate matters and temperature interactions on schizophrenia admission in Tongling City of Anhui Province, 2014–2017. Zhonghua Yu Fang Yi Xue Za Zhi 2019, 53, 51–56. [Google Scholar] [CrossRef]
- Qiu, H.; Zhu, X.; Wang, L.; Pan, J.; Pu, X.; Zeng, X.; Zhang, L.; Peng, Z.; Zhou, L. Attributable risk of hospital admissions for overall and specific mental disorders due to particulate matter pollution: A time-series study in Chengdu, China. Environ. Res. 2019, 170, 230–237. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, C.; Cao, Y.; Kan, H.D.; Chen, R.J.; Yao, C.Y.; Liu, X.L.; Xiang, Y.; Wu, N.; Wu, L.; et al. The association between short-term ambient air pollution and daily outpatient visits for schizophrenia: A hospital-based study. Environ. Pollut. 2019, 244, 102–108. [Google Scholar] [CrossRef]
- Duan, J.; Cheng, Q.; Luo, X.; Bai, L.; Zhang, H.; Wang, S.; Xu, Z.; Gao, J.; Zhang, Y.; Su, H. Is the serious ambient air pollution associated with increased admissions for schizophrenia? Sci. Total Environ. 2018, 644, 14–19. [Google Scholar] [CrossRef]
- Eguchi, R.; Onozuka, D.; Ikeda, K.; Kuroda, K.; Ieiri, I.; Hagihara, A. The relationship between fine particulate matter (PM2.5) and schizophrenia severity. Int. Arch. Occup. Environ. Health 2018, 91, 613–622. [Google Scholar] [CrossRef]
- Yackerson, N.S.; Zilberman, A.; Todder, D.; Kaplan, Z. The influence of air-suspended particulate concentration on the incidence of suicide attempts and exacerbation of schizophrenia. Int. J. Biometeorol. 2014, 58, 61–67. [Google Scholar] [CrossRef]
- Pedersen, C.B.; Raaschou-Nielsen, O.; Hertel, O.; Mortensen, P.B. Air pollution from traffic and schizophrenia risk. Schizophr. Res. 2004, 66, 83–85. [Google Scholar] [CrossRef]
- Pinares-Garcia, P.; Stratikopoulos, M.; Zagato, A.; Loke, H.; Lee, J. Sex: A significant risk factor for neurodevelopmental and neurodegenerative disorders. Brain Sci. 2018, 8, 154. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.L.; Lin, Y.T.; Hwang, B.F.; Nakayama, S.F.; Tsai, C.H.; Sun, X.L.; Ma, C.; Jung, C.R. Fine particulate matter is a potential determinant of Alzheimer’s disease: A systemic review and meta-analysis. Environ. Res. 2019, 177, 108638. [Google Scholar] [CrossRef]
- Younan, D.; Petkus, A.J.; Widaman, K.F.; Wang, X.; Casanova, R.; Espeland, M.A.; Gatz, M.; Henderson, V.W.; Manson, J.E.; Rapp, S.R.; et al. Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain 2020, 143, 289–302. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Guo, X.; Cheung, F.M.H.; Yung, K.K.L. The association between PM(2.5) exposure and neurological disorders: A systematic review and meta-analysis. Sci. Total Environ. 2019, 655, 1240–1248. [Google Scholar] [CrossRef]
- Oudin, A.; Forsberg, B.; Adolfsson, A.N.; Lind, N.; Modig, L.; Nordin, M.; Nordin, S.; Adolfsson, R.; Nilsson, L.G. Traffic-related air pollution and dementia incidence in northern Sweden: A longitudinal study. Environ. Health Perspect. 2016, 124, 306–312. [Google Scholar] [CrossRef]
- Jung, C.R.; Lin, Y.T.; Hwang, B.F. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based cohort study in Taiwan. J. Alzheimers Dis. 2015, 44, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajmani, G.S.; Suh, H.H.; Pinto, J.M. Effects of ambient air pollution exposure on olfaction: A review. Environ. Health Perspect. 2016, 124, 1683–1693. [Google Scholar] [CrossRef]
- Han, C.; Lu, Y.; Cheng, H.; Wang, C.; Chan, P. The impact of long-term exposure to ambient air pollution and second-hand smoke on the onset of Parkinson disease: A review and meta-analysis. Public Health 2020, 179, 100–110. [Google Scholar] [CrossRef]
- Kasdagli, M.I.; Katsouyanni, K.; Dimakopoulou, K.; Samoli, E. Air pollution and Parkinson’s disease: A systematic review and meta-analysis up to 2018. Int. J. Hyg. Environ. Health 2019, 222, 402–409. [Google Scholar] [CrossRef]
- Hu, C.Y.; Fang, Y.; Li, F.L.; Dong, B.; Hua, X.G.; Jiang, W.; Zhang, H.; Lyu, Y.; Zhang, X.J. Association between ambient air pollution and Parkinson’s disease: Systematic review and meta-analysis. Environ. Res. 2019, 168, 448–459. [Google Scholar] [CrossRef]
- Shin, S.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; van Donkelaar, A.; Brook, J.R.; Copes, R.; Tu, K.; Goldberg, M.S.; Villeneuve, P.J.; et al. Effects of ambient air pollution on incident Parkinson’s disease in Ontario, 2001 to 2013: A population-based cohort study. Int. J. Epidemiol. 2018, 47, 2038–2048. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hung, H.J.; Chang, K.H.; Hsu, C.Y.; Muo, C.H.; Tsai, C.H.; Wu, T.N. Long-term exposure to air pollution and the incidence of Parkinson’s disease: A nested case-control study. PLoS ONE 2017, 12, e0182834. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.C.; Liu, L.L.; Sun, Y.; Chen, Y.A.; Liu, C.C.; Li, C.Y.; Yu, H.L.; Ritz, B. Traffic-related air pollution increased the risk of Parkinson’s disease in Taiwan: A nationwide study. Environ. Int. 2016, 96, 75–81. [Google Scholar] [CrossRef]
- Ritz, B.; Lee, P.C.; Hansen, J.; Lassen, C.F.; Ketzel, M.; Sorensen, M.; Raaschou-Nielsen, O. Traffic-related air pollution and Parkinson’s disease in Denmark: A case-control study. Environ. Health Perspect. 2016, 124, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Toro, R.; Downward, G.S.; van der Mark, M.; Brouwer, M.; Huss, A.; Peters, S.; Hoek, G.; Nijssen, P.; Mulleners, W.M.; Sas, A.; et al. Parkinson’s disease and long-term exposure to outdoor air pollution: A matched case-control study in The Netherlands. Environ. Int. 2019, 129, 28–34. [Google Scholar] [CrossRef]
- Palacios, N.; Fitzgerald, K.C.; Hart, J.E.; Weisskopf, M.; Schwarzschild, M.A.; Ascherio, A.; Laden, F. Air pollution and risk of Parkinson’s disease in a large prospective study of men. Environ. Health Perspect. 2017, 125, 087011. [Google Scholar] [CrossRef]
- Palacios, N.; Fitzgerald, K.C.; Hart, J.E.; Weisskopf, M.G.; Schwarzschild, M.A.; Ascherio, A.; Laden, F. Particulate matter and risk of Parkinson disease in a large prospective study of women. Environ. Health 2014, 13, 80. [Google Scholar] [CrossRef] [Green Version]
- Tateo, F.; Grassivaro, F.; Ermani, M.; Puthenparampil, M.; Gallo, P. PM2.5 levels strongly associate with multiple sclerosis prevalence in the Province of Padua, Veneto Region, North-East Italy. Mult. Scler. J. 2019, 25, 1719–1727. [Google Scholar] [CrossRef]
- Jeanjean, M.; Bind, M.A.; Roux, J.; Ongagna, J.C.; de Sèze, J.; Bard, D.; Leray, E. Ozone, NO(2) and PM(10) are associated with the occurrence of multiple sclerosis relapses. Evidence from seasonal multi-pollutant analyses. Environ. Res. 2018, 163, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschi, R.; Cortese, A.; Pichiecchio, A.; Berzolari, F.G.; Borrelli, P.; Mallucci, G.; Bollati, V.; Romani, A.; Nosari, G.; Villa, S.; et al. Air pollution is associated to the multiple sclerosis inflammatory activity as measured by brain MRI. Mult. Scler. J. 2018, 24, 1578–1584. [Google Scholar] [CrossRef]
- Roux, J.; Bard, D.; Le Pabic, E.; Segala, C.; Reis, J.; Ongagna, J.C.; de Sèze, J.; Leray, E. Air pollution by particulate matter PM(10) may trigger multiple sclerosis relapses. Environ. Res. 2017, 156, 404–410. [Google Scholar] [CrossRef]
- Heydarpour, P.; Amini, H.; Khoshkish, S.; Seidkhani, H.; Sahraian, M.A.; Yunesian, M. Potential impact of air pollution on multiple sclerosis in Tehran, Iran. Neuroepidemiology 2014, 43, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Oikonen, M.; Laaksonen, M.; Laippala, P.; Oksaranta, O.; Lilius, E.M.; Lindgren, S.; Rantio-Lehtimaki, A.; Anttinen, A.; Koski, K.; Eralinna, J.P. Ambient air quality and occurrence of multiple sclerosis relapse. Neuroepidemiology 2003, 22, 95–99. [Google Scholar] [CrossRef]
- Palacios, N.; Munger, K.L.; Fitzgerald, K.C.; Hart, J.E.; Chitnis, T.; Ascherio, A.; Laden, F. Exposure to particulate matter air pollution and risk of multiple sclerosis in two large cohorts of US nurses. Environ. Int. 2017, 109, 64–72. [Google Scholar] [CrossRef]
- Visser, A.E.; D’Ovidio, F.; Peters, S.; Vermeulen, R.C.; Beghi, E.; Chiò, A.; Veldink, J.H.; Logroscino, G.; Hardiman, O.; van den Berg, L.H. Multicentre, population-based, case-control study of particulates, combustion products and amyotrophic lateral sclerosis risk. J. Neurol. Neurosurg. Psychiatry 2019, 90, 854–860. [Google Scholar] [CrossRef]
- Myung, W.; Lee, H.; Kim, H. Short-term air pollution exposure and emergency department visits for amyotrophic lateral sclerosis: A time-stratified case-crossover analysis. Environ. Int. 2019, 123, 467–475. [Google Scholar] [CrossRef]
- Seelen, M.; Toro Campos, R.A.; Veldink, J.H.; Visser, A.E.; Hoek, G.; Brunekreef, B.; van der Kooi, A.J.; de Visser, M.; Raaphorst, J.; van den Berg, L.H.; et al. Long-term air pollution exposure and amyotrophic lateral sclerosis in Netherlands: A population-based case-control study. Environ. Health Perspect. 2017, 125, 097023. [Google Scholar] [CrossRef] [Green Version]
- Pujol, J.; Martinez-Vilavella, G.; Macia, D.; Fenoll, R.; Alvarez-Pedrerol, M.; Rivas, I.; Forns, J.; Blanco-Hinojo, L.; Capellades, J.; Querol, X.; et al. Traffic pollution exposure is associated with altered brain connectivity in school children. NeuroImage 2016, 129, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Pujol, J.; Fenoll, R.; Macia, D.; Martinez-Vilavella, G.; Alvarez-Pedrerol, M.; Rivas, I.; Forns, J.; Deus, J.; Blanco-Hinojo, L.; Querol, X.; et al. Airborne copper exposure in school environments associated with poorer motor performance and altered basal ganglia. Brain Behav. 2016, 6, e00467. [Google Scholar] [CrossRef] [Green Version]
- Mezzaroba, L.; Alfieri, D.F.; Simão, A.N.C.; Reiche, E.M.V. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Guxens, M.; Lubczynska, M.J.; Muetzel, R.L.; Dalmau-Bueno, A.; Jaddoe, V.W.V.; Hoek, G.; van der Lugt, A.; Verhulst, F.C.; White, T.; Brunekreef, B.; et al. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol. Psychiatry 2018, 84, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Harris, M.H.; Gold, D.R.; Rifas-Shiman, S.L.; Melly, S.J.; Zanobetti, A.; Coull, B.A.; Schwartz, J.D.; Gryparis, A.; Kloog, I.; Koutrakis, P.; et al. Prenatal and childhood traffic-related pollution exposure and childhood cognition in the Project viva cohort (Massachusetts, USA). Environ. Health Perspect. 2015, 123, 1072–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.C.; Chen, S.J.; Huang, K.L.; Hwang, W.I.; Chang-Chien, G.P.; Lin, W.Y. Characteristics of metals in nano/ultrafine/fine/coarse particles collected beside a heavily trafficked road. Environ. Sci. Technol. 2005, 39, 8113–8122. [Google Scholar] [CrossRef]
- Friedlander, S.K. Chemical element balances and identification of air pollution sources. Environ. Sci. Technol. 1973, 7, 235–240. [Google Scholar] [CrossRef]
- Ruuskanen, J.; Tuch, T.; Brink, T.; Peters, A.; Khlystov, A.; Mirme, A.; Kos, G.P.A.; Brunekreef, B.; Wichmann, H.E.; Buzorius, G.; et al. Concentrations of ultrafine, fine and PM2.5 particles in three European cities. Atmos. Environ. 2001, 35, 3729–3738. [Google Scholar] [CrossRef]
- Tuch, T.; Brand, P.; Wichmann, H.E.; Heyder, J. Variation of particle number and mass concentration in various size ranges of ambient aerosols in Eastern Germany. Atmos. Environ. 1997, 31, 4193–4197. [Google Scholar] [CrossRef]
- Knibbs, L.D.; de Dear, R.J. Exposure to ultrafine particles and PM2.5 in four Sydney transport modes. Atmos. Environ. 2010, 44, 3224–3227. [Google Scholar] [CrossRef] [Green Version]
- Friedlander, S.K. Gas-to-particle conversion. In Smoke, Dust and Haze: Fundamentals of Aerosol Dynamics; Oxford University Press: New York, NY, USA, 2000; pp. 275–305. [Google Scholar]
- Kulmala, M.; Kerminen, V.M.; Petäjä, T.; Ding, A.J.; Wang, L. Atmospheric gas-to-particle conversion: Why NPF events are observed in megacities? Faraday Discuss. 2017, 200, 271–288. [Google Scholar] [CrossRef]
- Reff, A.; Bhave, P.V.; Simon, H.; Pace, T.G.; Pouliot, G.A.; Mobley, J.D.; Houyoux, M. Emissions inventory of PM2.5 trace elements across the United States. Environ. Sci. Technol. 2009, 43, 5790–5796. [Google Scholar] [CrossRef]
- Fang, T.; Guo, H.Y.; Zeng, L.H.; Verma, V.; Nenes, A.; Weber, R.J. Highly acidic ambient particles, soluble metals, and oxidative potential: A link between sulfate and aerosol toxicity. Environ. Sci. Technol. 2017, 51, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.Y.; Brain, J.D. The effect of SO2 on the uptake of particles by mouse bronchial epithelium. Exp. Lung Res. 1980, 1, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Conrad, K.; Oberdorster, G.; Johnston, C.J.; Sleezer, B.; Cory-Slechta, D.A. Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environ. Health Perspect. 2013, 121, 32–38. [Google Scholar] [CrossRef]
- Allen, J.L.; Klocke, C.; Morris-Schaffer, K.; Conrad, K.; Sobolewski, M.; Cory-Slechta, D.A. Cognitive effects of air pollution exposures and potential mechanistic underpinnings. Curr. Environ. Health Rep. 2017, 4, 180–191. [Google Scholar] [CrossRef]
- Allen, J.L.; Liu, X.; Pelkowski, S.; Palmer, B.; Conrad, K.; Oberdorster, G.; Weston, D.; Mayer-Proschel, M.; Cory-Slechta, D.A. Early postnatal exposure to ultrafine particulate matter air pollution: Persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environ. Health Perspect. 2014, 122, 939–945. [Google Scholar] [CrossRef]
