Efficacy of Radiant Catalytic Ionization in Reduction of Enterococcus spp., Clostridioides difficile and Staphylococcus aureus in Indoor Air
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
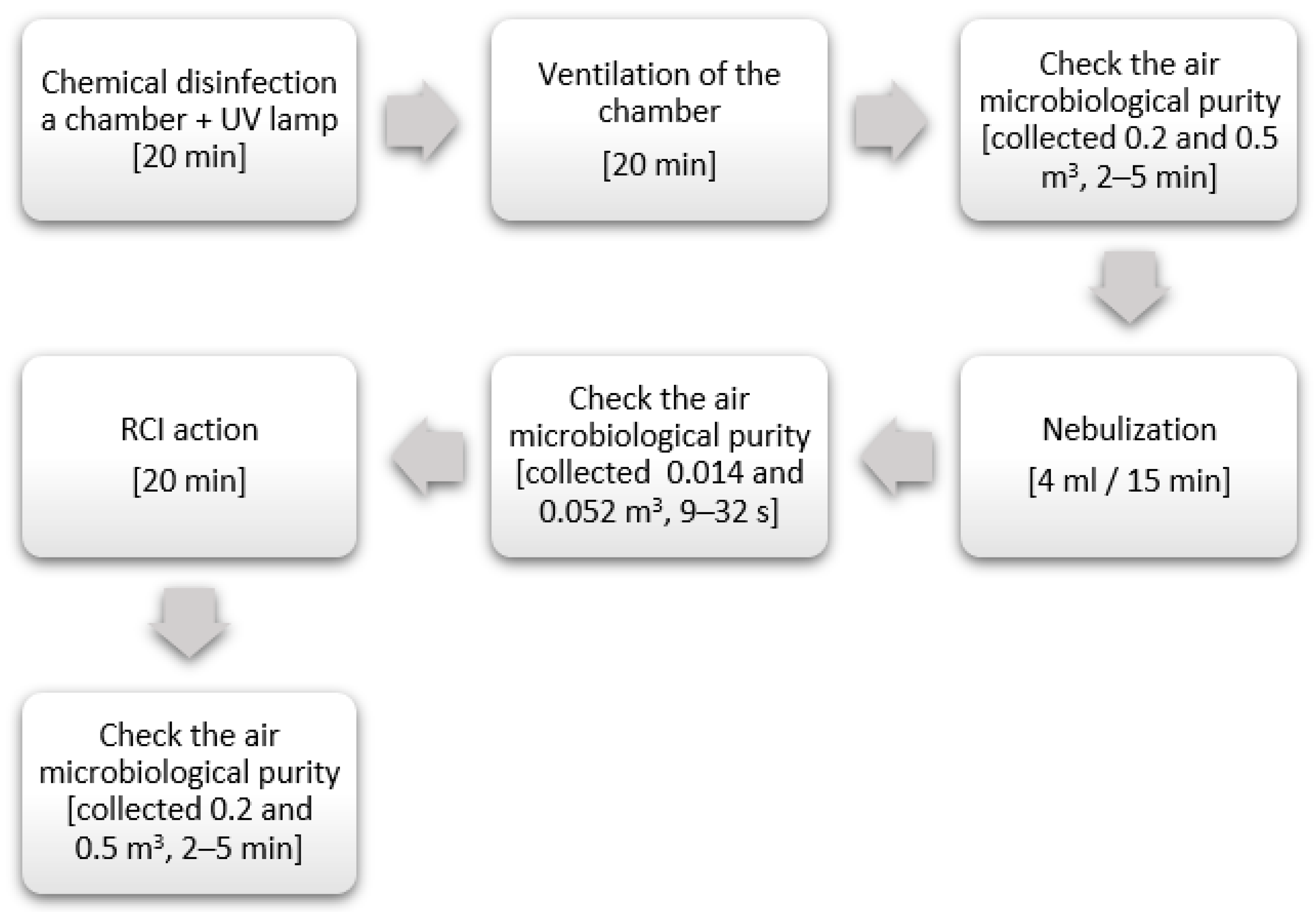
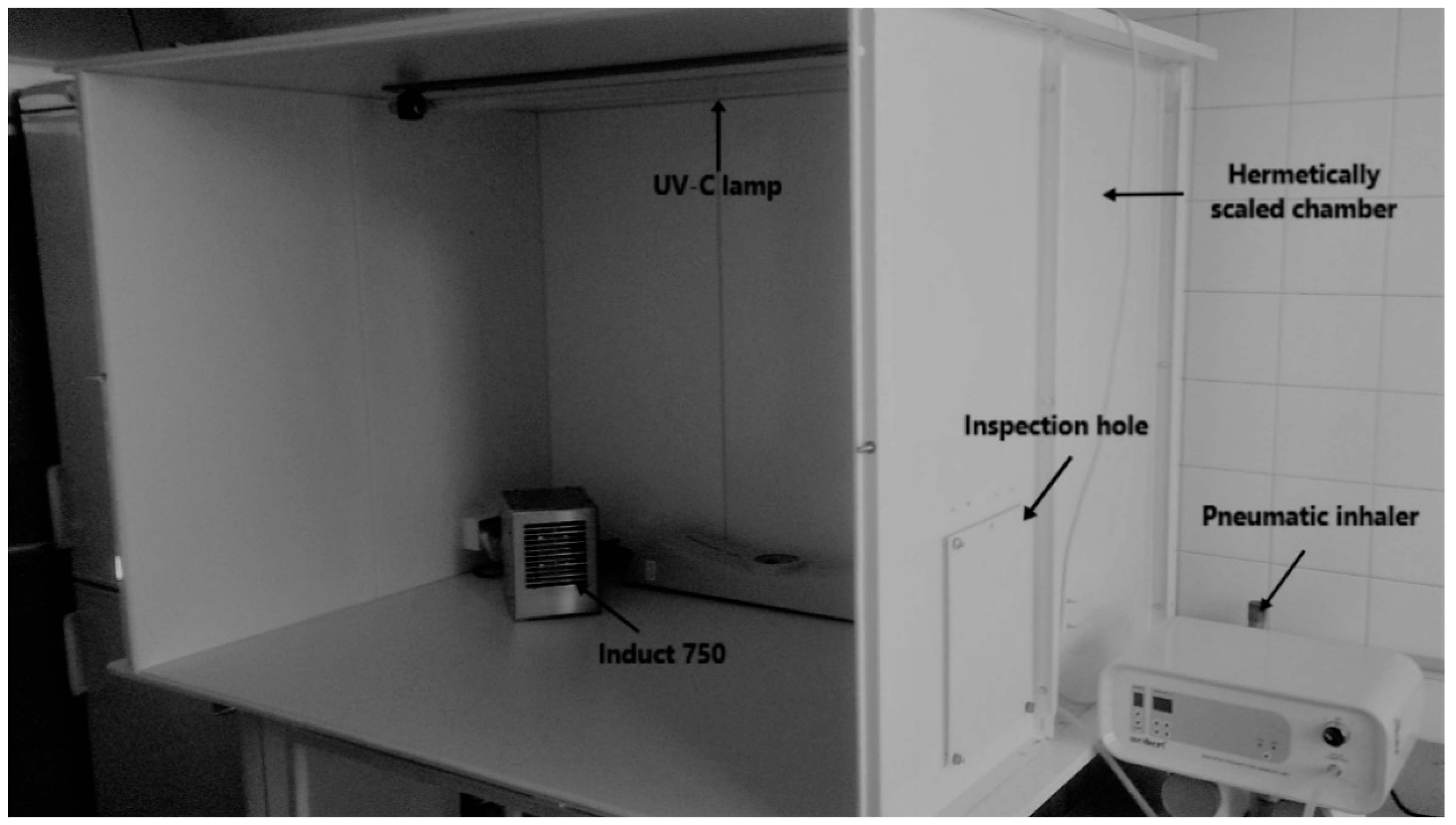
2.2. RCI Efficiently
2.3. Experimental Environment Conditions
2.4. Statistical Analysis
3. Results
3.1. Experimental Environment Coditions
3.2. Changes in the Number of Enterococcus spp. in the Air
3.3. Changes in the Number of C. difficile in the Air
3.4. Changes in the Number of Staphylococcus aureus in the Air
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beggs, C.; Knibbs, L.D.; Johnson, G.R.; Morawska, L. Environmental contamination and hospital-acquired infection: Factors that are easily overlooked. Indoor Air 2015, 25, 462–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.C.; Wu, P.C.; Tseng, C.H.; Su, H.J. Indoor air quality varies with ventilation types and working areas in hospitals. Build. Environ. 2015, 85, 190–195. [Google Scholar] [CrossRef]
- Gilbert, Y.; Veillette, M.; Duchaine, C. Airborne bacteria and antibiotic resistance genes in hospital rooms. Aerobiologia 2010, 26, 185–194. [Google Scholar] [CrossRef]
- Park, D.U.; Yeom, J.K.; Lee, W.J.; Lee, K.M. Assessment of the levels of airborne bacteria, gram-negative bacteria and fungi in hospital lobbies. Int. J. Environ. Res. Publ. Health 2013, 10, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, S.; Cencetti, S.; Rovesti, S.; Marchesi, I.; Bargellini, A.; Borella, P. Risk factors for particulate and microbial contamination of air in operating theatres. J. Hosp. Infect. 2007, 66, 320–326. [Google Scholar] [CrossRef]
- Wan, G.H.; Chung, F.F.; Tang, C.S. Long-term surveillance of air quality in medical center operating rooms. Am. J. Infect. Control 2011, 39, 302–308. [Google Scholar] [CrossRef]
