Direct Measurement of Adhesion Force of Individual Aerosol Particles by Atomic Force Microscopy
Abstract
:1. Introduction
2. Sampling and Analysis
2.1. Sample Preparation
2.2. Collecting Atomic Force Microscopy Images of Aerosol Particles
2.3. Protocol of Force–Distance Curve Mapping for Individual Aerosol Particles
2.4. Chemical Composition Analysis of Individual Aerosol Particles
3. Results and Discussion
3.1. Force–Distance Curve Mapping on PSL
3.2. Force–Distance Curve Mapping on Standard Dust Particles
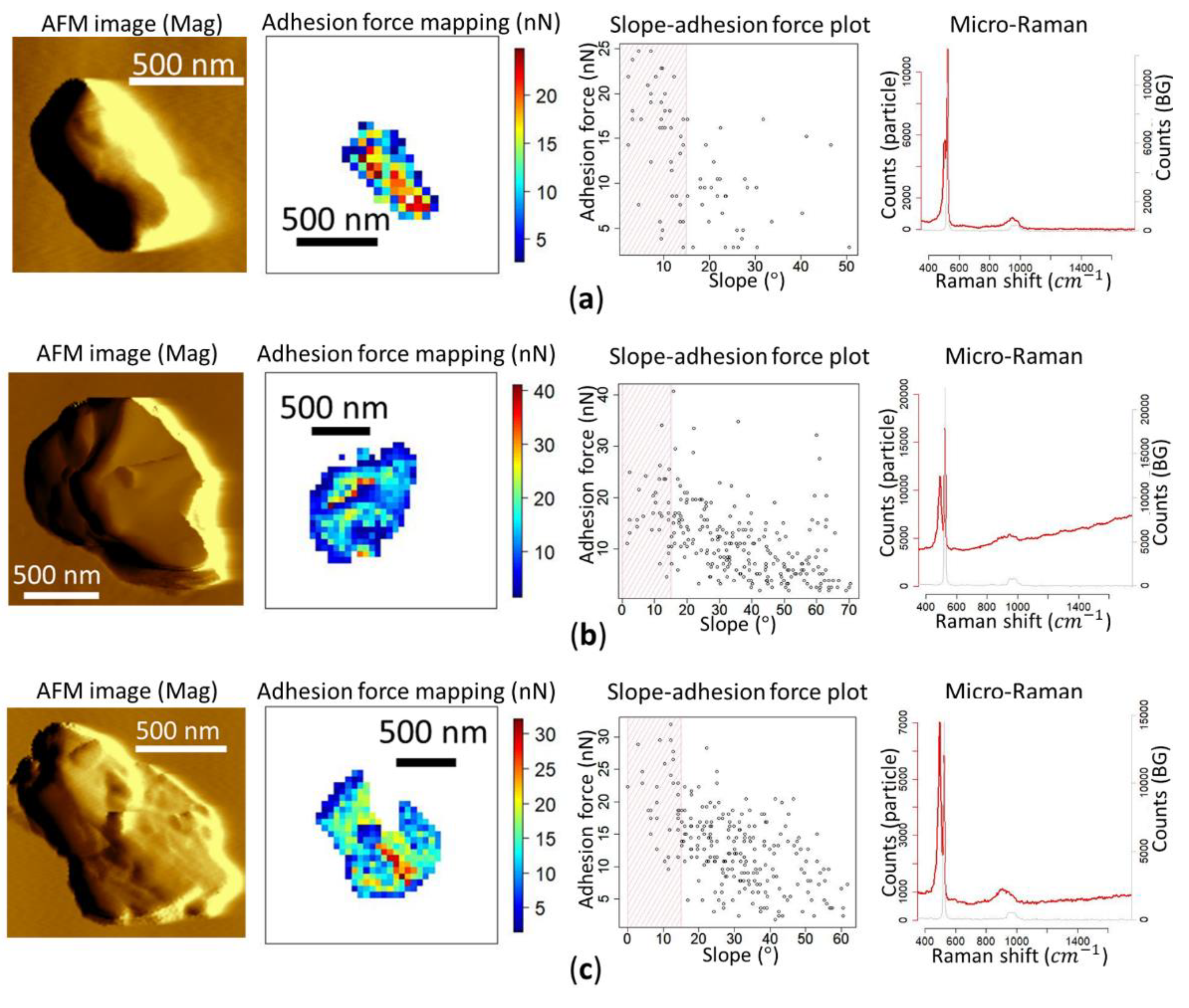
3.3. Force–Distance Curve Mapping on Inorganic Aerosol Particles
3.4. Chemical Analysis and Force–Distance Curve Mapping of Ambient Aerosol Particles
3.5. Comparison of Adhesion Forces among Different Particle Types
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hinds, W.C. Aerosol Technology: Properties, Behavior, and Measurement of AIrborne Particles, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Virtanen, A.; Joutsensaari, J.; Koop, T.; Kannosto, J.; Yli-Pirilä, P.; Leskinen, J.; Mäkelä, J.M.; Holopainen, J.K.; Pöschl, U.; Kulmala, M.; et al. An amorphous solid state of biogenic secondary organic aerosol particles. Nature 2010, 467, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, S.; Speckart, S.; Brown, M.J. Adhesion and aerodynamic forces for the resuspension of non-spherical particles in outdoor environments. J. Aerosol Sci. 2017, 112, 52–67. [Google Scholar] [CrossRef]
- Fukuyama, T.; Fujiwara, H. Contribution of Asian dust to atmospheric deposition of radioactive cesium (137Cs). Sci. Total Environ. 2008, 405, 389–395. [Google Scholar] [CrossRef]
- Sayyah, A.; Horenstein, M.N.; Mazumder, M.K. Energy yield loss caused by dust deposition on photovoltaic panels. Sol. Energy 2014, 107, 576–604. [Google Scholar] [CrossRef]
- Chesnutt, J.K.W.; Ashkanani, H.; Guo, B.; Wu, C.Y. Simulation of microscale particle interactions for optimization of an electrodynamic dust shield to clean desert dust from solar panels. Sol. Energy 2017, 155, 1197–1207. [Google Scholar] [CrossRef]
- Petean, P.G.C.; Aguiar, M.L. Determining the adhesion force between particles and rough surfaces. Powder Technol. 2015, 274, 67–76. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.; Chu, D.; Wang, X.; Sun, F.; Guo, Z. An experimental study of ash particles adhesion force in flue gas. Adv. Powder Technol. 2017, 28, 1435–1442. [Google Scholar] [CrossRef]
- Leung, W.T.; Fu, S.C.; Sze To, G.N.; Chao, C.Y.H. Comparison of the resuspension behavior between liquid and solid aerosols. Aerosol Sci. Technol. 2013, 47, 1239–1247. [Google Scholar] [CrossRef]