- Allen, J.L.; Liu, X.; Weston, D.; Conrad, K.; Oberdorster, G.; Cory-Slechta, D.A. Consequences of developmental exposure to concentrated ambient ultrafine particle air pollution combined with the adult paraquat and maneb model of the Parkinson’s disease phenotype in male mice. Neurotoxicology 2014, 41, 80–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.L.; Liu, X.; Weston, D.; Prince, L.; Oberdorster, G.; Finkelstein, J.N.; Johnston, C.J.; Cory-Slechta, D.A. Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol. Sci. 2014, 140, 160–178. [Google Scholar] [CrossRef] [Green Version]
- Cory-Slechta, D.A.; Allen, J.L.; Conrad, K.; Marvin, E.; Sobolewski, M. Developmental exposure to low level ambient ultrafine particle air pollution and cognitive dysfunction. Neurotoxicology 2017. [Google Scholar] [CrossRef]
- Cory-Slechta, D.A.; Sobolewski, M.; Marvin, E.; Conrad, K.; Merrill, A.; Anderson, T.; Jackson, B.P.; Oberdorster, G. The impact of inhaled ambient ultrafine particulate matter on developing brain: Potential importance of elemental contaminants. Toxicol. Pathol. 2019, 47, 976–992. [Google Scholar] [CrossRef]
- Sobolewski, M.; Anderson, T.; Conrad, K.; Marvin, E.; Klocke, C.; Morris-Schaffer, K.; Allen, J.L.; Cory-Slechta, D.A. Developmental exposures to ultrafine particle air pollution reduces early testosterone levels and adult male social novelty preference: Risk for children’s sex-biased neurobehavioral disorders. NeuroToxicology 2018, 68, 203–211. [Google Scholar] [CrossRef]
- Bandeira, F.; Lent, R.; Herculano-Houzel, S. Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc. Natl. Acad. Sci. USA 2009, 106, 14108–14113. [Google Scholar] [CrossRef] [Green Version]
- Klocke, C.; Allen, J.L.; Sobolewski, M.; Blum, J.L.; Zelikoff, J.T.; Cory-Slechta, D.A. Exposure to fine and ultrafine particulate matter during gestation alters postnatal oligodendrocyte maturation, proliferation capacity, and myelination. Neurotoxicology 2018, 65, 196–206. [Google Scholar] [CrossRef]
- Klocke, C.; Allen, J.L.; Sobolewski, M.; Mayer-Pröschel, M.; Blum, J.L.; Lauterstein, D.; Zelikoff, J.T.; Cory-Slechta, D.A. Neuropathological consequences of gestational exposure to concentrated ambient fine and ultrafine particles in the mouse. Toxicol. Sci. 2017, 156, 492–508. [Google Scholar] [CrossRef]
- Klocke, C.; Sherina, V.; Graham, U.M.; Gunderson, J.; Allen, J.L.; Sobolewski, M.; Blum, J.L.; Zelikoff, J.T.; Cory-Slechta, D.A. Enhanced cerebellar myelination with concomitant iron elevation and ultrastructural irregularities following prenatal exposure to ambient particulate matter in the mouse. Inhal. Toxicol. 2018, 30, 381–396. [Google Scholar] [CrossRef]
- Scassellati, C.; Bonvicini, C.; Benussi, L.; Ghidoni, R.; Squitti, R. Neurodevelopmental disorders: Metallomics studies for the identification of potential biomarkers associated to diagnosis and treatment. J. Trace Elem. Med. Biol. 2020, 60, 126499. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biol. Trace Elem. Res. 2013, 151, 171–180. [Google Scholar] [CrossRef]
- Fiore, M.; Barone, R.; Copat, C.; Grasso, A.; Cristaldi, A.; Rizzo, R.; Ferrante, M. Metal and essential element levels in hair and association with autism severity. J. Trace Elem. Med. Biol. 2020, 57, 126409. [Google Scholar] [CrossRef]
- Lakshmi Priya, M.D.; Geetha, A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol. Trace Elem. Res. 2011, 142, 148–158. [Google Scholar] [CrossRef]
- Li, S.O.; Wang, J.L.; Bjørklund, G.; Zhao, W.N.; Yin, C.H. Serum copper and zinc levels in individuals with autism spectrum disorders. Neuroreport 2014, 25, 1216–1220. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Ahangari, N.; Hendi, K.; Saleh, F.; Rezaei, N. Status of essential elements in autism spectrum disorder: Systematic review and meta-analysis. Rev. Neurosci. 2017, 28, 783–809. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Simashkova, N.V.; Klyushnik, T.P.; Grabeklis, A.R.; Radysh, I.V.; Skalnaya, M.G.; Tinkov, A.A. Analysis of hair trace elements in children with autism spectrum disorders and communication disorders. Biol. Trace Elem. Res. 2017, 177, 215–223. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Skalnaya, M.G.; Simashkova, N.V.; Klyushnik, T.P.; Skalnaya, A.A.; Bjørklund, G.; Notova, S.V.; Kiyaeva, E.V.; Skalny, A.V. Association between catatonia and levels of hair and serum trace elements and minerals in autism spectrum disorder. Biomed. Pharmacother. 2019, 109, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, D.J.; Shou, X.J.; Zhang, J.S.; Meng, F.C.; Liu, Y.Q.; Han, S.P.; Zhang, R.; Jia, J.Z.; Wang, J.Y.; et al. Chinese children with autism: A multiple chemical elements profile in erythrocytes. Autism Res. 2018, 11, 834–845. [Google Scholar] [CrossRef]
- Zhai, Q.; Cen, S.; Jiang, J.; Zhao, J.; Zhang, H.; Chen, W. Disturbance of trace element and gut microbiota profiles as indicators of autism spectrum disorder: A pilot study of Chinese children. Environ. Res. 2019, 171, 501–509. [Google Scholar] [CrossRef]
- Skalny, A.V.; Simashkova, N.V.; Skalnaya, A.A.; Klyushnik, T.P.; Bjørklund, G.; Skalnaya, M.G.; Tinkov, A.A. Assessment of gender and age effects on serum and hair trace element levels in children with autism spectrum disorder. Metab. Brain Dis. 2017, 32, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Mold, M.; Umar, D.; King, A.; Exley, C. Aluminium in brain tissue in autism. J. Trace Elem. Med. Biol. 2018, 46, 76–82. [Google Scholar] [CrossRef]
- Exley, C.; Clarkson, E. Aluminium in human brain tissue from donors without neurodegenerative disease: A comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci. Rep. 2020, 10, 7770. [Google Scholar] [CrossRef]
- Skalny, A.V.; Simashkova, N.V.; Skalnaya, A.A.; Klyushnik, T.P.; Zhegalova, I.V.; Grabeklis, A.R.; Skalnaya, M.G.; Tinkov, A.A. Trace element levels are associated with neuroinflammatory markers in children with autistic spectrum disorder. J. Trace Elem. Med. Biol. 2018, 50, 622–628. [Google Scholar] [CrossRef]
- Cai, L.; Chen, T.; Yang, J.; Zhou, K.; Yan, X.; Chen, W.; Sun, L.; Li, L.; Qin, S.; Wang, P.; et al. Serum trace element differences between Schizophrenia patients and controls in the Han Chinese population. Sci. Rep. 2015, 5, 15013. [Google Scholar] [CrossRef] [Green Version]
- Gillin, J.C.; Carpenter, W.T.; Hambidge, K.M.; Wyatt, R.J.; Henkin, R.I. Zinc and copper in patients with schizophrenia. Encephale 1982, 8, 435–444. [Google Scholar]
- Joe, P.; Petrilli, M.; Malaspina, D.; Weissman, J. Zinc in schizophrenia: A meta-analysis. Gen. Hosp. Psychiatry 2018, 53, 19–24. [Google Scholar] [CrossRef]
- Ma, J.; Wang, B.; Gao, X.; Wu, H.; Wang, D.; Li, N.; Tan, J.; Wang, J.; Yan, L. A comparative study of the typical toxic metals in serum by patients of schizophrenia and healthy controls in China. Psychiatry Res. 2018, 269, 558–564. [Google Scholar] [CrossRef]
- Ma, J.; Yan, L.; Guo, T.; Yang, S.; Guo, C.; Liu, Y.; Xie, Q.; Wang, J. Association of typical toxic heavy metals with Schizophrenia. Int. J. Environ. Res. Public Health 2019, 16, 4200. [Google Scholar] [CrossRef] [Green Version]
- Modabbernia, A.; Velthorst, E.; Gennings, C.; De Haan, L.; Austin, C.; Sutterland, A.; Mollon, J.; Frangou, S.; Wright, R.; Arora, M.; et al. Early-life metal exposure and schizophrenia: A proof-of-concept study using novel tooth-matrix biomarkers. Eur. Psychiatry 2016, 36, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Vidović, B.; Dorđević, B.; Milovanović, S.; Škrivanj, S.; Pavlović, Z.; Stefanović, A.; Kotur-Stevuljević, J. Selenium, zinc, and copper plasma levels in patients with schizophrenia: Relationship with metabolic risk factors. Biol. Trace Elem. Res. 2013, 156, 22–28. [Google Scholar] [CrossRef]
- Yanik, M.; Kocyigit, A.; Tutkun, H.; Vural, H.; Herken, H. Plasma manganese, selenium, zinc, copper, and iron concentrations in patients with schizophrenia. Biol. Trace Elem. Res. 2004, 98, 109–117. [Google Scholar] [CrossRef]
- Adisetiyo, V.; Helpern, J.A. Brain iron: A promising noninvasive biomarker of attention-deficit/hyperactivity disorder that warrants further investigation. Biomark. Med. 2015, 9, 403–406. [Google Scholar] [CrossRef]
- Adisetiyo, V.; Jensen, J.H.; Tabesh, A.; Deardorff, R.L.; Fieremans, E.; Di Martino, A.; Gray, K.M.; Castellanos, F.X.; Helpern, J.A. Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: A noninvasive biomarker that responds to psychostimulant treatment? Radiology 2014, 272, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Arnold, L.E.; DiSilvestro, R.A. Zinc in attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2005, 15, 619–627. [Google Scholar] [CrossRef]
- Cortese, S.; Azoulay, R.; Castellanos, F.X.; Chalard, F.; Lecendreux, M.; Chechin, D.; Delorme, R.; Sebag, G.; Sbarbati, A.; Mouren, M.C.; et al. Brain iron levels in attention-deficit/hyperactivity disorder: A pilot MRI study. World J. Biol. Psychiatry 2012, 13, 223–231. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zeng, B.Y.; Li, D.J.; Cheng, Y.S.; Chen, T.Y.; Liang, H.Y.; Yang, W.C.; Lin, P.Y.; Chen, Y.W.; Tseng, P.T.; et al. Significantly lower serum and hair magnesium levels in children with attention deficit hyperactivity disorder than controls: A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 134–141. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; El-Mazary, A.A.; Maher, R.M.; Saber, M.M. Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Ital. J. Pediatr. 2011, 37, 60. [Google Scholar] [CrossRef] [Green Version]
- Scassellati, C.; Bonvicini, C.; Faraone, S.V.; Gennarelli, M. Biomarkers and attention-deficit/hyperactivity disorder: A systematic review and meta-analyses. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 1003–1019.e1020. [Google Scholar] [CrossRef] [PubMed]
- Garza-Lombo, C.; Posadas, Y.; Quintanar, L.; Gonsebatt, M.E.; Franco, R. Neurotoxicity linked to dysfunctional metal ion homeostasis and xenobiotic metal exposure: Redox signaling and oxidative stress. Antioxid. Redox Signal. 2018. [Google Scholar] [CrossRef] [Green Version]
- Abeyawardhane, D.L.; Lucas, H.R. Iron redox chemistry and implications in the Parkinson’s disease brain. Oxid. Med. Cell. Longev. 2019, 2019, 4609702. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Hofer, T.; Nurchi, V.M.; Aaseth, J. Iron and other metals in the pathogenesis of Parkinson’s disease: Toxic effects and possible detoxification. J. Inorg. Biochem. 2019, 199, 110717. [Google Scholar] [CrossRef]
- Bu, X.L.; Xiang, Y.; Guo, Y. The role of iron in amyotrophic Lateral sclerosis. Adv. Exp. Med. Biol. 2019, 1173, 145–152. [Google Scholar] [CrossRef]
- Rao, S.S.; Adlard, P.A. Untangling tau and iron: Exploring the interaction between iron and tau in neurodegeneration. Front. Mol. Neurosci. 2018, 11, 276. [Google Scholar] [CrossRef]
- Du, L.; Zhao, Z.; Cui, A.; Zhu, Y.; Zhang, L.; Liu, J.; Shi, S.; Fu, C.; Han, X.; Gao, W.; et al. Increased iron deposition on brain quantitative Susceptibility mapping correlates with decreased cognitive function in Alzheimer’s disease. ACS Chem. Neurosci. 2018, 9, 1849–1857. [Google Scholar] [CrossRef]
- Bondy, S.C. Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. Neurotoxicology 2016, 52, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Kandimalla, R.; Vallamkondu, J.; Corgiat, E.B.; Gill, K.D. Understanding aspects of aluminum exposure in Alzheimer’s disease development. Brain Pathol. 2016, 26, 139–154. [Google Scholar] [CrossRef]
- Maya, S.; Prakash, T.; Madhu, K.D.; Goli, D. Multifaceted effects of aluminium in neurodegenerative diseases: A review. Biomed. Pharmacother. 2016, 83, 746–754. [Google Scholar] [CrossRef]
- Chen, B.; Wen, X.; Jiang, H.; Wang, J.; Song, N.; Xie, J. Interactions between iron and alpha-synuclein pathology in Parkinson’s disease. Free Radic. Biol. Med. 2019, 141, 253–260. [Google Scholar] [CrossRef]
- Peres, T.V.; Schettinger, M.R.; Chen, P.; Carvalho, F.; Avila, D.S.; Bowman, A.B.; Aschner, M. Manganese-induced neurotoxicity: A review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol. Toxicol. 2016, 17, 57. [Google Scholar] [CrossRef] [Green Version]
- Binolfi, A.; Rasia, R.M.; Bertoncini, C.W.; Ceolin, M.; Zweckstetter, M.; Griesinger, C.; Jovin, T.M.; Fernandez, C.O. Interaction of alpha-synuclein with divalent metal ions reveals key differences: A link between structure, binding specificity and fibrillation enhancement. J. Am. Chem. Soc. 2006, 128, 9893–9901. [Google Scholar] [CrossRef]
- Huang, C.C. Parkinsonism induced by chronic manganese intoxication—An experience in Taiwan. Chang Gung Med. J. 2007, 30, 385–395. [Google Scholar]
- Guilarte, T.R.; Burton, N.C.; McGlothan, J.L.; Verina, T.; Zhou, Y.; Alexander, M.; Pham, L.; Griswold, M.; Wong, D.F.; Syversen, T.; et al. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): Implications to manganese-induced parkinsonism. J. Neurochem. 2008, 107, 1236–1247. [Google Scholar] [CrossRef] [Green Version]
- Sheykhansari, S.; Kozielski, K.; Bill, J.; Sitti, M.; Gemmati, D.; Zamboni, P.; Singh, A.V. Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: A review. Cell Death Dis. 2018, 9, 348. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic, A.; Stevic, Z.; Lavrnic, S.; Dakovic, M.; Bacic, G. Brain iron MRI: A biomarker for amyotrophic lateral sclerosis. J. Magn. Reson. Imaging 2013, 38, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Hadzhieva, M.; Kirches, E.; Mawrin, C. Review: Iron metabolism and the role of iron in neurodegenerative disorders. Neuropathol. Appl. Neurobiol. 2014, 40, 240–257. [Google Scholar] [CrossRef]