- Shiomor, T.; Miyamoto, H.; Makishima, K.; Yoshida, M.; Fujiyoshi, T.; Udaka, T.; Inaba, T.; Hiraki, N. Evaluation of bedmaking-related airborne and surface methicillin-resistant Staphylococcus aureus contamination. J. Hosp. Infect. 2002, 50, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, J.; Chartier, Y.; Pessoa-Silva, C.L.; Jensen, P.; Li, Y.; Seto, W.H. Natural Ventilation for Infection Control in Health-Care Settings; World Health Organization: Geneva, Switzerland, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK143277/ (accessed on 8 April 2020).
- Lim, T.; Chob, J.; Kim, B.S. The predictions of infection risk of indoor airborne transmission of diseases in high-rise hospitals: Tracer gas simulation. Energy Build. 2010, 42, 1172–1181. [Google Scholar] [CrossRef]
- Wallner, P.; Munoz, U.; Tappler, P.; Wanka, A.; Kundi, M.; Shelton, J.F.; Hutter, H.P. Indoor environmental quality in mechanically ventilated, energy-efficient buildings vs. conventional buildings. Int. J. Environ. Res. Public Health 2015, 12, 14132–14147. [Google Scholar] [CrossRef]
- Gao, M.; An, T.; Li, G.; Nie, X.; Yip, H.L.; Zhao, H.; Wong, P.K. Genetic studies of the role of fatty acid and coenzyme A in photocatalytic inactivation of Escherichia coli. Water Res. 2012, 46, 3951–3957. [Google Scholar] [CrossRef]
- Hayden, M.K. Insights into the epidemiology and control of infection with vancomycin-resistant enterococci. Clin. Infect. Dis. 2000, 31, 1058–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathnayake, I.U.; Hargreaves, M.; Huygens, F. Antibiotic resistance and virulence traits in clinical and environmental Enterococcus faecalis and Enterococcus faecium isolates. Syst. Appl. Microbiol. 2012, 35, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Auchtung, J.M. Control of Clostridium difficile infection by defined microbial communities. Microbiol. Spectr. 2017, 5, 1–25. [Google Scholar]
- Awad, M.M.; Johanesen, P.A.; Carter, G.P.; Rose, E.; Lyras, D. Clostridium difficile virulence factors: Insights into an anaerobic spore-forming pathogen. Gut Microbes 2014, 5, 579–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada-Gómez, C.; López-Ureña, D.; Acuña-Amador, L.; Villalobos-Zúñiga, M.; Du, T.; Freire, R.; Guzmán-Verri, C.; del Mar Gamboa-Coronado, M.; Lawley, T.D.; Moreno, E.; et al. Emergence of an outbreak-associated Clostridium difficile variant with increased virulence. J. Clin. Microbiol. 