Grazing under Irrigation Affects N2O-Emissions Substantially in South Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Description
2.2. Experimental Layout and Treatments
2.3. Pasture and Grazing Management
2.4. N2O Measurements
2.5. Flux Calculations
2.6. Nitrogen Balance
2.7. Regression Analysis and Emission Factors
2.8. Pasture Sampling
2.8.1. Herbage Yield and Forage Quality
2.8.2. Soil Sampling
2.9. Weather Data, Soil Temperature, and Soil Water
2.10. Statistical Analyses
3. Results
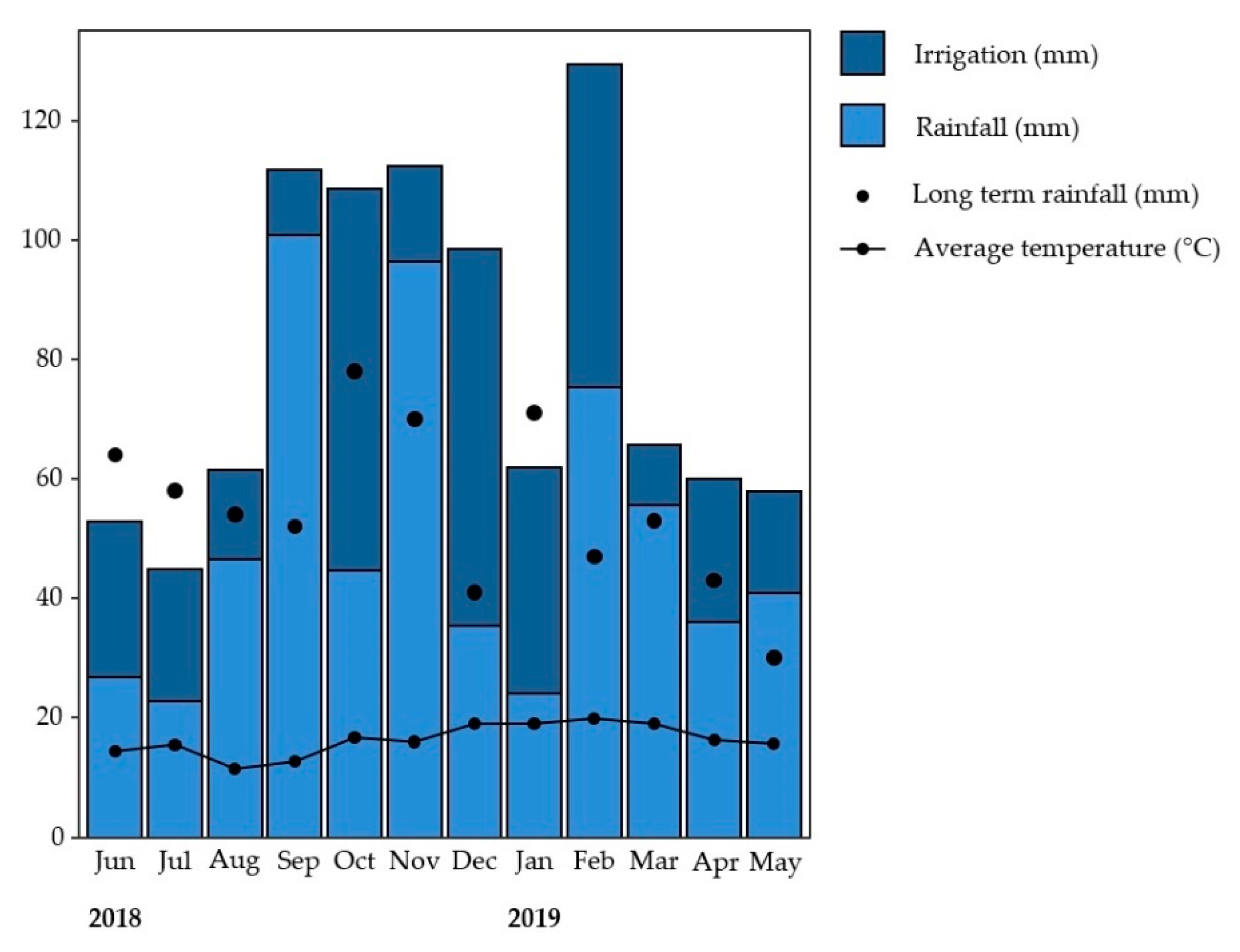
3.1. Weather Results and Environmental Variables
3.2. Herbage and Nitrogen Yield
3.3. Daily N2O Fluxes
3.4. Accumulated N2O-Losses
3.5. Regressions and Emission Factors
4. Discussion
4.1. Nitrogen Yield
4.2. Daily N2O Fluxes
4.3. Accumulated N2O-losses
4.4. Predictions of Annual N2O Emissions from Pastures
4.5. Mitigation Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006. [Google Scholar]
- Stevens, R.J.; Laughlin, R.J.; Burns, L.C.; Arah, J.R.M.; Hood, R.C. Measuring the contributions of nitrification and denitrification to the flux of nitrous oxide from soil. Soil Biol. Biochem. 1997, 29, 139–151. [Google Scholar] [CrossRef]
- Team, C.W.; Pachauri, R.K.; Meyer, L.A. IPCC 2014, Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Chang. 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Bellarby, J.; Foereid, B.; Hastings, A.F.S.J.; Smith, P. Cool Farming: Climate Impacts of Agriculture and Mitigation Potential; Greenpeace International: Amsterdam, The Netherlands, 2008; Volume 44. [Google Scholar]
- DEA. Greenhouse Gas Inventory for South Africa: 2000 to 2015; National Inventory Report; Department of Environmental Affairs: Pretoria, Republic of South Africa, 2016. Available online: https://www.environment.gov.za/sites/default/files/reports/GHG-National-Inventory-Report-SouthAfrica-2000-2015.pdf (accessed on 13 January 2020).
- Department of Environmental Affairs. South Africa’s Low-Emission Development Strategy 2050; Department of Environmental Affairs: Pretoria, South Africa, 2018. [Google Scholar]
- Aneja, V.P.; Schlesinger, W.H.; Li, Q.; Nahas, A.; Battye, W.H. Characterization of atmospheric nitrous oxide emissions from global agricultural soils. SN Appl. Sci. 2019, 1, 1622. [Google Scholar] [CrossRef] [Green Version]