- Peters, T.L.; Beard, J.D.; Umbach, D.M.; Allen, K.; Keller, J.; Mariosa, D.; Sandler, D.P.; Schmidt, S.; Fang, F.; Ye, W.; et al. Blood levels of trace metals and amyotrophic lateral sclerosis. Neurotoxicology 2016, 54, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Kamel, F.; Umbach, D.M.; Hu, H.; Munsat, T.L.; Shefner, J.M.; Taylor, J.A.; Sandler, D.P. Lead exposure as a risk factor for amyotrophic lateral sclerosis. Neuro Degener. Dis. 2005, 2, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, Y. Genetic associations between voltage-gated calcium channels and autism spectrum disorder: A systematic review. Mol. Brain 2020, 13, 96. [Google Scholar] [CrossRef]
- Abraham, J.R.; Szoko, N.; Barnard, J.; Rubin, R.A.; Schlatzer, D.; Lundberg, K.; Li, X.; Natowicz, M.R. Proteomic investigations of autism brain identify known and novel pathogenetic processes. Sci. Rep. 2019, 9, 13118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal-Domènech, F.; Riquelme, G.; Pinacho, R.; Rodriguez-Mias, R.; Vera, A.; Monje, A.; Ferrer, I.; Callado, L.F.; Meana, J.J.; Villén, J.; et al. Calcium-binding proteins are altered in the cerebellum in schizophrenia. PLoS ONE 2020, 15, e0230400. [Google Scholar] [CrossRef]
- Dey, K.; Bazala, M.A.; Kuznicki, J. Targeting mitochondrial calcium pathways as a potential treatment against Parkinson’s disease. Cell Calcium 2020, 89, 102216. [Google Scholar] [CrossRef]
- Alzheimer’s Association Calcium Hypothesis, W.; Khachaturian, Z.S. Calcium hypothesis of Alzheimer’s disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimer’s Dement. 2017, 13, 178–182.e117. [Google Scholar] [CrossRef] [PubMed]
- Grosskreutz, J.; Van Den Bosch, L.; Keller, B.U. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium 2010, 47, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Q.; Zhang, M. Age, gender, and hemispheric differences in iron deposition in the human brain: An in vivo MRI study. NeuroImage 2008, 40, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, B.; Sourander, P. THE effect of age on the non-haemin iron in the human brain. J. Neurochem. 1958, 3, 41–51. [Google Scholar] [CrossRef]
- Loeffler, D.A.; Connor, J.R.; Juneau, P.L.; Snyder, B.S.; Kanaley, L.; DeMaggio, A.J.; Nguyen, H.; Brickman, C.M.; LeWitt, P.A. Transferrin and iron in normal, Alzheimer’s disease, and Parkinson’s disease brain regions. J. Neurochem. 1995, 65, 710–716. [Google Scholar] [CrossRef]
- Bartzokis, G.; Mintz, J.; Sultzer, D.; Marx, P.; Herzberg, J.S.; Phelan, C.K.; Marder, S.R. In vivo MR evaluation of age-related increases in brain iron. Am. J. Neuroradiol. 1994, 15, 1129–1138. [Google Scholar]
- Martin, W.R.W.; Ye, F.Q.; Allen, P.S. Increasing striatal iron content associated with normal aging. Mov. Disord. 1998, 13, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Zecca, L.; Zucca, F.A.; Toscani, M.; Adorni, F.; Giaveri, G.; Rizzio, E.; Gallorini, M. Iron, copper and their proteins in substantia nigra of human brain during aging. J. Radioanal. Nucl. Chem. 2005, 263, 733–737. [Google Scholar] [CrossRef]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhães, T.; Almeida, A. Anatomical region differences and age-related changes in copper, zinc, and manganese levels in the human brain. Biol. Trace Elem. Res. 2014, 161, 190–201. [Google Scholar] [CrossRef]
- Vasudevaraju, P.; Bharathi, G.; Jyothsna, T.; Shamasundar, N.M.; Subba Rao, K.; Balaraj, B.M.; Rao, K.S.J.; Sathyanarayana Rao, T.S. New evidence on iron, copper accumulation and zinc depletion and its correlation with DNA integrity in aging human brain regions. Indian J. Psychiatry 2010, 52, 140–144. [Google Scholar] [CrossRef]
- Bolognin, S.; Messori, L.; Zatta, P. Metal ion physiopathology in neurodegenerative disorders. Neuromol. Med. 2009, 11, 223–238. [Google Scholar] [CrossRef]
- Jellinger, K.A. Chapter one—The relevance of metals in the pathophysiology of neurodegeneration, pathological considerations. In International Review of Neurobiology; Bhatia, K.P., Schneider, S.A., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 110, pp. 1–47. [Google Scholar]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Gambling, L.; Lang, C.; McArdle, H.J. Fetal regulation of iron transport during pregnancy. Am. J. Clin. Nutr. 2011, 94, 1903S–1907S. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Fleming, M.D. The placenta: The forgotten essential organ of iron transport. Nutr. Rev. 2016, 74, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Moos, T. Developmental profile of non-heme iron distribution in the rat brain during ontogenesis. Dev. Brain Res. 1995, 87, 203–213. [Google Scholar] [CrossRef]
- Dawson, P.A.; Elliott, A.; Bowling, F.G. Sulphate in pregnancy. Nutrients 2015, 7, 1594–1606. [Google Scholar] [CrossRef] [Green Version]
- Semmler-Behnke, M.; Lipka, J.; Wenk, A.; Hirn, S.; Schaffler, M.; Tian, F.; Schmid, G.; Oberdorster, G.; Kreyling, W.G. Size dependent translocation and fetal accumulation of gold nanoparticles from maternal blood in the rat. Part Fibre Toxicol. 2014, 11, 33. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Campagnolo, L.; Fadeel, B. Interactions of engineered nanoparticles with organs protected by internal biological barriers. Small 2013, 9, 1557–1572. [Google Scholar] [CrossRef] [Green Version]
- McArdle, H.J.; Andersen, H.S.; Jones, H.; Gambling, L. Copper and iron transport across the placenta: Regulation and interactions. J. Neuroendocrinol. 2008, 20, 427–431. [Google Scholar] [CrossRef]
- Edel, J.; Sabbioni, E. Vanadium transport across placenta and milk of rats to the fetus and newborn. Biol. Trace Elem. Res. 1989, 22, 265–275. [Google Scholar] [CrossRef]
- Goyer, R.A. Transplacental transport of lead. Environ. Health Perspect. 1990, 89, 101–105. [Google Scholar] [CrossRef]
- Gundacker, C.; Hengstschlager, M. The role of the placenta in fetal exposure to heavy metals. Wien Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef]
- Iwai-Shimada, M.; Kameo, S.; Nakai, K.; Yaginuma-Sakurai, K.; Tatsuta, N.; Kurokawa, N.; Nakayama, S.F.; Satoh, H. Exposure profile of mercury, lead, cadmium, arsenic, antimony, copper, selenium and zinc in maternal blood, cord blood and placenta: The Tohoku Study of Child Development in Japan. Environ. Health Prev. Med. 2019, 24, 35. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Zhuang, T.; Shi, J.; Liang, Y.; Song, M. Heavy metals in maternal and cord blood in Beijing and their efficiency of placental transfer. J. Environ. Sci. (China) 2019, 80, 99–106. [Google Scholar] [CrossRef]
- Chen, Z.; Myers, R.; Wei, T.; Bind, E.; Kassim, P.; Wang, G.; Ji, Y.; Hong, X.; Caruso, D.; Bartell, T.; et al. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, M.; Yasutake, A.; Domingo, J.L.; Chan, H.M.; Kubota, M.; Murata, K. Relationships between trace element concentrations in chorionic tissue of placenta and umbilical cord tissue: Potential use as indicators for prenatal exposure. Environ. Int. 2013, 60, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moos, T.; Morgan, E.H. A morphological study of the developmentally regulated transport of iron into the brain. Dev. Neurosci. 2002, 24, 99–105. [Google Scholar] [CrossRef]
- Siddappa, A.J.; Rao, R.B.; Wobken, J.D.; Leibold, E.A.; Connor, J.R.; Georgieff, M.K. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J. Neurosci. Res. 2002, 68, 761–775. [Google Scholar] [CrossRef]
- Lonnerdal, B.; Georgieff, M.K.; Hernell, O. Developmental physiology of iron absorption, homeostasis, and metabolism in the healthy term infant. J. Pediatr. 2015, 167, S8–S14. [Google Scholar] [CrossRef] [Green Version]
- Hoet, P.H.; Bruske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and unknown health risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Geiser, M.; Rothen-Rutishauser, B.; Kapp, N.; Schurch, S.; Kreyling, W.; Schulz, H.; Semmler, M.; Im Hof, V.; Heyder, J.; Gehr, P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005, 113, 1555–1560. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Hu, C.; Chen, A.; Feng, X.; Liang, H.; Yin, S.; Zhang, G.; Shao, L. Neurotoxicity of nanoparticles entering the brain via sensory nerve-to-brain pathways: Injuries and mechanisms. Arch. Toxicol. 2020, 94, 1479–1495. [Google Scholar] [CrossRef] [Green Version]
- Tjalve, H.; Henriksson, J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology 1999, 20, 181–195. [Google Scholar]
- Wang, B.; Feng, W.Y.; Wang, M.; Shi, J.W.; Zhang, F.; Ouyang, H.; Zhao, Y.L.; Chai, Z.F.; Huang, Y.Y.; Xie, Y.N.; et al. Transport of intranasally instilled fine Fe2O3 particles into the brain: Micro-distribution, chemical states, and histopathological observation. Biol. Trace Elem. Res. 2007, 118, 233–243. [Google Scholar] [CrossRef]
- Petters, C.; Irrsack, E.; Koch, M.; Dringen, R. Uptake and metabolism of iron oxide nanoparticles in brain cells. Neurochem. Res. 2014, 39, 1648–1660. [Google Scholar] [CrossRef]
- Persson, E.; Henriksson, J.; Tjalve, H. Uptake of cobalt from the nasal mucosa into the brain via olfactory pathways in rats. Toxicol. Lett. 2003, 145, 19–27. [Google Scholar] [CrossRef]
- Sunderman, F.W., Jr. Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann. Clin. Lab. Sci. 2001, 31, 3–24. [Google Scholar]
- Perl, D.; Good, P. Uptake of aluminium into central nervous system along nasal-olfactory pathways. Lancet 1987, 329, 1028. [Google Scholar] [CrossRef]
- Ibanez, C.; Suhard, D.; Tessier, C.; Delissen, O.; Lestaevel, P.; Dublineau, I.; Gourmelon, P. Intranasal exposure to uranium results in direct transfer to the brain along olfactory nerve bundles. Neuropathol. Appl. Neurobiol. 2014, 40, 477–488. [Google Scholar] [CrossRef]
- Lucchini, R.G.; Dorman, D.C.; Elder, A.; Veronesi, B. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology 2012, 33, 838–841. [Google Scholar] [CrossRef] [Green Version]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Askri, D.; Ouni, S.; Galai, S.; Arnaud, J.; Chovelon, B.; Lehmann, S.G.; Sturm, N.; Sakly, M.; Seve, M.; Amara, S. Intranasal instillation of iron oxide nanoparticles induces inflammation and perturbation of trace elements and neurotransmitters, but not behavioral impairment in rats. Environ. Sci. Pollut. Res. Int. 2018. [Google Scholar] [CrossRef]
- Costa-Mallen, P.; Gatenby, C.; Friend, S.; Maravilla, K.R.; Hu, S.-C.; Cain, K.C.; Agarwal, P.; Anzai, Y. Brain iron concentrations in regions of interest and relation with serum iron levels in Parkinson disease. J. Neurol. Sci. 2017, 378, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wang, J.; Zhao, L.; Jin, H.; Fei, G.; Zhang, Y.; Zeng, M.; Zhong, C. Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson’s disease. Brain 2011, 134, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Logroscino, G.; Marder, K.; Graziano, J.; Freyer, G.; Slavkovich, V.; LoIacono, N.; Cote, L.; Mayeux, R. Altered systemic iron metabolism in Parkinson’s disease. Neurology 1997, 49, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.D.; Dey, R.D. Identification and neuropeptide content of trigeminal neurons innervating the rat nasal epithelium. Neuroscience 1998, 83, 591–599. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s disease: A dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007, 33, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Chounlamountry, K.; Boyer, B.; Penalba, V.; François-Bellan, A.M.; Bosler, O.; Kessler, J.P.; Strube, C. Remodeling of glial coverage of glutamatergic synapses in the rat nucleus tractus solitarii after ozone inhalation. J. Neurochem. 2015, 134, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.K.; Birrell, M.A.; Adcock, J.J.; Wortley, M.A.; Dubuis, E.D.; Chen, S.; McGilvery, C.M.; Hu, S.; Shaffer, M.S.P.; Bonvini, S.J.; et al. Mechanistic link between diesel exhaust particles and respiratory reflexes. J. Allergy Clin. Immunol. 2018, 141, 1074–1084.e1079. [Google Scholar] [CrossRef] [Green Version]
- Villarreal-Calderon, R.; Torres-Jardón, R.; Palacios-Moreno, J.; Osnaya, N.; Pérez-Guillé, B.; Maronpot, R.R.; Reed, W.; Zhu, H.; Calderón-Garcidueñas, L. Urban air pollution targets the dorsal vagal complex and dark chocolate offers neuroprotection. Int. J. Toxicol. 2010, 29, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.K.; Rasool, C.G.; Bradley, W.G.; Munsat, T.L. Retrograde axonal transport of lead in rat sciatic nerve. Neurology 1981, 31, 612. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Bell, R.; Jones, A.L. Endogenous doesn’t always mean innocuous: A scoping review of iron toxicity by inhalation. J. Toxicol. Environ. Health B 2020, 23, 107–136. [Google Scholar] [CrossRef]
- Verkhlyutov, V.M.; Gapienko, G.V.; Ushakov, V.L.; Portnova, G.V.; Verkhlyutova, I.A.; Anisimov, N.V.; Pirogov, Y.A. MRI morphometry of the cerebral ventricles in patients with attention deficit hyperactivity disorder. Neurosci. Behav. Physiol. 2010, 40, 295–303. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, T.; Cao, Q.; Wang, Y. Characterizing anatomic differences in boys with attention-deficit/hyperactivity disorder with the use of deformation-based morphometry. AJNR Am. J. Neuroradiol. 2007, 28, 543–547. [Google Scholar]
- Cheung, C.; Yu, K.; Fung, G.; Leung, M.; Wong, C.; Li, Q.; Sham, P.; Chua, S.; McAlonan, G. Autistic disorders and schizophrenia: Related or remote? An anatomical likelihood estimation. PLoS ONE 2010, 5, e12233. [Google Scholar] [CrossRef] [Green Version]