2015, 53, 1216–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, K.; Smith, C.F.; Snelling, A.M.; Kerr, K.G.; Banfield, K.R.; Sleigh, P.A.; Beggs, C.B. Aerial dissemination of Clostridium difficile spores. BMC Infect. Dis. 2008, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Best, E.L.; Fawley, W.N.; Parnell, P.; Wilcox, M.H. The potential for airborne dispersal of Clostridium difficile from symptomatic patients. Clin. Infect. Dis. 2010, 50, 1450–1457. [Google Scholar] [CrossRef] [Green Version]
- Carlson, P.E.; Kaiser, A.M.; McColm, S.A.; Bauer, J.M.; Young, V.B.; Aronoff, D.M.; Hanna, P.C. Variation in germination of Clostridium difficile clinical isolates correlates to disease severity. Anaerobe 2015, 33, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Gerding, D.N.; Muto, C.A.; Owens, R.C. Measures to control and prevent Clostridium difficile infection. Clin. Infect. Dis. 2008, 46, S43–S49. [Google Scholar] [CrossRef] [Green Version]
- Fawley, W.N.; Parnell, P.; Verity, P.; Freeman, J.; Wilcox, M.H. Molecular epidemiology of endemic Clostridium difficile infection and the significance of subtypes of the United Kingdom epidemic strain (PCR ribotype 1). J. Clin. Microbiol. 2005, 43, 2685–2696. [Google Scholar] [CrossRef] [Green Version]
- Snelling, A.M.; Beggs, C.B.; Kerr, K.G.; Shepherd, S.J. Spores of Clostridium difficile in Hospital Air. Clin. Infect. Dis. 2010, 51, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Aires de Sousa, M.; Lencastre, H. Bridges from hospitals to the laboratory: Genetic portraits of methicillin-resistant Staphylococcus aureus clones. Pathog. Dis. 2004, 40, 101–111. [Google Scholar]
- Berning, C.; Lanckohr, C.; Baumgartner, H.; Drescher, M.; Becker, K.; Peters, G.; Köck, R.; Kahl, B.C. Fatal infections caused by methicillin-resistant Staphylococcus aureus of clonal complex 398: Case presentations and molecular epidemiology. JMM Case Rep. 2015, 2, e000024. [Google Scholar] [CrossRef]