- Nishikawa, M.; Hao, Q.; Morita, M. Preparation and evaluation of certified reference materials for asian mineral dust. Glob. Environ. Res. 2000, 4, 103–113. [Google Scholar]
- Mori, I.; Nishikawa, M.; Quan, H.; Morita, M. Estimation of the concentration and chemical composition of kosa aerosols at their origin. Atmos. Environ. 2002, 36, 4569–4575. [Google Scholar] [CrossRef]
- Murray, B.J.; O’Sullivan, D.; Atkinson, J.D.; Webb, M.E. Ice nucleation by particles immersed in supercooled cloud droplets. Chem. Soc. Rev. 2012, 41, 6519–6554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, T.; Buseck, P.R.; Iwasaka, Y.; Matsuki, A.; Trochkine, D. Sulfate-coated dust particles in the free troposphere over Japan. Atmos. Res. 2006, 82, 698–708. [Google Scholar] [CrossRef]
- Trochkine, D.; Iwasaka, Y.; Matsuki, A.; Yamada, M.; Kim, Y.S.; Nagatani, T.; Zhang, D.; Shi, G.Y.; Shen, Z. Mineral aerosol particles collected in Dunhuang, China, and their comparison with chemically modified particles collected over Japan. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zang, J.; Shi, G.; Iwasaka, Y.; Matsuki, A.; Trochkine, D. Mixture state of individual Asian dust particles at a coastal site of Qingdao, China. Atmos. Environ. 2003, 37, 3895–3901. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Iwasaka, Y.; Shi, G.; Zang, J.; Matsuki, A.; Trochkine, D. Mixture state and size of Asian dust particles collected at southwestern Japan in spring 2000. J. Geophys. Res. Atmos. 2003, 108, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Matsuki, A.; Iwasaka, Y.; Shi, G.; Zhang, D.; Trochkine, D.; Yamada, M.; Yoon-Suk, K.; Chen, B.; Nagatani, T.; Miyazawa, T.; et al. Morphological and chemical modification of mineral dust: Observational insight into the heterogeneous uptake of acidic gases. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Tobo, Y.; Zhang, D.; Matsuki, A.; Iwasaka, Y. Asian dust particles converted into aqueous droplets under remote marine atmospheric conditions. Proc. Natl. Acad. Sci. USA 2010, 107, 17905–17910. [Google Scholar] [CrossRef] [Green Version]
- Cappella, B.; Dietler, G. Force-distance curves by atomic force microscopy. Surf. Sci. Rep. 1999, 34, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Chow, E.H.H.; Bučar, D.K.; Jones, W. New opportunities in crystal engineering—The role of atomic force microscopy in studies of molecular crystals. Chem. Commun. 2012, 48, 9210–9226. [Google Scholar] [CrossRef]