- Cuesta, M.J.; Lecumberri, P.; Cabada, T.; Moreno-Izco, L.; Ribeiro, M.; Lopez-Ilundain, J.M.; Peralta, V.; Lorente-Omenaca, R.; Sanchez-Torres, A.M.; Gomez, M. Basal ganglia and ventricle volume in first-episode psychosis. A family and clinical study. Psychiatry Res. Neuroimaging 2017, 269, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Del Re, E.C.; Konishi, J.; Bouix, S.; Blokland, G.A.; Mesholam-Gately, R.I.; Goldstein, J.; Kubicki, M.; Wojcik, J.; Pasternak, O.; Seidman, L.J.; et al. Enlarged lateral ventricles inversely correlate with reduced corpus callosum central volume in first episode schizophrenia: Association with functional measures. Brain Imaging Behav. 2016, 10, 1264–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milovanovic, N.; Damjanovic, A.; Puskas, L.; Milovanovic, S.; Barisic, J.; Malis, M.; Stankovic, G.; Rankovic, A.; Latas, M.; Filipovic, B.F.; et al. Reliability of the bicaudate parameter in the revealing of the enlarged lateral Ventricles in schizophrenia patients. Psychiatr. Danub. 2018, 30, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Fukunaga, M.; Yamashita, F.; Koshiyama, D.; Yamamori, H.; Ohi, K.; Yasuda, Y.; Fujimoto, M.; Watanabe, Y.; Yahata, N.; et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol. Psychiatry 2016, 21, 1460–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Erp, T.G.; Greve, D.N.; Rasmussen, J.; Turner, J.; Calhoun, V.D.; Young, S.; Mueller, B.; Brown, G.G.; McCarthy, G.; Glover, G.H.; et al. A multi-scanner study of subcortical brain volume abnormalities in schizophrenia. Psychiatry Res. 2014, 222, 10–16. [Google Scholar] [CrossRef] [Green Version]
- van Erp, T.G.; Hibar, D.P.; Rasmussen, J.M.; Glahn, D.C.; Pearlson, G.D.; Andreassen, O.A.; Agartz, I.; Westlye, L.T.; Haukvik, U.K.; Dale, A.M.; et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol. Psychiatry 2016, 21, 585. [Google Scholar] [CrossRef]
- Lange, N.; Travers, B.G.; Bigler, E.D.; Prigge, M.B.; Froehlich, A.L.; Nielsen, J.A.; Cariello, A.N.; Zielinski, B.A.; Anderson, J.S.; Fletcher, P.T.; et al. Longitudinal volumetric brain changes in autism spectrum disorder ages 6-35 years. Autism Res. 2015, 8, 82–93. [Google Scholar] [CrossRef]
- Aoki, Y.; Yoncheva, Y.N.; Chen, B.; Nath, T.; Sharp, D.; Lazar, M.; Velasco, P.; Milham, M.P.; Di Martino, A. Association of white matter structure with autism spectrum disorder and attention-deficit/hyperactivity disorder. JAMA Psychiatry 2017. [Google Scholar] [CrossRef]
- Di, X.; Azeez, A.; Li, X.; Haque, E.; Biswal, B.B. Disrupted focal white matter integrity in autism spectrum disorder: A voxel-based meta-analysis of diffusion tensor imaging studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 82, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Fingher, N.; Dinstein, I.; Ben-Shachar, M.; Haar, S.; Dale, A.M.; Eyler, L.; Pierce, K.; Courchesne, E. Toddlers later diagnosed with autism exhibit multiple structural abnormalities in temporal corpus callosum fibers. Cortex 2017, 97, 291–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karahanoglu, F.I.; Baran, B.; Nguyen, Q.T.H.; Meskaldji, D.E.; Yendiki, A.; Vangel, M.; Santangelo, S.L.; Manoach, D.S. Diffusion-weighted imaging evidence of altered white matter development from late childhood to early adulthood in autism spectrum disorder. Neuroimage Clin. 2018, 19, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, M.D.; Hudziak, J.J.; Ing, A.; Chaarani, B.; Barker, E.; Jia, T.; Lemaitre, H.; Watts, R.; Orr, C.; Spechler, P.A.; et al. White matter microstructure is associated with hyperactive/inattentive symptomatology and polygenic risk for attention-deficit/hyperactivity disorder in a population-based sample of adolescents. Neuropsychopharmacology 2019, 44, 1597–1603. [Google Scholar] [CrossRef]
- Cropley, V.L.; Klauser, P.; Lenroot, R.K.; Bruggemann, J.; Sundram, S.; Bousman, C.; Pereira, A.; Di Biase, M.A.; Weickert, T.W.; Weickert, C.S.; et al. Accelerated gray and white matter deterioration with age in schizophrenia. Am. J. Psychiatry 2017, 174, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitt, J.J.; Nestor, P.G.; Levin, L.; Pelavin, P.; Lin, P.; Kubicki, M.; McCarley, R.W.; Shenton, M.E.; Rathi, Y. Reduced structural connectivity in frontostriatal white matter tracts in the associative loop in schizophrenia. Am. J. Psychiatry 2017, 174, 1102–1111. [Google Scholar] [CrossRef]
- Price, G.; Cercignani, M.; Parker, G.J.; Altmann, D.R.; Barnes, T.R.; Barker, G.J.; Joyce, E.M.; Ron, M.A. Abnormal brain connectivity in first-episode psychosis: A diffusion MRI tractography study of the corpus callosum. Neuroimage 2007, 35, 458–466. [Google Scholar] [CrossRef]
- Seal, M.L.; Yucel, M.; Fornito, A.; Wood, S.J.; Harrison, B.J.; Walterfang, M.; Pell, G.S.; Pantelis, C. Abnormal white matter microstructure in schizophrenia: A voxelwise analysis of axial and radial diffusivity. Schizophr. Res. 2008, 101, 106–110. [Google Scholar] [CrossRef]
- Siffredi, V.; Anderson, V.; McIlroy, A.; Wood, A.G.; Leventer, R.J.; Spencer-Smith, M.M. A neuropsychological profile for agenesis of the corpus callosum? Cognitive, academic, executive, social, and behavioral functioning in school-age children. J. Int. Neuropsychol. Soc. 2018, 24, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.B.; Paul, L.K.; Brown, W.S. Emotional intelligence in agenesis of the corpus callosum. Arch. Clin. Neuropsychol. 2017, 32, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Blackmon, K.; Ben-Avi, E.; Wang, X.; Pardoe, H.R.; Di Martino, A.; Halgren, E.; Devinsky, O.; Thesen, T.; Kuzniecky, R. Periventricular white matter abnormalities and restricted repetitive behavior in autism spectrum disorder. NeuroImage Clin. 2016, 10, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Chen, B. Abnormal regions in functional connectivity of chronic schizophrenia. Int. J. Neurosci. 2018, 128, 1150–1156. [Google Scholar] [CrossRef]
- Frith, C. Is autism a disconnection disorder? Lancet Neurol. 2004, 3, 577. [Google Scholar] [CrossRef]
- Geschwind, D.H.; Levitt, P. Autism spectrum disorders: Developmental disconnection syndromes. Curr. Opin. Neurobiol. 2007, 17, 103–111. [Google Scholar] [CrossRef]
- Cai, W.; Chen, T.; Szegletes, L.; Supekar, K.; Menon, V. Aberrant time-varying cross-network interactions in children with attention-deficit/hyperactivity disorder and the relation to attention deficits. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 263–273. [Google Scholar] [CrossRef]
- Griffa, A.; Baumann, P.S.; Klauser, P.; Mullier, E.; Cleusix, M.; Jenni, R.; van den Heuvel, M.P.; Do, K.Q.; Conus, P.; Hagmann, P. Brain connectivity alterations in early psychosis: From clinical to neuroimaging staging. Transl. Psychiatry 2019, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Joo, S.W.; Yoon, W.; Shon, S.H.; Kim, H.; Cha, S.; Park, K.J.; Lee, J. Altered white matter connectivity in patients with schizophrenia: An investigation using public neuroimaging data from SchizConnect. PLoS ONE 2018, 13, e0205369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Li, S.; Wang, X.; Gong, Y.; Yao, L.; Xiao, Y.; Liu, J.; Keedy, S.K.; Gong, Q.; Sweeney, J.A.; et al. Abnormal dynamic functional connectivity between speech and auditory areas in schizophrenia patients with auditory hallucinations. Neuroimage Clin. 2018, 19, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Liu, F.; Guo, W.; Chen, J.; Su, Q.; Zhang, Z.; Li, H.; Fan, X.; Zhao, J. Disrupted asymmetry of inter- and intra-hemispheric functional connectivity in patients with drug-naive, first-episode schizophrenia and their unaffected siblings. EBioMedicine 2018, 36, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Cochran, D.M.; Sikoglu, E.M.; Hodge, S.M.; Edden, R.A.; Foley, A.; Kennedy, D.N.; Moore, C.M.; Frazier, J.A. Relationship among glutamine, gamma-aminobutyric acid, and social cognition in autism spectrum disorders. J. Child Adolesc. Psychopharmacol. 2015, 25, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Drenthen, G.S.; Barendse, E.M.; Aldenkamp, A.P.; van Veenendaal, T.M.; Puts, N.A.; Edden, R.A.; Zinger, S.; Thoonen, G.; Hendriks, M.P.; Kessels, R.P.; et al. Altered neurotransmitter metabolism in adolescents with high-functioning autism. Psychiatry Res. 2016, 256, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Ford, T.C.; Nibbs, R.; Crewther, D.P. Glutamate/GABA+ ratio is associated with the psychosocial domain of autistic and schizotypal traits. PLoS ONE 2017, 12, e0181961. [Google Scholar] [CrossRef] [Green Version]
- Rosch, K.S.; Mostofsky, S.H.; Nebel, M.B. ADHD-related sex differences in fronto-subcortical intrinsic functional connectivity and associations with delay discounting. J. Neurodev. Disord. 2018, 10, 34. [Google Scholar] [CrossRef]
- Zepf, F.D.; Bubenzer-Busch, S.; Runions, K.C.; Rao, P.; Wong, J.W.Y.; Mahfouda, S.; Morandini, H.A.E.; Stewart, R.M.; Moore, J.K.; Biskup, C.S.; et al. Functional connectivity of the vigilant-attention network in children and adolescents with attention-deficit/hyperactivity disorder. Brain Cogn. 2019, 131, 56–65. [Google Scholar] [CrossRef]
- Al-Otaish, H.; Al-Ayadhi, L.; Bjorklund, G.; Chirumbolo, S.; Urbina, M.A.; El-Ansary, A. Relationship between absolute and relative ratios of glutamate, glutamine and GABA and severity of autism spectrum disorder. Metab. Brain Dis. 2018. [Google Scholar] [CrossRef]
- Avissar, M.; Javitt, D. Mismatch negativity: A simple and useful biomarker of N-methyl-d-aspartate receptor (NMDAR)-type glutamate dysfunction in schizophrenia. Schizophr. Res. 2018, 191, 1–4. [Google Scholar] [CrossRef]
- Bauer, J.; Werner, A.; Kohl, W.; Kugel, H.; Shushakova, A.; Pedersen, A.; Ohrmann, P. Hyperactivity and impulsivity in adult attention-deficit/hyperactivity disorder is related to glutamatergic dysfunction in the anterior cingulate cortex. World J. Biol. Psychiatry 2018, 19, 538–546. [Google Scholar] [CrossRef]
- Chang, J.P.; Lane, H.Y.; Tsai, G.E. Attention deficit hyperactivity disorder and N-methyl-D-aspartate (NMDA) dysregulation. Curr. Pharm. Des. 2014, 20, 5180–5185. [Google Scholar] [CrossRef]
- Duarte, J.M.N.; Xin, L. Magnetic resonance spectroscopy in schizophrenia: Evidence for glutamatergic dysfunction and impaired energy metabolism. Neurochem. Res. 2019, 44, 102–116. [Google Scholar] [CrossRef] [Green Version]
- Hegarty, J.P., 2nd; Weber, D.J.; Cirstea, C.M.; Beversdorf, D.Q. Cerebro-cerebellar functional connectivity is associated with cerebellar excitation-inhibition balance in autism spectrum disorder. J. Autism Dev. Disord. 2018, 48, 3460–3473. [Google Scholar] [CrossRef]
- Hoftman, G.D.; Dienel, S.J.; Bazmi, H.H.; Zhang, Y.; Chen, K.; Lewis, D.A. Altered gradients of glutamate and gamma-aminobutyric acid transcripts in the cortical visuospatial working Memory network in schizophrenia. Biol. Psychiatry 2017. [Google Scholar] [CrossRef]
- Jelen, L.A.; King, S.; Horne, C.M.; Lythgoe, D.J.; Young, A.H.; Stone, J.M. Functional magnetic resonance spectroscopy in patients with schizophrenia and bipolar affective disorder: Glutamate dynamics in the anterior cingulate cortex during a working memory task. Eur. Neuropsychopharmacol. 2019, 29, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Uno, Y.; Coyle, J.T. Glutamate hypothesis in schizophrenia. Psychiatry Clin. Neurosci. 2019, 73, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Batool, S.; Raza, H.; Zaidi, J.; Riaz, S.; Hasan, S.; Syed, N.I. Synapse formation: From cellular and molecular mechanisms to neurodevelopmental and neurodegenerative disorders. J. Neurophysiol. 2019, 121, 1381–1397. [Google Scholar] [CrossRef]
- Bennett, M.R.; Lagopoulos, J. Neurodevelopmental sequelae associated with gray and white matter changes and their cellular basis: A comparison between autism spectrum disorder, ADHD and dyslexia. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2015, 46, 132–143. [Google Scholar] [CrossRef]
- Bunney, B.G.; Potkin, S.G.; Bunney, W.E., Jr. New morphological and neuropathological findings in schizophrenia: A neurodevelopmental perspective. Clin. Neurosci. 1995, 3, 81–88. [Google Scholar]
- Connor, C.M.; Crawford, B.C.; Akbarian, S. White matter neuron alterations in schizophrenia and related disorders. Int. J. Dev. Neurosci. 2011, 29, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, J.H.; Jarskog, L.F.; Vadlamudi, S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J. Neuroimmunol. 2005, 159, 106–112. [Google Scholar] [CrossRef]
- Glantz, L.A.; Gilmore, J.H.; Lieberman, J.A.; Jarskog, L.F. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr. Res. 2006, 81, 47–63. [Google Scholar] [CrossRef]
- Moretto, E.; Murru, L.; Martano, G.; Sassone, J.; Passafaro, M. Glutamatergic synapses in neurodevelopmental disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84, 328–342. [Google Scholar] [CrossRef]
- Rankovic, M.; Zweckstetter, M. Upregulated levels and pathological aggregation of abnormally phosphorylated Tau-protein in children with neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2019, 98, 1–9. [Google Scholar] [CrossRef]
- Vilalta, A.; Brown, G.C. Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J. 2018, 285, 3566–3575. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Alberts, I.; Li, X. The apoptotic perspective of autism. Int. J. Dev. Neurosci. 2014, 36, 13–18. [Google Scholar] [CrossRef]