- Shirmori, T.; Miyamoto, H.; Makishima, K. Significance of airbone transmission of methicillin-resistant Staphylococus aureus in an otolaryngology-head and neck surgery unit. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 644–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Space Fundation, Radiant Catalytic Ionization Air & Water Purification. Available online: http://www.spacefoundation.org/programs/space-certification/certified-products/space-technology/radiant-catalytic-ionization-air (accessed on 10 February 2020).
- Grinshpun, S.A.; Adhikari, A.; Honda, T.; Kim, K.Y.; Toivola, M.; Rao, K.S. Reponen, Control of aerosol contaminants in indoor air: Combining the particle concentration reduction with microbial inactivation. Environ. Sci. Technol. 2007, 41, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Materiomics 2017, 3, 3–16. [Google Scholar] [CrossRef]
- Gao, W.; Howden, B.P.; Stinear, T.P. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr. Opin. Microbiol. 2017, 41, 76–82. [Google Scholar] [CrossRef]
- Hodgson, A.I.; Destaillats, H.; Sullivan, D.P.; Fisk, W.J. Performance of ultraviolet photocatalytic oxidation for indoor air cleaning applications. Indoor Air 2007, 17, 305–316. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Huang, G.; Xia, D.; An, T.; Ng, T.W.; Yip, H.Y.; Li, G.; Zhao, H.; Wong, P.K. Dual roles of capsular extracellular polymeric substances in photocatalytic inactivation of Escherichia coli: Comparison of E. coli BW25113 and isogenic mutants. Appl. Environ. Microbiol. 2015, 81, 5174–5183. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wang, Y. Photocatalytic effect on plasmid DNA damage under different UV irradiation time. Build. Environ. 2008, 43, 253–257. [Google Scholar] [CrossRef]
- Ortega, M.T.; Franken, L.J.; Hatesohl, P.R.; Marsden, L.J. Efficacy of ecoquest radiant catalytic Ionization cell and breeze at ozone generator at reducing microbial populations on stainless steel surfaces. J. Rapid Meth. Aut. Mic. 2007, 5, 359–368. [Google Scholar] [CrossRef]
- ActivTek. Instruction of Use Induct 750 Product. Available online: http://activtek.pl/wp-content/uploads/2014/12/INDUCT-750-Spec-Sheet.pdf (accessed on 10 February 2020).