- De Falco, G.; Commodo, M.; Minutolo, P.; Danna, A. Flame-formed carbon nanoparticles: Morphology, interaction forces, and hamaker constant from AFM. Aerosol Sci. Technol. 2015, 49, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.L.C.; Gao, S.; Wee, B.S.; Asa-Awuku, A.; Thio, B.J.R. Adhesion of dust particles to common indoor surfaces in an air-conditioned environment. Aerosol Sci. Technol. 2014, 48, 541–551. [Google Scholar] [CrossRef]
- Moutinho, H.R.; Jiang, C.S.; To, B.; Perkins, C.; Muller, M.; Al-Jassim, M.M.; Simpson, L. Adhesion mechanisms on solar glass: Effects of relative humidity, surface roughness, and particle shape and size. Sol. Energy Mater. Sol. Cells 2017, 172, 145–153. [Google Scholar] [CrossRef]
- Yabuki, S.; Kanayama, S.; Honda, M. Mineral composition of certified reference 18 material: China loess (CJ1) and Simulated Asian Mineral Dust (CJ2). Chikyu Kankyo 2002, 7, 171–179. [Google Scholar]
- Louis, S.; Matter, I. Product specification. Build. Res. Inf. 1993, 21, 21–22. [Google Scholar]
- Hutter, J.L.; Bechhoefer, J. Measurement and manipulation of van der Waals forces in atomic-force microscopy. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. Process. Meas. Phenom. 1994, 12, 2251–2253. [Google Scholar] [CrossRef]
- Iwata, A.; Matsuki, A. Characterization of individual ice residual particles by the single droplet freezing method: A case study in the Asian dust outflow region. Atmos. Chem. Phys. 2018, 18, 1785–1804. [Google Scholar] [CrossRef] [Green Version]
- Laskina, O.; Young, M.A.; Kleiber, P.D.; Grassian, V.H. Infrared extinction spectroscopy and micro-Raman spectroscopy of select components of mineral dust mixed with organic compounds. J. Geophys. Res. Atmos. 2013, 118, 6593–6606. [Google Scholar] [CrossRef]
- Freeman, J.J.; Wang, A.; Kuebler, K.E.; Jolliff, B.L.; Haskin, L.A. Characterization of natural feldspars by raman spectroscopy for future planetary exploration. Can. Mineral. 2008, 46, 1477–1500. [Google Scholar] [CrossRef]
- Tang, I.N.; Fung, K.H. Characterization of inorganic salt particles by Raman spectroscopy. J. Aerosol Sci. 1989, 20, 609–617. [Google Scholar] [CrossRef]
- Daly, F.P.; Brown, C.W.; Kester, D.R. Sodium and magnesium sulfate ion pairing: Evidence from raman spectroscopy. J. Phys. Chem. 1972, 76, 3664–3668. [Google Scholar] [CrossRef]
- Hiranuma, N.; Brooks, S.D.; Gramann, J.; Auvermann, B.W. High concentrations of coarse particles emitted from a cattle feeding operation. Atmos. Chem. Phys. 2011, 11, 8809–8823. [Google Scholar] [CrossRef] [Green Version]