- King, B.H.; Lord, C. Is schizophrenia on the autism spectrum? Brain Res. 2011, 1380, 34–41. [Google Scholar] [CrossRef]
- Meyer, U.; Feldon, J.; Dammann, O. Schizophrenia and autism: Both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatric Res. 2011, 69, 26R–33R. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.H.; Goldstein, B.I. Inflammation in children and adolescents with neuropsychiatric disorders: A systematic review. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 274–296. [Google Scholar] [CrossRef]
- Prata, J.; Santos, S.G.; Almeida, M.I.; Coelho, R.; Barbosa, M.A. Bridging autism spectrum disorders and schizophrenia through inflammation and biomarkers—Pre-clinical and clinical investigations. J. Neuroinflammation 2017, 14, 179. [Google Scholar] [CrossRef] [Green Version]
- Rogers, J.C.; De Brito, S.A. Cortical and subcortical gray matter volume in youths with conduct problems: A meta-analysis. JAMA Psychiatry 2016, 73, 64–72. [Google Scholar] [CrossRef]
- Smaga, I.; Niedzielska, E.; Gawlik, M.; Moniczewski, A.; Krzek, J.; Przegalinski, E.; Pera, J.; Filip, M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol. Rep. 2015, 67, 569–580. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yu, Z.; Sakai, M.; Tomita, H. Linking activation of microglia and peripheral monocytic cells to the pathophysiology of psychiatric disorders. Front. Cell. Neurosci. 2016, 10, 144. [Google Scholar] [CrossRef]
- Krzysztofik, K.; Otrębski, W. Measurement tools of autism syndrome severity and selected neurocognitive processes in individuals with ASD. Psychiatr. Pol. 2018, 52, 641–650. [Google Scholar] [CrossRef]
- Leffa, D.T.; Torres, I.L.S.; Rohde, L.A. A Review on the role of inflammation in attention-deficit/hyperactivity disorder. Neuroimmunomodulation 2018, 25, 328–333. [Google Scholar] [CrossRef]
- Maas, D.A.; Vallès, A.; Martens, G.J.M. Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl. Psychiatry 2017, 7, e1171. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, Y.; Chiba, K. Involvement of neuroinflammation during brain development in social cognitive deficits in autism spectrum disorder and schizophrenia. J. Pharmacol. Exp. Ther. 2016, 358, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Rommelse, N.N.; Geurts, H.M.; Franke, B.; Buitelaar, J.K.; Hartman, C.A. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci. Biobehav. Rev. 2011, 35, 1363–1396. [Google Scholar] [CrossRef]
- Upthegrove, R.; Khandaker, G.M. Cytokines, oxidative stress and cellular markers of inflammation in schizophrenia. Curr. Top. Behav. Neurosci. 2020, 44, 49–66. [Google Scholar] [CrossRef]
- Waligora, A.; Waligora, S.; Kozarska, M.; Damasiewicz-Bodzek, A.; Gorczyca, P.; Tyrpien-Golder, K. Autism spectrum disorder (ASD)—Biomarkers of oxidative stress and methylation and transsulfuration cycle. Psychiatr. Pol. 2019, 53, 771–788. [Google Scholar] [CrossRef]
- Yui, K.; Kawasaki, Y.; Yamada, H.; Ogawa, S. Oxidative stress and nitric oxide in autism spectrum disorder and other neuropsychiatric disorders. CNS Neurol. Disord. Drug Targets 2016, 15, 587–596. [Google Scholar] [CrossRef]
- Andrews, G.; Pine, D.S.; Hobbs, M.J.; Anderson, T.M.; Sunderland, M. Neurodevelopmental disorders: Cluster 2 of the proposed meta-structure for DSM-V and ICD-11. Psychol. Med. 2009, 39, 2013–2023. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.Y.; Ragland, J.D.; Carter, C.S. Memory and cognition in schizophrenia. Mol. Psychiatry 2019, 24, 633–642. [Google Scholar] [CrossRef]
- Ha, S.; Hu, H.; Roussos-Ross, D.; Haidong, K.; Roth, J.; Xu, X. The effects of air pollution on adverse birth outcomes. Environ. Res. 2014, 134, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Hollis, F.; Kanellopoulos, A.K.; Bagni, C. Mitochondrial dysfunction in autism spectrum disorder: Clinical features and perspectives. Curr. Opin. Neurobiol. 2017, 45, 178–187. [Google Scholar] [CrossRef]
- Legido, A.; Jethva, R.; Goldenthal, M.J. Mitochondrial dysfunction in autism. Semin. Pediatr. Neurol. 2013, 20, 163–175. [Google Scholar] [CrossRef]
- Noreika, V.; Falter, C.M.; Rubia, K. Timing deficits in attention-deficit/hyperactivity disorder (ADHD): Evidence from neurocognitive and neuroimaging studies. Neuropsychologia 2013, 51, 235–266. [Google Scholar] [CrossRef]
- Robbins, T.W. Pharmacological treatment of cognitive deficits in nondementing mental health disorders. Dialogues Clin. Neurosci. 2019, 21, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Sheffield, J.M.; Karcher, N.R.; Barch, D.M. Cognitive deficits in psychotic disorders: A lifespan perspective. Neuropsychol. Rev. 2018, 28, 509–533. [Google Scholar] [CrossRef]
- Sheppard, D.P.; Bruineberg, J.P.; Kretschmer-Trendowicz, A.; Altgassen, M. Prospective memory in autism: Theory and literature review. Clin. Neuropsychol. 2018, 32, 748–782. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.; Mollinedo-Gajate, I.; Peñagarikano, O. Neural circuits for social cognition: Implications for autism. Neuroscience 2018, 370, 148–162. [Google Scholar] [CrossRef]
- Marazziti, D.; Baroni, S.; Picchetti, M.; Landi, P.; Silvestri, S.; Vatteroni, E.; Catena Dell’Osso, M. Psychiatric disorders and mitochondrial dysfunctions. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 270–275. [Google Scholar]
- Mier, D.; Kirsch, P. Social-cognitive deficits in schizophrenia. Curr. Top. Behav. Neurosci. 2017, 30, 397–409. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Venkatasubramanian, G.; Berk, M.; Debnath, M. Mitochondrial dysfunction in schizophrenia: Pathways, mechanisms and implications. Neurosci. Biobehav. Rev. 2015, 48, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Buys, N. Early executive function deficit in preterm children and its association with neurodevelopmental disorders in childhood: A literature review. Int. J. Adolesc. Med. Health 2012, 24, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uittenbogaard, M.; Chiaramello, A. Mitochondrial biogenesis: A therapeutic target for neurodevelopmental disorders and neurodegenerative diseases. Curr. Pharm. Des. 2014, 20, 5574–5593. [Google Scholar] [CrossRef] [Green Version]
- Yui, K.; Sato, A.; Imataka, G. Mitochondrial dysfunction and its relationship with mTOR signaling and oxidative damage in autism spectrum disorders. Mini Rev. Med. Chem. 2015, 15, 373–389. [Google Scholar] [CrossRef]
- Mahone, E.M.; Denckla, M.B. Attention-deficit/hyperactivity disorder: A historical neuropsychological perspective. J. Int. Neuropsychol. Soc. 2017, 23, 916–929. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Singh, A.; Nthenge-Ngumbau, D.N.; Rajamma, U.; Sinha, S.; Mukhopadhyay, K.; Mohanakumar, K.P. Attention deficit-hyperactivity disorder suffers from mitochondrial dysfunction. BBA Clin. 2016, 6, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, M.; Cheng, H.; Mishra, V.; Gong, G.; Mosconi, M.W.; Sweeney, J.; Peng, Y.; Huang, H. Atypical age-dependent effects of autism on white matter microstructure in children of 2–7 years. Hum. Brain Mapp. 2016, 37, 819–832. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.H.; Greenspan, K.S.; van Erp, T.G.M. Pallidum and lateral ventricle volume enlargement in autism spectrum disorder. Psychiatry Res. Neuroimaging 2016, 252, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Gaffney, G.R.; Kuperman, S.; Tsai, L.Y.; Minchin, S. Forebrain structure in infantile autism. J. Am. Acad. Child Adolesc. Psychiatry 1989, 28, 534–537. [Google Scholar] [CrossRef]
- Piven, J.; Arndt, S.; Bailey, J.; Havercamp, S.; Andreasen, N.C.; Palmer, P. An MRI study of brain size in autism. Am. J. Psychiatry 1995, 152, 1145–1149. [Google Scholar]
- Bouziane, C.; Caan, M.W.A.; Tamminga, H.G.H.; Schrantee, A.; Bottelier, M.A.; de Ruiter, M.B.; Kooij, S.J.J.; Reneman, L. ADHD and maturation of brain white matter: A DTI study in medication naive children and adults. Neuroimage Clin. 2018, 17, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; McAnulty, G.; Hamoda, H.M.; Sarill, K.; Karmacharya, S.; Gagoski, B.; Ning, L.; Grant, P.E.; Shenton, M.E.; Waber, D.P.; et al. Detecting microstructural white matter abnormalities of frontal pathways in children with ADHD using advanced diffusion models. Brain Imaging Behav. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, T.; Acer, N.; Koseoglu, E.; Zararsiz, G.; Sonmez, A.; Gumus, K.; Kurtoglu, E. Total intracranial and lateral ventricle volumes measurement in Alzheimer’s disease: A methodological study. J. Clin. Neurosci. 2016, 34, 133–139. [Google Scholar] [CrossRef]
- Bartos, A.; Gregus, D.; Ibrahim, I.; Tintera, J. Brain volumes and their ratios in Alzheimer s disease on magnetic resonance imaging segmented using Freesurfer 6.0. Psychiatry Res. Neuroimaging 2019, 287, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Marizzoni, M.; Ferrari, C.; Jovicich, J.; Albani, D.; Babiloni, C.; Cavaliere, L.; Didic, M.; Forloni, G.; Galluzzi, S.; Hoffmann, K.T.; et al. Predicting and tracking short term disease progression in amnestic mild cognitive impairment patients with prodromal Alzheimer’s disease: Structural brain biomarkers. J. Alzheimers Dis. 2019, 69, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Tan, M.Z.; Qiu, A. CSF and brain structural imaging markers of the Alzheimer’s pathological cascade. PLoS ONE 2012, 7, e47406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, M.M.; Smith, A.B.; Styner, M.; Gu, H.; Poole, R.; Zhu, H.; Li, Y.; Barbero, X.; Gouttard, S.; McKeown, M.J.; et al. Asymmetrical lateral ventricular enlargement in Parkinson’s disease. Eur. J. Neurol. 2009, 16, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Costa, J.F.; Carratala-Bosca, S.; Tembl, J.I.; Fornes-Ferrer, V.; Perez-Tur, J.; Marti-Bonmati, L.; Sevilla, T. The width of the third ventricle associates with cognition and behaviour in motor neuron disease. Acta Neurol. Scand. 2019, 139, 118–127. [Google Scholar] [CrossRef]
- Westeneng, H.J.; Verstraete, E.; Walhout, R.; Schmidt, R.; Hendrikse, J.; Veldink, J.H.; van den Heuvel, M.P.; van den Berg, L.H. Subcortical structures in amyotrophic lateral sclerosis. Neurobiol. Aging 2015, 36, 1075–1082. [Google Scholar] [CrossRef]
- Sinnecker, T.; Ruberte, E.; Schädelin, S.; Canova, V.; Amann, M.; Naegelin, Y.; Penner, I.K.; Müller, J.; Kuhle, J.; Décard, B.; et al. New and enlarging white matter lesions adjacent to the ventricle system and thalamic atrophy are independently associated with lateral ventricular enlargement in multiple sclerosis. J. Neurol. 2020, 267, 192–202. [Google Scholar] [CrossRef]
- Gaetano, L.; Häring, D.A.; Radue, E.W.; Mueller-Lenke, N.; Thakur, A.; Tomic, D.; Kappos, L.; Sprenger, T. Fingolimod effect on gray matter, thalamus, and white matter in patients with multiple sclerosis. Neurology 2018, 90, e1324–e1332. [Google Scholar] [CrossRef]
- Kim, J.H.; Cumming, P.; Son, Y.D.; Kim, H.K.; Joo, Y.H.; Kim, J.H. Altered connectivity between striatal and extrastriatal regions in patients with schizophrenia on maintenance antipsychotics: An [(18) F]fallypride PET and functional MRI study. Synapse 2018, 72, e22064. [Google Scholar] [CrossRef] [Green Version]
- Mayo, C.D.; Mazerolle, E.L.; Ritchie, L.; Fisk, J.D.; Gawryluk, J.R. Longitudinal changes in microstructural white matter metrics in Alzheimer’s disease. Neuroimage Clin. 2017, 13, 330–338. [Google Scholar] [CrossRef]
- Tang, X.; Qin, Y.; Zhu, W.; Miller, M.I. Surface-based vertexwise analysis of morphometry and microstructural integrity for white matter tracts in diffusion tensor imaging: With application to the corpus callosum in Alzheimer’s disease. Hum. Brain Mapp. 2017, 38, 1875–1893. [Google Scholar] [CrossRef] [Green Version]
- Tomimoto, H.; Lin, J.X.; Matsuo, A.; Ihara, M.; Ohtani, R.; Shibata, M.; Miki, Y.; Shibasaki, H. Different mechanisms of corpus callosum atrophy in Alzheimer’s disease and vascular dementia. J. Neurol. 2004, 251, 398–406. [Google Scholar] [CrossRef]
- Bledsoe, I.O.; Stebbins, G.T.; Merkitch, D.; Goldman, J.G. White matter abnormalities in the corpus callosum with cognitive impairment in Parkinson disease. Neurology 2018, 91, e2244–e2255. [Google Scholar] [CrossRef]
- Goldman, J.G.; Bledsoe, I.O.; Merkitch, D.; Dinh, V.; Bernard, B.; Stebbins, G.T. Corpus callosal atrophy and associations with cognitive impairment in Parkinson disease. Neurology 2017, 88, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Gorges, M.; Muller, H.P.; Liepelt-Scarfone, I.; Storch, A.; Dodel, R.; Consortium, L.; Hilker-Roggendorf, R.; Berg, D.; Kunz, M.S.; Kalbe, E.; et al. Structural brain signature of cognitive decline in Parkinson’s disease: DTI-based evidence from the LANDSCAPE study. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419843447. [Google Scholar] [CrossRef]
- Koirala, N.; Anwar, A.R.; Ciolac, D.; Glaser, M.; Pintea, B.; Deuschl, G.; Muthuraman, M.; Groppa, S. Alterations in white matter network and microstructural integrity differentiate Parkinson’s disease patients and healthy subjects. Front. Aging Neurosci. 2019, 11, 191. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.T.; Zhang, Y.X.; Zheng, Y.; Fang, W.; Ding, M.P. Callosal lesions on magnetic resonance imaging with multiple sclerosis, neuromyelitis optica spectrum disorder and acute disseminated encephalomyelitis. Mult. Scler. Relat. Disord. 2019, 32, 41–45. [Google Scholar] [CrossRef]