- WHO. Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide Global Update 2005 Summary of Risk Assessment; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Skowron, K.; Grudlewska, K.; Kwiecińska-Piróg, J.; Gryń, G.; Śrutek, M.; Gospodarek-Komkowska, E. Efficacy of radiant catalytic ionization to reduce bacterial populations in air and on different surfaces. Sci. Total Environ. 2018, 610–611, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Leung, N.H.; Cowling, B.J.; Yang, Z.F. Role of viral bioaerosols in nosocomial infections and measures for prevention and control. J. Aerosol Sci. 2018, 117, 200–211. [Google Scholar]
- Fletcher, L.A.; Noakes, C.J.; Beggs, C.B.; Sleigh, P.A. The importance of bioaerosols in hospital infections and the potential for control using germicidal ultraviolet irradiation. In Proceedings of the 1st Seminar on Applied Aerobiology, Murcia, Spain, 20 May 2004. [Google Scholar]
- Lai, K.; Nasir, Z.A.; Taylor, J. Bioaerosols and hospital infections. Aerosol Sci. 2014, 117, 271–289. [Google Scholar]
- Matsunaga, T.; Tamoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powers. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Gogniat, G.; Thyssen, M.; Denis, M.; Pulgarin, C.; Dukan, S. The bactericidal effect of TiO2 photocatalysis in volves adsorption on to catalyst and the loss of membrane integrity. FEMS Microbiol. Lett. 2006, 258, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Paspaltsis, I.; Kotta, K.; Lagoudaki, R.; Grigoriadis, N.; Poulios, I.; Sklaviadis, T. Titanium dioxide photocatalytic inactivation of prions. J. Gen. Virol. 2006, 87, 3125–3130. [Google Scholar] [CrossRef]
- Skowron, K.; Wiktorczyk, N.; Kwiecińska-Piróg, J.; Sękowska, A.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. Elimination of Klebsiella pneumoniae NDM from the air and selected surfaces in hospital using radiant catalytic ionization. Lett. Appl. Microbiol. 2019, 69, 333–338. [Google Scholar] [CrossRef]
- McDonald, L.C.; Killgore, G.E.; Thompson, A.; Owens, R.C.; Kazakova, S.V.; Sambol, S.P.; Johnson, S.; Gerding, D.N. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 2005, 353, 2433–2441. [Google Scholar] [CrossRef] [Green Version]
- Smits, W.K.; Lyras, D.; Lacy, D.B.; Wilcox, M.H.; Kuijper, E.J. Clostridium difficile infection. Nat. Rev. Dis. Primers 2012, 2, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warny, M.; Pepin, J.; Fang, A.; Killgore, G.; Thompson, A.; Brazier, J.; Frost, E.; McDonald, L.C. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005, 36, 1079–1084. [Google Scholar] [CrossRef]
- Barabasz, W. The Results of the Research Carried out in University of Agriculture in Krakow about Efficacy of RCI Technology. 2014. Available online: http://activtek.pl/wp-content/uploads/2014/07/Uniwersytet-Rolniczy.pdf (accessed on 10 February 2020).
- Wiktorczyk, N.; Kwiecińska-Piróg, J.; Skowron, K.; Michalska, A.; Zalas-Więcek, P.; Białucha, A.; Budzyńska, A.; Grudlewska-Buda, K.; Prażyńska, M.; Gospodarek-Komkowska, E. Assessment of endoscope cleaning and disinfection efficacy, and the impact of endoscope storage on the microbiological safety level. J. App. Microb. 2019, 128, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Wałecka-Zacharska, E.; Grudlewska, K.; Kwiecińska-Piróg, J.; Wiktorczyk, N.; Kowalska, M.; Paluszak, Z.; Kosek-Paszkowska, K.; Brożek, K.; Korkus, J.; et al. Effect of selected environmental factors on the microbicidal effectiveness of radiant catalytic ionization. Front. Microbiol. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- ActivTek. Documents About Use of Product. Available online: http://activtek.pl/dokumenty/ (accessed on 10 February 2020).