- Baustian, K.J.; Cziczo, D.J.; Wise, M.E.; Pratt, K.A.; Kulkarni, G.; Hallar, A.G.; Tolbert, M.A. Importance of aerosol composition, mixing state, and morphology for heterogeneous ice nucleation: A combined field and laboratory approach. J. Geophys. Res. Atmos. 2012, 117, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Iwata, A.; Imura, M.; Hama, M.; Maki, T.; Tsuchiya, N.; Kunihisa, R.; Matsuki, A. Release of highly active ice nucleating biological particles associated with rain. Atmosphere 2019, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Kumakura, T.; Sekikawa, T. The Effect of Continental Air Mass on the Modification of Individual Sea-Salt Particles Collected over the Coast and the Open Sea. J. Meteorol. Soc. Japan 1991, 69, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Laskin, A.; Iedema, M.J.; Ichkovich, A.; Graber, E.R.; Taraniuk, I.; Rudich, Y. Direct observation of completely processed calcium carbonate dust particles. Faraday Discuss. 2005, 130, 453–468. [Google Scholar] [CrossRef]
- Matsuki, A.; Schwarzenboeck, A.; Venzac, H.; Laj, P.; Crumeyrolle, S.; Gomes, L. Cloud processing of mineral dust: Direct comparison of cloud residual and clear sky particles during AMMA aircraft campaign in summer 2006. Atmos. Chem. Phys. 2010, 10, 1057–1069. [Google Scholar] [CrossRef] [Green Version]
- Miura, K. Production, and Chemical and Physical Properties of Sea Salt Particles. Bull. Soc. Sea Water Sci. Japan 2007, 61, 102–109. [Google Scholar]






| Raman Peak Assignments | |
|---|---|
| Species | Raman Shift (cm−1) |
| Quartz | 465 [28] |
| Feldspar | 485 [29] |
| (NH4)2SO4 | 975 [30] |
| Na2SO4(aq) | 982 [31] |
| CaSO4 | 1005 [32] |
| Ca(NO3)24H2O | 1050 [30] |
| Mg(NO3)26H2O | 1059 [30] |
| NaNO3 | 1069 [30] |
| Na2CO3 | 1080 [28] |
| CaCO3 | 1089 [28] |
| Bonded OH stretch | 3200–3650 [33] |
| Fluorescence | −4000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, K.; Mizushima, Y.; Furuya, M.; Kunihisa, R.; Tsuchiya, N.; Fukuma, T.; Iwata, A.; Matsuki, A. Direct Measurement of Adhesion Force of Individual Aerosol Particles by Atomic Force Microscopy. Atmosphere 2020, 11, 489. https://doi.org/10.3390/atmos11050489
Ono K, Mizushima Y, Furuya M, Kunihisa R, Tsuchiya N, Fukuma T, Iwata A, Matsuki A. Direct Measurement of Adhesion Force of Individual Aerosol Particles by Atomic Force Microscopy. Atmosphere. 2020; 11(5):489. https://doi.org/10.3390/atmos11050489
Chicago/Turabian StyleOno, Kohei, Yuki Mizushima, Masaki Furuya, Ryota Kunihisa, Nozomu Tsuchiya, Takeshi Fukuma, Ayumi Iwata, and Atsushi Matsuki. 2020. "Direct Measurement of Adhesion Force of Individual Aerosol Particles by Atomic Force Microscopy" Atmosphere 11, no. 5: 489. https://doi.org/10.3390/atmos11050489