- Garg, N.; Reddel, S.W.; Miller, D.H.; Chataway, J.; Riminton, D.S.; Barnett, Y.; Masters, L.; Barnett, M.H.; Hardy, T.A. The corpus callosum in the diagnosis of multiple sclerosis and other CNS demyelinating and inflammatory diseases. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1374–1382. [Google Scholar] [CrossRef]
- Gonçalves, L.I.; Dos Passos, G.R.; Conzatti, L.P.; Burger, J.L.P.; Tomasi, G.H.; Zandoná, M.; Azambuja, L.S.; Gomes, I.; Franco, A.; Sato, D.K.; et al. Correlation between the corpus callosum index and brain atrophy, lesion load, and cognitive dysfunction in multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 20, 154–158. [Google Scholar] [CrossRef]
- Granberg, T.; Bergendal, G.; Shams, S.; Aspelin, P.; Kristoffersen-Wiberg, M.; Fredrikson, S.; Martola, J. MRI-defined corpus callosal atrophy in multiple sclerosis: A comparison of volumetric measurements, corpus callosum area and index. J. Neuroimaging 2015, 25, 996–1001. [Google Scholar] [CrossRef]
- Gabel, M.C.; Broad, R.J.; Young, A.L.; Abrahams, S.; Bastin, M.E.; Menke, R.A.L.; Al-Chalabi, A.; Goldstein, L.H.; Tsermentseli, S.; Alexander, D.C.; et al. Evolution of white matter damage in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 722–732. [Google Scholar] [CrossRef]
- Hübers, A.; Böckler, B.; Abaei, A.; Rasche, V.; Lulé, D.; Ercan, E.; Doorenweerd, N.; Müller, H.P.; Dreyhaupt, J.; Kammer, T.; et al. Functional and structural impairment of transcallosal motor fibres in ALS: A study using transcranial magnetic stimulation, diffusion tensor imaging, and diffusion weighted spectroscopy. Brain Imaging Behav. 2020. [Google Scholar] [CrossRef]
- Tu, S.; Wang, C.; Menke, R.A.L.; Talbot, K.; Barnett, M.; Kiernan, M.C.; Turner, M.R. Regional callosal integrity and bilaterality of limb weakness in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener 2020. [Google Scholar] [CrossRef]
- Benarroch, E.E. Glutamatergic synaptic plasticity and dysfunction in Alzheimer disease: Emerging mechanisms. Neurology 2018, 91, 125–132. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Pocernich, C.B. The glutamatergic system and Alzheimer’s disease: Therapeutic implications. CNS Drugs 2003, 17, 641–652. [Google Scholar] [CrossRef]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef]
- Ahmed, I.; Bose, S.K.; Pavese, N.; Ramlackhansingh, A.; Turkheimer, F.; Hotton, G.; Hammers, A.; Brooks, D.J. Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain 2011, 134, 979–986. [Google Scholar] [CrossRef]
- Obal, I.; Majlath, Z.; Toldi, J.; Vecsei, L. Mental disturbances in Parkinson’s disease and related disorders: The role of excitotoxins. J. Parkinsons Dis. 2014, 4, 139–150. [Google Scholar] [CrossRef]
- Frigo, M.; Cogo, M.G.; Fusco, M.L.; Gardinetti, M.; Frigeni, B. Glutamate and multiple sclerosis. Curr. Med. Chem. 2012, 19, 1295–1299. [Google Scholar] [CrossRef]
- Levite, M. Glutamate, T cells and multiple sclerosis. J. Neural. Transm. 2017, 124, 775–798. [Google Scholar] [CrossRef]
- Macrez, R.; Stys, P.K.; Vivien, D.; Lipton, S.A.; Docagne, F. Mechanisms of glutamate toxicity in multiple sclerosis: Biomarker and therapeutic opportunities. Lancet Neurol. 2016, 15, 1089–1102. [Google Scholar] [CrossRef]
- Stojanovic, I.R.; Kostic, M.; Ljubisavljevic, S. The role of glutamate and its receptors in multiple sclerosis. J. Neural. Transm. 2014, 121, 945–955. [Google Scholar] [CrossRef]
- Fogarty, M.J. Driven to decay: Excitability and synaptic abnormalities in amyotrophic lateral sclerosis. Brain Res. Bull. 2018, 140, 318–333. [Google Scholar] [CrossRef]
- Holecek, V.; Rokyta, R. Possible etiology and treatment of amyotrophic lateral sclerosis. Neuroendocrinol. Lett. 2018, 38, 528–531. [Google Scholar]
- Shaw, P.J.; Ince, P.G. Glutamate, excitotoxicity and amyotrophic lateral sclerosis. J. Neurol. 1997, 244, S3–S14. [Google Scholar] [CrossRef]
- Øie, M.G.; Andersen, P.N.; Hovik, K.T.; Skogli, E.W.; Rund, B.R. Similar impairments shown on a neuropsychological test battery in adolescents with high-functioning autism and early onset schizophrenia: A two-year follow-up study. Cogn. Neuropsychiatry 2020, 25, 163–178. [Google Scholar] [CrossRef]
- Rabiee, A.; Vasaghi-Gharamaleki, B.; Samadi, S.A.; Amiri-Shavaki, Y.; Alaghband-Rad, J. Working memory deficits and its relationship to autism spectrum disorders. Iran. J. Med. Sci. 2020, 45, 100–109. [Google Scholar] [CrossRef]
- Seng, G.J.; Tseng, W.L.; Chiu, Y.N.; Tsai, W.C.; Wu, Y.Y.; Gau, S.S. Executive functions in youths with autism spectrum disorder and their unaffected siblings. Psychol. Med. 2020. [Google Scholar] [CrossRef]
- Valeri, G.; Casula, L.; Napoli, E.; Stievano, P.; Trimarco, B.; Vicari, S.; Scalisi, T.G. Executive functions and symptom severity in an Italian sample of intellectually able preschoolers with autism spectrum disorder. J. Autism Dev. Disord. 2020, 50, 3207–3215. [Google Scholar] [CrossRef] [Green Version]
- Teigset, C.M.; Mohn, C.; Rund, B.R. Perinatal complications and executive dysfunction in early-onset schizophrenia. BMC Psychiatry 2020, 20, 103. [Google Scholar] [CrossRef] [Green Version]
- Vahdani, B.; Armani Kian, A.; Esmaeilzadeh, A.; Zenoozian, S.; Yousefi, V.; Mazloomzadeh, S. Adjunctive raloxifene and isradipine improve cognitive functioning in patients with schizophrenia: A pilot study. J. Clin. Psychopharmacol. 2020. [Google Scholar] [CrossRef]
- Kaga, Y.; Ueda, R.; Tanaka, M.; Kita, Y.; Suzuki, K.; Okumura, Y.; Egashira, Y.; Shirakawa, Y.; Mitsuhashi, S.; Kitamura, Y.; et al. Executive dysfunction in medication-naïve children with ADHD: A multi-modal fNIRS and EEG study. Brain Dev. 2020, 42, 555–563. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, H.Y.; Yoo, E.Y.; Kim, J.R. Effects of a cognitive-functional intervention method on improving executive function and self-directed learning in school-aged children with attention deficit hyperactivity disorder: A single-subject design study. Occup. Ther. Int. 2020, 2020, 1250801. [Google Scholar] [CrossRef]
- Taş Torun, Y.; Işik Taner, Y.; Güney, E.; İseri, E. Osmotic release oral system-methylphenidate hydrochloride (OROS-MPH) versus atomoxetine on executive function improvement and clinical effectiveness in ADHD: A randomized controlled trial. Appl. Neuropsychol. Child 2020. [Google Scholar] [CrossRef]
- Besag, F.M.C.; Vasey, M.J. Social cognition and psychopathology in childhood and adolescence. Epilepsy Behav. E&B 2019, 100, 106210. [Google Scholar] [CrossRef]
- Pallathra, A.A.; Cordero, L.; Wong, K.; Brodkin, E.S. Psychosocial interventions targeting social functioning in adults on the autism spectrum: A literature review. Curr. Psychiatry Rep. 2019, 21, 5. [Google Scholar] [CrossRef]
- Reyes, N.M.; Pickard, K.; Reaven, J. Emotion regulation: A treatment target for autism spectrum disorder. Bull. Menninger Clin. 2019, 83, 205–234. [Google Scholar] [CrossRef]
- Kimoto, S.; Makinodan, M.; Kishimoto, T. Neurobiology and treatment of social cognition in schizophrenia: Bridging the bed-bench gap. Neurobiol. Dis. 2019, 131, 104315. [Google Scholar] [CrossRef]
- Aman, M.G.; Farmer, C.A.; Hollway, J.; Arnold, L.E. Treatment of inattention, overactivity, and impulsiveness in autism spectrum disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2008, 17, 713–738. [Google Scholar] [CrossRef] [Green Version]
- Skoretz, P.; Tang, C. Stimulants for impulsive violence in schizophrenia spectrum disordered women: A case series and brief review. CNS Spectr. 2016, 21, 445–449. [Google Scholar] [CrossRef]
- Patros, C.H.; Alderson, R.M.; Kasper, L.J.; Tarle, S.J.; Lea, S.E.; Hudec, K.L. Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clin. Psychol. Rev. 2016, 43, 162–174. [Google Scholar] [CrossRef]
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed. Res. Int. 2015, 2015, 748212. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Q.; Tan, L.; Wang, H.F.; Tan, M.S.; Tan, L.; Xu, W.; Zhao, Q.F.; Wang, J.; Jiang, T.; Yu, J.T. Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: A systematic review and meta-analysis of cohort studies. J. Neurol. Neurosurg. Psychiatry 2016, 87, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Ding, L.J.; Li, F.F.; Han, Y.; Mu, L. Neurodegeneration and cognition in Parkinson’s disease: A review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2275–2281. [Google Scholar]
- Hoogland, J.; van Wanrooij, L.L.; Boel, J.A.; Goldman, J.G.; Stebbins, G.T.; Dalrymple-Alford, J.C.; Marras, C.; Adler, C.H.; Junque, C.; Pedersen, K.F.; et al. Detecting mild cognitive deficits in Parkinson’s disease: Comparison of neuropsychological tests. Mov. Disord. 2018, 33, 1750–1759. [Google Scholar] [CrossRef]
- Chiaravalloti, N.D.; DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar] [CrossRef]
- Grzegorski, T.; Losy, J. Cognitive impairment in multiple sclerosis—A review of current knowledge and recent research. Rev. Neurosci. 2017, 28, 845–860. [Google Scholar] [CrossRef]
- Koutsouraki, E.; Kalatha, T.; Grosi, E.; Koukoulidis, T.; Michmizos, D. Cognitive decline in Multiple Sclerosis patients. Hell. J. Nucl. Med. 2019, 22, 75–81. [Google Scholar]
- Miller, E.; Morel, A.; Redlicka, J.; Miller, I.; Saluk, J. Pharmacological and non-pharmacological therapies of cognitive impairment in multiple sclerosis. Curr. Neuropharmacol. 2018, 16, 475–483. [Google Scholar] [CrossRef]
- Beeldman, E.; Raaphorst, J.; Klein Twennaar, M.; de Visser, M.; Schmand, B.A.; de Haan, R.J. The cognitive profile of ALS: A systematic review and meta-analysis update. J. Neurol. Neurosurg. Psychiatry 2016, 87, 611–619. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, L.H.; Abrahams, S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: Nature of impairment and implications for assessment. Lancet Neurol. 2013, 12, 368–380. [Google Scholar] [CrossRef]
- Irwin, D.; Lippa, C.F.; Swearer, J.M. Cognition and amyotrophic lateral sclerosis (ALS). Am. J. Alzheimers Dis. Other Demen. 2007, 22, 300–312. [Google Scholar] [CrossRef]
- Khin Khin, E.; Minor, D.; Holloway, A.; Pelleg, A. Decisional capacity in amyotrophic lateral sclerosis. J. Am. Acad. Psychiatry Law 2015, 43, 210–217. [Google Scholar]
- Phukan, J.; Pender, N.P.; Hardiman, O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007, 6, 994–1003. [Google Scholar] [CrossRef]
- Vance, D.E.; Moore, B.S.; Farr, K.F.; Struzick, T. Procedural memory and emotional attachment in Alzheimer disease: Implications for meaningful and engaging activities. J. Neurosci. Nurs. 2008, 40, 96–102. [Google Scholar] [CrossRef]
- Palmeri, R.; Lo Buono, V.; Corallo, F.; Foti, M.; Di Lorenzo, G.; Bramanti, P.; Marino, S. Nonmotor symptoms in Parkinson disease: A descriptive review on social cognition ability. J. Geriatr. Psychiatry Neurol. 2017, 30, 109–121. [Google Scholar] [CrossRef]
- Sofologi, M.; Koutsouraki, E.; Tsolaki, M.; Tsolaki, A.; Koukoulidis, T.; Theofilidis, A.; Papantoniou, G.; Moraitou, D. Analyzing social cognition and understanding of social inferences in patients with multiple sclerosis. A comparative study. Hell. J. Nucl. Med. 2019, 22, 15–26. [Google Scholar]
- Dondu, A.; Sevincoka, L.; Akyol, A.; Tataroglu, C. Is obsessive-compulsive symptomatology a risk factor for Alzheimer-type dementia? Psychiatry Res. 2015, 225, 381–386. [Google Scholar] [CrossRef]
- Keszycki, R.M.; Fisher, D.W.; Dong, H. The hyperactivity-impulsivity-irritiability-disinhibition-aggression-agitation domain in Alzheimer’s disease: Current management and future directions. Front. Pharmacol. 2019, 10, 1109. [Google Scholar] [CrossRef] [Green Version]
- Jeon, N.; Bortolato, M. What drugs modify the risk of iatrogenic impulse-control disorders in Parkinson’s disease? A preliminary pharmacoepidemiologic study. PLoS ONE 2020, 15, e0227128. [Google Scholar] [CrossRef]
- O’Callaghan, C. The multifaceted nature of impulsivity in Parkinson’s disease. Brain 2019, 142, 3666–3669. [Google Scholar] [CrossRef]
- Toro, J.; Blanco, L.; Orozco-Cabal, L.F.; Díaz, C.; Reyes, S.; Burbano, L.; Cuéllar-Giraldo, D.F.; Duque, A.; Patiño, J.; Cortés, F. Impulsivity traits in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 22, 148–152. [Google Scholar] [CrossRef]
- Schumann, C.M.; Amaral, D.G. Stereological analysis of amygdala neuron number in autism. J. Neurosci. 2006, 26, 7674. [Google Scholar] [CrossRef]
- Falkai, P.; Bogerts, B. Cell loss in the hippocampus of schizophrenics. Eur. Arch. Psychiatry Neurol. Sci. 1986, 236, 154–161. [Google Scholar] [CrossRef]
- Ardalan, M.; Chumak, T.; Vexler, Z.; Mallard, C. Sex-dependent effects of perinatal inflammation on the brain: Implication for neuro-psychiatric disorders. Int. J. Mol. Sci. 2019, 20, 2270. [Google Scholar] [CrossRef] [Green Version]
- Flinkkila, E.; Keski-Rahkonen, A.; Marttunen, M.; Raevuori, A. Prenatal inflammation, infections and mental disorders. Psychopathology 2016, 49, 317–333. [Google Scholar] [CrossRef]
- Cortelazzo, A.; De Felice, C.; Guerranti, R.; Signorini, C.; Leoncini, S.; Zollo, G.; Leoncini, R.; Timperio, A.M.; Zolla, L.; Ciccoli, L.; et al. Expression and oxidative modifications of plasma proteins in autism spectrum disorders: Interplay between inflammatory response and lipid peroxidation. Proteom. Clin. Appl. 2016, 10, 1103–1112. [Google Scholar] [CrossRef]