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef] [PubMed]
| Parameter | RCI Technology Usage | |
|---|---|---|
| Before | After | |
| Negative ions concentration [ion cm−3] | <1.0 × 104 | 7.2 × 105 (±0.7 × 105) |
| Temperature [°C] | 25.3 (±0.4) | 24.8 (±0.2) |
| Relative humidity [%] | 50.4 (±1.1) | 49.2 (±0.8) |
| Strain | The Average Number of Bacteria after Nebulization [CFU m−3] | The Average Number of Bacteria after 20 min without RCI | Precipitate Factor RwithoutRCI (K+) [%] | The Average Number of Bacteria after Using the Induct 750 [CFU m−3] | Percentage of Reduction in the Number of Bacteria RRCI [%] |
|---|---|---|---|---|---|
| Enterococcus faecalis PCM 1861 | 3.64 × 105 (±5.31 × 104) * | 2.89 × 105 (±2.31 × 104) | 20.60 a | 8.72 × 102 (±7.05 × 103) | 99.76 b |
| Enterococcus faecalis ATCC 51299 | 3.91 × 105 (±7.13 × 104) * | 3.04 × 105 (±3.16 × 104) | 22.25 a | 3.89 × 104 (±7.05 × 103) | 90.05 b |
| Enterococcus faecium ATCC 51559 | 3.18 × 105 (±8.23 × 104) | 2.58 × 105 (±5.31 × 104) | 18.99 a | 1.25 × 103 (±9.63 × 102) | 99.61 b |
| Strain | The Average Number of Bacteria after Nebulization [CFU m−3] | The Average Number of Bacteria after 20 min without RCI | Precipitate Factor RwithoutRCI (K+) [%] | The Average Number of Bacteria after Using the Induct 750 [CFU m−3] | Percentage of Reduction in the Number of Bacteria RRCI [%] |
|---|---|---|---|---|---|
| CDI tox(−) | 3.14 × 104 (±3.54 × 102) * | 2.28 × 104 (±1.11 × 102) | 27.36 a | 5.15 × 103 (±7.21 × 102) | 83.57 b |
| CDI MXF-R/ tox A/B/bin(+) | 2.43 × 105 (±2.47 × 104) | 1.74 × 105 (±2.26 × 104) | 28.42 a | 1.30 × 104 (±1.85 × 103) | 94.65 b |
| CDI PCR 027 | 8.20 × 103 (±4.24 × 102) | 6.03 × 104 (±3.81 × 102) | 26.46 a | 2.92 × 103 (±1.87 × 102) | 64.45 b |
| Strain | The Average Number of Bacteria after Nebulization [CFU m−3] | The Average Number of Bacteria after 20 min without RCI | Precipitate Factor RwithoutRCI (K+) [%] | The Average Number of Bacteria after Using the Induct 750 [CFU m−3] | Percentage of Reduction in the Number of Bacteria RRCI [%] |
|---|---|---|---|---|---|
| Staphylococcus aureus MRSA | 4.70 × 105 (±7.16 × 104) * | 3.65 × 105 (±3.44 × 104) | 22.34 a | 2.06 × 104 (±1.72 × 104) | 95.61 b |
| Staphylococcus aureus non-MRSA | 5.10 × 105 (±1.12 × 105) | 3.90 × 105 (±1.89 × 105) | 23.60 a | 6.50 × 102 (±9.22 × 101) | 99.87 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowron, K.; Grudlewska-Buda, K.; Kożuszko, S.; Wiktorczyk, N.; Skowron, K.J.; Mikucka, A.; Bernaciak, Z.; Gospodarek-Komkowska, E. Efficacy of Radiant Catalytic Ionization in Reduction of Enterococcus spp., Clostridioides difficile and Staphylococcus aureus in Indoor Air. Atmosphere 2020, 11, 764. https://doi.org/10.3390/atmos11070764
Skowron K, Grudlewska-Buda K, Kożuszko S, Wiktorczyk N, Skowron KJ, Mikucka A, Bernaciak Z, Gospodarek-Komkowska E. Efficacy of Radiant Catalytic Ionization in Reduction of Enterococcus spp., Clostridioides difficile and Staphylococcus aureus in Indoor Air. Atmosphere. 2020; 11(7):764. https://doi.org/10.3390/atmos11070764
Chicago/Turabian StyleSkowron, Krzysztof, Katarzyna Grudlewska-Buda, Sylwia Kożuszko, Natalia Wiktorczyk, Karolina Jadwiga Skowron, Agnieszka Mikucka, Zuzanna Bernaciak, and Eugenia Gospodarek-Komkowska. 2020. "Efficacy of Radiant Catalytic Ionization in Reduction of Enterococcus spp., Clostridioides difficile and Staphylococcus aureus in Indoor Air" Atmosphere 11, no. 7: 764. https://doi.org/10.3390/atmos11070764
APA StyleSkowron, K., Grudlewska-Buda, K., Kożuszko, S., Wiktorczyk, N., Skowron, K. J., Mikucka, A., Bernaciak, Z., & Gospodarek-Komkowska, E. (2020). Efficacy of Radiant Catalytic Ionization in Reduction of Enterococcus spp., Clostridioides difficile and Staphylococcus aureus in Indoor Air. Atmosphere, 11(7), 764. https://doi.org/10.3390/atmos11070764