- El-Ansary, A.; Bjorklund, G.; Chirumbolo, S.; Alnakhli, O.M. Predictive value of selected biomarkers related to metabolism and oxidative stress in children with autism spectrum disorder. Metab. Brain Dis. 2017, 32, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.E.; James, S.J. Metabolic pathology of autism in relation to redox metabolism. Biomark. Med. 2014, 8, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Instanes, J.T.; Halmøy, A.; Engeland, A.; Haavik, J.; Furu, K.; Klungsøyr, K. Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol. Psychiatry 2017, 81, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Eckert, A.; Marques, C.A.; Keil, U.; Schussel, K.; Muller, W.E. Increased apoptotic cell death in sporadic and genetic Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2003, 1010, 604–609. [Google Scholar] [CrossRef]
- Herrup, K. The contributions of unscheduled neuronal cell cycle events to the death of neurons in Alzheimer’s disease. Front. Biosci. (Elite Ed) 2012, 4, 2101–2109. [Google Scholar] [CrossRef]
- Michel, P.P.; Hirsch, E.C.; Hunot, S. Understanding dopaminergic cell death pathways in Parkinson disease. Neuron 2016, 90, 675–691. [Google Scholar] [CrossRef] [Green Version]
- Surmeier, D.J. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 2018, 285, 3657–3668. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, D.; Dukes, S.; Patel, R.; Nicholas, R.; Vora, A.; Reynolds, R. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009, 19, 238–253. [Google Scholar] [CrossRef]
- Schirmer, L.; Albert, M.; Buss, A.; Schulz-Schaeffer, W.J.; Antel, J.P.; Brück, W.; Stadelmann, C. Substantial early, but nonprogressive neuronal loss in multiple sclerosis (MS) spinal cord. Ann. Neurol. 2009, 66, 698–704. [Google Scholar] [CrossRef]
- Wegner, C.; Esiri, M.M.; Chance, S.A.; Palace, J.; Matthews, P.M. Neocortical neuronal, synaptic, and glial loss in multiple sclerosis. Neurology 2006, 67, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Shibuya, K.; Misawa, S.; Sekiguchi, Y.; Watanabe, K.; Amino, H.; Kuwabara, S. Axonal dysfunction precedes motor neuronal death in amyotrophic lateral sclerosis. PLoS ONE 2016, 11, e0158596. [Google Scholar] [CrossRef]
- Macdonald, R.; Barnes, K.; Hastings, C.; Mortiboys, H. Mitochondrial abnormalities in Parkinson’s disease and Alzheimer’s disease: Can mitochondria be targeted therapeutically? Biochem. Soc. Trans. 2018, 46, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H. Mitochondria and mitochondrial cascades in Alzheimer’s disease. J. Alzheimers Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobore, T.O. On the central role of mitochondria dysfunction and oxidative stress in Alzheimer’s disease. Neurol. Sci. 2019, 40, 1527–1540. [Google Scholar] [CrossRef]
- Bose, A.; Beal, M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016, 139, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.J.; Lin, K.L.; Chen, S.D.; Liou, C.W.; Chuang, Y.C.; Lin, H.Y.; Lin, T.K. The overcrowded crossroads: Mitochondria, alpha-synuclein, and the endo-lysosomal system interaction in Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 5312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, G.; Mahad, D.J. Mitochondrial dysfunction and axon degeneration in progressive multiple sclerosis. FEBS Lett. 2018, 592, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Rajda, C.; Pukoli, D.; Bende, Z.; Majláth, Z.; Vécsei, L. Excitotoxins, mitochondrial and redox disturbances in multiple sclerosis. Int. J. Mol. Sci. 2017, 18, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witte, M.E.; Mahad, D.J.; Lassmann, H.; van Horssen, J. Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol. Med. 2014, 20, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Greco, V.; Longone, P.; Spalloni, A.; Pieroni, L.; Urbani, A. Crosstalk between oxidative stress and mitochondrial damage: Focus on amyotrophic lateral sclerosis. Adv. Exp. Med. Biol. 2019, 1158, 71–82. [Google Scholar] [CrossRef]
- Hervias, I.; Beal, M.F.; Manfredi, G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle Nerve 2006, 33, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.F.; Shaw, P.J.; De Vos, K.J. The role of mitochondria in amyotrophic lateral sclerosis. Neurosci. Lett. 2019, 710, 132933. [Google Scholar] [CrossRef]
- Dani, M.; Wood, M.; Mizoguchi, R.; Fan, Z.; Walker, Z.; Morgan, R.; Hinz, R.; Biju, M.; Kuruvilla, T.; Brooks, D.J.; et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain 2018, 141, 2740–2754. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Brooks, D.J.; Okello, A.; Edison, P. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain 2017, 140, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Regen, F.; Hellmann-Regen, J.; Costantini, E.; Reale, M. Neuroinflammation and Alzheimer’s Disease: Implications for microglial activation. Curr. Alzheimer Res. 2017, 14, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.S. Microglia in Parkinson’s disease. Adv. Exp. Med. Biol. 2019, 1175, 335–353. [Google Scholar] [CrossRef]
- Sanchez-Guajardo, V.; Tentillier, N.; Romero-Ramos, M. The relation between alpha-synuclein and microglia in Parkinson’s disease: Recent developments. Neuroscience 2015, 302, 47–58. [Google Scholar] [CrossRef]
- Vivekanantham, S.; Shah, S.; Dewji, R.; Dewji, A.; Khatri, C.; Ologunde, R. Neuroinflammation in Parkinson’s disease: Role in neurodegeneration and tissue repair. Int. J. Neurosci. 2015, 125, 717–725. [Google Scholar] [CrossRef]
- Correale, J.; Gaitán, M.I.; Ysrraelit, M.C.; Fiol, M.P. Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain 2017, 140, 527–546. [Google Scholar] [CrossRef] [Green Version]
- Matthews, P.M. Chronic inflammation in multiple sclerosis—Seeing what was always there. Nat. Rev. Neurol. 2019, 15, 582–593. [Google Scholar] [CrossRef]
- Patejdl, R.; Penner, I.K.; Noack, T.K.; Zettl, U.K. Multiple sclerosis and fatigue: A review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun. Rev. 2016, 15, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Voet, S.; Prinz, M.; van Loo, G. Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol. Med. 2019, 25, 112–123. [Google Scholar] [CrossRef]
- Evans, M.C.; Couch, Y.; Sibson, N.; Turner, M.R. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol. Cell Neurosci. 2013, 53, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.S.; Wosiski-Kuhn, M.; Gillespie, R.; Caress, J.; Milligan, C. Inflammation, immunity, and amyotrophic lateral sclerosis: I. Etiology and pathology. Muscle Nerve 2019, 59, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Thonhoff, J.R.; Simpson, E.P.; Appel, S.H. Neuroinflammatory mechanisms in amyotrophic lateral sclerosis pathogenesis. Curr. Opin. Neurol. 2018, 31, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, G.; Chakrabarti, S.; Chatterjee, U.; Saso, L. Proteinopathy, oxidative stress and mitochondrial dysfunction: Cross talk in Alzheimer’s disease and Parkinson’s disease. Drug Des. Dev. Ther. 2017, 11, 797–810. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Tonnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibitoye, R.; Kemp, K.; Rice, C.; Hares, K.; Scolding, N.; Wilkins, A. Oxidative stress-related biomarkers in multiple sclerosis: A review. Biomark. Med. 2016, 10, 889–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassmann, H.; van Horssen, J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim. Biophys. Acta 2016, 1862, 506–510. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, F.; Mirra, A.; Carrì, M.T. Oxidative stress and mitochondrial damage in the pathogenesis of ALS: New perspectives. Neurosci. Lett. 2017, 636, 3–8. [Google Scholar] [CrossRef]
- D’Amico, E.; Factor-Litvak, P.; Santella, R.M.; Mitsumoto, H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2013, 65, 509–527. [Google Scholar] [CrossRef] [Green Version]
- Ault, A.P.; Peters, T.M.; Sawvel, E.J.; Casuccio, G.S.; Willis, R.D.; Norris, G.A.; Grassian, V.H. Single-particle SEM-EDX analysis of iron-containing coarse particulate matter in an urban environment: Sources and distribution of iron within Cleveland, Ohio. Environ. Sci. Technol. 2012, 46, 4331–4339. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Q.; Chen, H.; Zhou, X.; Wang, H.; Wang, H.; Zhang, J.; Feng, W. Size-dependent translocation pattern, chemical and biological transformation of nano- and submicron-sized ferric oxide particles in the central nervous system. J. Nanosci. Nanotechnol. 2016, 16, 5553–5561. [Google Scholar] [CrossRef] [PubMed]
- Sutunkova, M.P.; Katsnelson, B.A.; Privalova, L.I.; Gurvich, V.B.; Konysheva, L.K.; Shur, V.Y.; Shishkina, E.V.; Minigalieva, I.A.; Solovjeva, S.N.; Grebenkina, S.V.; et al. On the contribution of the phagocytosis and the solubilization to the iron oxide nanoparticles retention in and elimination from lungs under long-term inhalation exposure. Toxicology 2016, 363–364, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, L.E.; Laing, E.A.; Peake, J.L.; Uyeminami, D.; Mack, S.M.; Li, X.; Smiley-Jewell, S.; Pinkerton, K.E. Repeated iron-soot exposure and nose-to-brain transport of inhaled ultrafine particles. Toxicol. Pathol. 2018, 46, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, B.A.; Ahmed, I.A.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.; Torres-Jardon, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Ding, T.; Sun, J. Neurotoxic potential of iron oxide nanoparticles in the rat brain striatum and hippocampus. Neurotoxicology 2013, 34, 243–253. [Google Scholar] [CrossRef]
- Lubczynska, M.J.; Sunyer, J.; Tiemeier, H.; Porta, D.; Kasper-Sonnenberg, M.; Jaddoe, V.W.V.; Basagana, X.; Dalmau-Bueno, A.; Forastiere, F.; Wittsiepe, J.; et al. Exposure to elemental composition of outdoor PM2.5 at birth and cognitive and psychomotor function in childhood in four European birth cohorts. Environ. Int. 2017, 109, 170–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.H.; Singh, N.; Tay, H.; Walczyk, T. Imbalance of iron influx and efflux causes brain iron accumulation over time in the healthy adult rat. Metallomics 2014, 6, 1417–1426. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Fasold, M.B.; Barrera, C.M.; Augereau, G. Studies of the slow bidirectional transport of iron and transferrin across the blood-brain barrier. Brain Res. Bull. 1988, 21, 881–885. [Google Scholar] [CrossRef]
- Dallman, P.R.; Spirito, R.A. Brain iron in the rat: Extremely slow turnover in normal rats may explain long-lasting effects of early iron deficiency. J. Nutr. 1977, 107, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Chenery, S.R.N.; Sarkar, S.K.; Chatterjee, M.; Marriott, A.L.; Watts, M.J. Heavy metals in urban road dusts from Kolkata and Bengaluru, India: Implications for human health. Environ. Geochem. Health 2020, 42, 2627–2643. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, R.; Liu, Y.; Meng, L.; Li, B.; Wang, L.; Xu, L.; Le Guyader, L.; Chen, C. The dose-dependent toxicological effects and potential perturbation on the neurotransmitter secretion in brain following intranasal instillation of copper nanoparticles. Nanotoxicology 2012, 6, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Zhang, L.; Liu, Y.; Li, B.; Wang, L.; Wang, P.; Autrup, H.; Beer, C.; Chen, C. Integrated analytical techniques with high sensitivity for studying brain translocation and potential impairment induced by intranasally instilled copper nanoparticles. Toxicol. Lett. 2014, 226, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, Y.; Liu, Y.; Li, B.; Chen, C.; Wu, G. Oxidative stress and acute changes in murine brain tissues after nasal instillation of copper particles with different sizes. J. Nanosci. Nanotechnol. 2014, 14, 4534–4540. [Google Scholar] [CrossRef]
- Alemany, S.; Vilor-Tejedor, N.; Bustamante, M.; Álvarez-Pedrerol, M.; Rivas, I.; Forns, J.; Querol, X.; Pujol, J.; Sunyer, J. Interaction between airborne copper exposure and ATP7B polymorphisms on inattentiveness in scholar children. Int. J. Hyg. Environ. Health 2017, 220, 51–56. [Google Scholar] [CrossRef]
- Persson, E.; Henriksson, J.; Tallkvist, J.; Rouleau, C.; Tjalve, H. Transport and subcellular distribution of intranasally administered zinc in the olfactory system of rats and pikes. Toxicology 2003, 191, 97–108. [Google Scholar] [CrossRef]
- Kao, Y.Y.; Cheng, T.J.; Yang, D.M.; Wang, C.T.; Chiung, Y.M.; Liu, P.S. Demonstration of an olfactory bulb-brain translocation pathway for ZnO nanoparticles in rodent cells in vitro and in vivo. J. Mol. Neurosci. 2012, 48, 464–471. [Google Scholar] [CrossRef]
- Liu, H.; Yang, H.; Fang, Y.; Li, K.; Tian, L.; Liu, X.; Zhang, W.; Tan, Y.; Lai, W.; Bian, L.; et al. Neurotoxicity and biomarkers of zinc oxide nanoparticles in main functional brain regions and dopaminergic neurons. Sci. Total Environ. 2020, 705, 135809. [Google Scholar] [CrossRef]
- Dorman, D.C.; Brenneman, K.A.; McElveen, A.M.; Lynch, S.E.; Roberts, K.C.; Wong, B.A. Olfactory transport: A direct route of delivery of inhaled manganese phosphate to the rat brain. J. Toxicol. Environ. Health Part A 2002, 65, 1493–1511. [Google Scholar] [CrossRef]
- Lewis, J.; Bench, G.; Myers, O.; Tinner, B.; Staines, W.; Barr, E.; Divine, K.K.; Barrington, W.; Karlsson, J. Trigeminal uptake and clearance of inhaled manganese chloride in rats and mice. Neurotoxicology 2005, 26, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colin-Barenque, L.; Souza-Gallardo, L.M.; Fortoul, T.I. Toxic effects of inhaled manganese on the olfactory bulb: An ultrastructural approach in mice. J. Electron. Microsc. 2011, 60, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Moberly, A.H.; Czarnecki, L.A.; Pottackal, J.; Rubinstein, T.; Turkel, D.J.; Kass, M.D.; McGann, J.P. Intranasal exposure to manganese disrupts neurotransmitter release from glutamatergic synapses in the central nervous system in vivo. Neurotoxicology 2012, 33, 996–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Normandin, L.; Carrier, G.; Gardiner, P.F.; Kennedy, G.; Hazell, A.S.; Mergler, D.; Butterworth, R.F.; Philippe, S.; Zayed, J. Assessment of bioaccumulation, neuropathology, and neurobehavior following subchronic (90 days) inhalation in Sprague-Dawley rats exposed to manganese phosphate. Toxicol. Appl. Pharmacol. 2002, 183, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Tapin, D.; Kennedy, G.; Lambert, J.; Zayed, J. Bioaccumulation and locomotor effects of manganese sulfate in Sprague-Dawley rats following subchronic (90 days) inhalation exposure. Toxicol. Appl. Pharmacol. 2006, 211, 166–174. [Google Scholar] [CrossRef]
- Dobson, A.W.; Weber, S.; Dorman, D.C.; Lash, L.K.; Erikson, K.M.; Aschner, M. Oxidative stress is induced in the rat brain following repeated inhalation exposure to manganese sulfate. Biol. Trace Elem. Res. 2003, 93, 113–126. [Google Scholar] [CrossRef]
- Taylor, M.D.; Erikson, K.M.; Dobson, A.W.; Fitsanakis, V.A.; Dorman, D.C.; Aschner, M. Effects of inhaled manganese on biomarkers of oxidative stress in the rat brain. Neurotoxicology 2006, 27, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Erikson, K.M.; Dorman, D.C.; Lash, L.H.; Dobson, A.W.; Aschner, M. Airborne manganese exposure differentially affects end points of oxidative stress in an age- and sex-dependent manner. Biol. Trace Elem. Res. 2004, 100, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Dorman, D.C.; McManus, B.E.; Marshall, M.W.; James, R.A.; Struve, M.F. Old age and gender influence the pharmacokinetics of inhaled manganese sulfate and manganese phosphate in rats. Toxicol. Appl. Pharmacol. 2004, 197, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Erikson, K.M.; Dorman, D.C.; Fitsanakis, V.; Lash, L.H.; Aschner, M. Alterations of oxidative stress biomarkers due to in utero and neonatal exposures of airborne manganese. Biol. Trace Elem. Res. 2006, 111, 199–215. [Google Scholar] [CrossRef] [Green Version]
- Erikson, K.M.; Dorman, D.C.; Lash, L.H.; Aschner, M. Persistent alterations in biomarkers of oxidative stress resulting from combined in utero and neonatal manganese inhalation. Biol. Trace Elem. Res. 2005, 104, 151–163. [Google Scholar] [CrossRef]
- Foster, M.L.; Rao, D.B.; Francher, T.; Traver, S.; Dorman, D.C. Olfactory toxicity in rats following manganese chloride nasal instillation: A pilot study. Neurotoxicology 2018, 64, 284–290. [Google Scholar] [CrossRef]
- Saputra, D.; Chang, J.; Lee, B.J.; Yoon, J.H.; Kim, J.; Lee, K. Short-term manganese inhalation decreases brain dopamine transporter levels without disrupting motor skills in rats. J. Toxicol. Sci. 2016, 41, 391–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Betancourt, J.; Anaya-Martinez, V.; Gutierrez-Valdez, A.L.; Ordonez-Librado, J.L.; Montiel-Flores, E.; Espinosa-Villanueva, J.; Reynoso-Erazo, L.; Avila-Costa, M.R. Manganese mixture inhalation is a reliable Parkinson disease model in rats. Neurotoxicology 2012, 33, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Elder, A.; Gelein, R.; Silva, V.; Feikert, T.; Opanashuk, L.; Carter, J.; Potter, R.; Maynard, A.; Ito, Y.; Finkelstein, J.; et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006, 114, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Dorman, D.C.; Struve, M.F.; Wong, B.A.; Dye, J.A.; Robertson, I.D. Correlation of brain magnetic resonance imaging changes with pallidal manganese concentrations in rhesus monkeys following subchronic manganese inhalation. Toxicol. Sci. 2006, 92, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erikson, K.M.; Dorman, D.C.; Lash, L.H.; Aschner, M. Manganese inhalation by rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Toxicol. Sci. 2007, 97, 459–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erikson, K.M.; Dorman, D.C.; Lash, L.H.; Aschner, M. Duration of airborne-manganese exposure in rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity. Neurotoxicology 2008, 29, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.F.; Menezes-Filho, J.A.; de Matos, V.P.; Bessa, J.R.; Coelho-Santos, J.; Viana, G.F.; Argollo, N.; Abreu, N. Elevated airborne manganese and low executive function in school-aged children in Brazil. Neurotoxicology 2014, 45, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y.; Kimura, H. Nutritional essentiality of sulfur in health and disease. Nutr. Rev. 2013, 71, 413–432. [Google Scholar] [CrossRef]
- Yargicoglu, P.; Agar, A.; Gumuslu, S.; Bilmen, S.; Oguz, Y. Age-related alterations in antioxidant enzymes, lipid peroxide levels, and somatosensory-evoked potentials: Effect of sulfur dioxide. Arch. Environ. Contam. Toxicol. 1999, 37, 554–560. [Google Scholar] [CrossRef]
- Kilic, D. The effects of ageing and sulfur dioxide inhalation exposure on visual-evoked potentials, antioxidant enzyme systems, and lipid-peroxidation levels of the brain and eye. Neurotoxicol. Teratol. 2003, 25, 587–598. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, B. Oxidative damage of sulfur dioxide inhalation on brains and livers of mice. Environ. Toxicol. Pharmacol. 2003, 13, 1–8. [Google Scholar] [CrossRef]
- Meng, Z. Oxidative damage of sulfur dioxide on various organs of mice: Sulfur dioxide is a systemic oxidative damage agent. Inhal. Toxicol. 2003, 15, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Meng, Z. Effect of sulfur dioxide inhalation on the glutathione redox system in mice and protective role of sea buckthorn seed oil. Arch. Environ. Contam. Toxicol. 2003, 45, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Li, R.; Zhang, X. Levels of sulfite in three organs from mice exposed to sulfur [corrected] dioxide. Inhal. Toxicol. 2005, 17, 309–313. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, Y. Cell morphological ultrastructural changes in various organs from mice exposed by inhalation to sulfur dioxide. Inhal. Toxicol. 2007, 19, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Wang, J.; Huo, Y.; Yan, H.; Jiang, C.; Zhou, J.; Wang, X.; Sang, N. Sulfur dioxide inhalation stimulated mitochondrial biogenesis in rat brains. Toxicology 2012, 300, 67–74. [Google Scholar] [CrossRef]
- Yun, Y.; Yao, G.; Yue, H.; Guo, L.; Qin, G.; Li, G.; Sang, N. SO(2) inhalation causes synaptic injury in rat hippocampus via its derivatives in vivo. Chemosphere 2013, 93, 2426–2432. [Google Scholar] [CrossRef]
- Yao, G.; Yun, Y.; Sang, N. Differential effects between one week and four weeks exposure to same mass of SO on synaptic plasticity in rat hippocampus. Environ. Toxicol. 2014. [Google Scholar] [CrossRef]
- Slikker, W., Jr.; Andersen, M.E.; Bogdanffy, M.S.; Bus, J.S.; Cohen, S.D.; Conolly, R.B.; David, R.M.; Doerrer, N.G.; Dorman, D.C.; Gaylor, D.W.; et al. Dose-dependent transitions in mechanisms of toxicity. Toxicol. Appl. Pharmacol. 2004, 201, 203–225. [Google Scholar] [CrossRef]
- Baisch, B.L.; Corson, N.M.; Wade-Mercer, P.; Gelein, R.; Kennell, A.J.; Oberdörster, G.; Elder, A. Equivalent titanium dioxide nanoparticle deposition by intratracheal instillation and whole body inhalation: The effect of dose rate on acute respiratory tract inflammation. Part. Fibre Toxicol. 2014, 11, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, G.; Yue, H.; Yun, Y.; Sang, N. Chronic SO inhalation above environmental standard impairs neuronal behavior and represses glutamate receptor gene expression and memory-related kinase activation via neuroinflammation in rats. Environ. Res. 2014, 137C, 85–93. [Google Scholar] [CrossRef]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, K.; Weistenhöfer, W.; Neff, F.; Hartwig, A.; van Thriel, C.; Drexler, H. The health effects of aluminum exposure. Dtsch. Arztebl. Int. 2017, 114, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Röllin, H.B.; Theodorou, P.; Kilroe-Smith, T.A. Deposition of aluminium in tissues of rabbits exposed to inhalation of low concentrations of Al2O3 dust. Br. J. Ind. Med. 1991, 48, 389–391. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.T.; Seo, G.B.; Jo, E.; Lee, M.; Kim, H.M.; Shim, I.; Lee, B.W.; Yoon, B.I.; Kim, P.; Choi, K. Aluminum nanoparticles induce ERK and p38MAPK activation in rat brain. Toxicol. Res. 2013, 29, 181–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Xu, Y.; Zhou, L.; Zhang, C.; Meng, Q.; Wu, S.; Wang, S.; Ding, Z.; Chen, X.; Li, X.; et al. Sex-dependent depression-like behavior induced by respiratory administration of aluminum oxide nanoparticles. Int. J. Environ. Res. Public Health 2015, 12, 15692–15705. [Google Scholar] [CrossRef]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef]
- Parveen, A.; Rizvi, S.H.; Sushma; Mahdi, F.; Ahmad, I.; Singh, P.P.; Mahdi, A.A. Intranasal exposure to silica nanoparticles induces alterations in pro-inflammatory environment of rat brain. Toxicol. Ind. Health 2017, 33, 119–132. [Google Scholar] [CrossRef]
- Lebedova, J.; Novakova, Z.; Vecera, Z.; Buchtova, M.; Dumkova, J.; Docekal, B.; Blahova, L.; Mikuska, P.; Misek, I.; Hampl, A.; et al. Impact of acute and subchronic inhalation exposure to PbO nanoparticles on mice. Nanotoxicology 2018, 12, 290–304. [Google Scholar] [CrossRef]
- Blahova, L.; Novakova, Z.; Vecera, Z.; Vrlikova, L.; Docekal, B.; Dumkova, J.; Krumal, K.; Mikuska, P.; Buchtova, M.; Hampl, A.; et al. The effects of nano-sized PbO on biomarkers of membrane disruption and DNA damage in a sub-chronic inhalation study on mice. Nanotoxicology 2020, 14, 214–231. [Google Scholar] [CrossRef]
- Sutunkova, M.P.; Solovyeva, S.N.; Chernyshov, I.N.; Klinova, S.V.; Gurvich, V.B.; Shur, V.Y.; Shishkina, E.V.; Zubarev, I.V.; Privalova, L.I.; Katsnelson, B.A. Manifestation of systemic toxicity in rats after a short-time inhalation of lead oxide nanoparticles. Int. J. Mol. Sci. 2020, 21, 690. [Google Scholar] [CrossRef] [Green Version]
- Ahsan, S.M.; Rao, C.M.; Ahmad, M.F. Nanoparticle-protein interaction: The significance and role of protein corona. Adv. Exp. Med. Biol. 2018, 1048, 175–198. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Rosfa, S.; Wlodarski, A.; Kuharev, J.; Rekik, A.; Knauer, S.K.; Bantz, C.; Nawroth, T.; Bier, C.; et al. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: A comprehensive quantitative proteomic analysis. ACS Nano 2011, 5, 7155–7167. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, G.; Sabella, S.; Sorce, B.; Brunetti, V.; Malvindi, M.A.; Cingolani, R.; Pompa, P.P. Effects of cell culture media on the dynamic formation of protein-nanoparticle complexes and influence on the cellular response. ACS Nano 2010, 4, 7481–7491. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, S.; Ogawara, K.; Fukuoka, Y.; Higaki, K.; Kimura, T. Time-dependent changes in opsonin amount associated on nanoparticles alter their hepatic uptake characteristics. Int. J. Pharm. 2007, 342, 215–221. [Google Scholar] [CrossRef]
- Cox, A.; Andreozzi, P.; Dal Magro, R.; Fiordaliso, F.; Corbelli, A.; Talamini, L.; Chinello, C.; Raimondo, F.; Magni, F.; Tringali, M.; et al. Evolution of nanoparticle protein corona across the blood–brain barrier. ACS Nano 2018, 12, 7292–7300. [Google Scholar] [CrossRef] [PubMed]


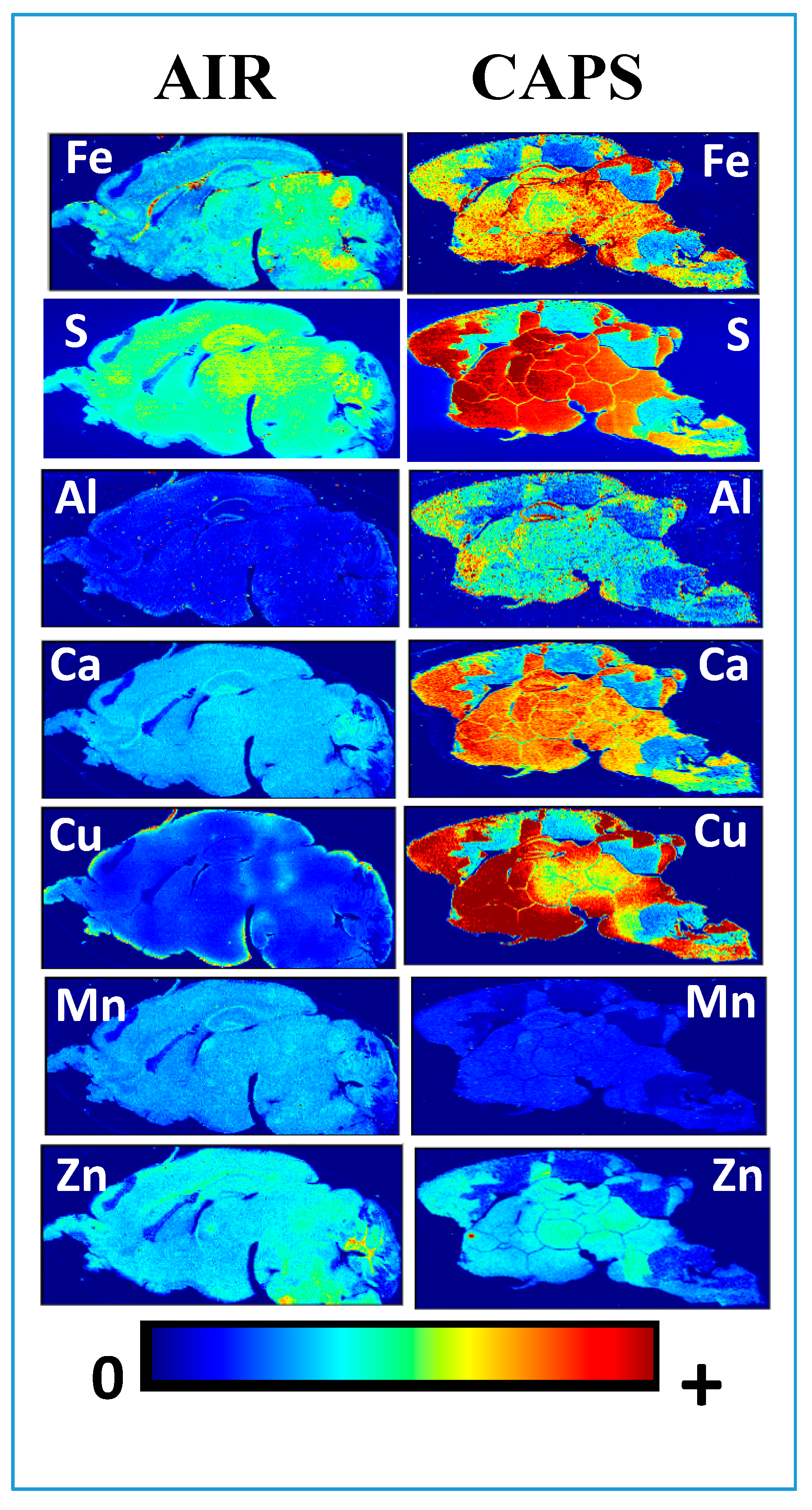
| Characteristic | NDDs | NDGDs | Fe | Cu | Zn | Mn | S | Pb | Al | Si | Ca |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ventriculomegaly | ASD, SCZ, ADHD | AD, PD, MS, ALS | |||||||||
| Altered Myelination | ASD, SCZ, ADHD | AD, PD, MS, ALS | √ | √ | √ | √ | |||||
| Interhemispheric Disconnection | ASD, SCZ, ADHD | AD, PD, MS, ALS | |||||||||
| Glutamatergic Dysfunction | ASD, SCZ, ADHD | AD, PD, MS, ALS | √ | √ | |||||||
| Neuronal Cell Death | ASD, SCZ | AD, PD, MS, ALS | √ | √ | √ | √ | √ | ||||
| Inflammation/Microglial Activation | ASD, SCZ, ADHD | AD, PD, MS, ALS | √ | √ | √ | ||||||
| Oxidative Stress | ASD, SCZ, ADHD | AD, PD, MS, ALS | √ | √ | √ | √ | √ | √ | |||
| Cognitive/Executive Deficits | ASD, SCZ, ADHD | AD, PD, MS, ALS | |||||||||
| Mitochondrial Dysfunction | ASD, SCZ, ADHD | AD, PD, MS, ALS | √ | √ | √ | √ | √ | ||||
| Social Behavioral Deficits | ASD, SCZ, ADHD | AD, PD, MS, ALS | |||||||||
| Impulsivity | ASD, SCZ, ADHD | AD, PD, MS | √ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cory-Slechta, D.A.; Sobolewski, M.; Oberdörster, G. Air Pollution-Related Brain Metal Dyshomeostasis as a Potential Risk Factor for Neurodevelopmental Disorders and Neurodegenerative Diseases. Atmosphere 2020, 11, 1098. https://doi.org/10.3390/atmos11101098
Cory-Slechta DA, Sobolewski M, Oberdörster G. Air Pollution-Related Brain Metal Dyshomeostasis as a Potential Risk Factor for Neurodevelopmental Disorders and Neurodegenerative Diseases. Atmosphere. 2020; 11(10):1098. https://doi.org/10.3390/atmos11101098
Chicago/Turabian StyleCory-Slechta, Deborah A., Marissa Sobolewski, and Günter Oberdörster. 2020. "Air Pollution-Related Brain Metal Dyshomeostasis as a Potential Risk Factor for Neurodevelopmental Disorders and Neurodegenerative Diseases" Atmosphere 11, no. 10: 1098. https://doi.org/10.3390/atmos11101098
APA StyleCory-Slechta, D. A., Sobolewski, M., & Oberdörster, G. (2020). Air Pollution-Related Brain Metal Dyshomeostasis as a Potential Risk Factor for Neurodevelopmental Disorders and Neurodegenerative Diseases. Atmosphere, 11(10), 1098. https://doi.org/10.3390/atmos11101098