- Ramanantenasoa, M.M.J.; Génermont, S.; Gilliot, J.M.; Bedos, C.; Makowski, D. Meta-modeling methods for estimating ammonia volatilization from nitrogen fertilizer and manure applications. J. Environ. Manag. 2019, 236, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Milk Producers’ Organization. Lacto Data. A Milk SA Publication Compiled by the Milk Producers’ Organization. Milk Producer Statistics. 2019. Available online: https://milksa.co.za/taxonomy/term/38 (accessed on 20 January 2020).
- Gertenbach, W. Dairy Farming in South Africa-Where to Now? Institute for Animal Production Western Cape Department of Agriculture. 2007. Available online: http://www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Dairy/Documents/18_William_Gertenbach__paper.pdf (accessed on 6 March 2020).
- Reinecke, R.; Casey, N.H. A whole farm model for quantifying total greenhouse gas emissions on South African dairy farms. S. Afr. J. Anim. Sci. 2017, 47, 883. [Google Scholar] [CrossRef]
- Milk Producers’ Organization. Lacto Data. A Milk SA Publication Compiled by the Milk Producers’ Organization. Milk Producer Statistics. 2017. Available online: https://milksa.co.za/taxonomy/term/38 (accessed on 20 January 2020).
- Van der Colf, J. The production Potential of Kikuyu (Pennisetum Clandestinum) Pastures Over-Sown with Ryegrass (Lolium spp.). Master´s Thesis, University of Pretoria, Pretoria, South Africa, 2011. [Google Scholar]
- Houlton, B.Z.; Almaraz, M.; Aneja, V.; Austin, A.T.; Bai, E.; Cassman, K.G.; Compton, J.E.; Davidson, E.A.; Erisman, J.W.; Galloway, J.N.; et al. A World of Cobenefits: Solving the Global Nitrogen Challenge. Earths Future 2019, 7, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Mosier, A.R.; Doran, J.W.; Freney, J.R. Managing soil denitrification. J. Soil Water Conserv. 2002, 57, 505–512. [Google Scholar]
- Signor, D.; Cerri, C.E.P. Nitrous oxide emissions in agricultural soils: A review. Pesqui. Agropecuária Trop. 2013, 43, 322–338. [Google Scholar] [CrossRef]
- Swanepoel, P.A.; Habig, J.; du Preez, C.C.; Botha, P.R.; Snyman, H.A. Biological quality of a podzolic soil after 19 years of irrigated minimum-till kikuyu-ryegrass pasture. Soil Res. 2014, 52, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Sutton, M.A.; Oenema, O.; Erisman, J.W.; Leip, A.; van Grinsven, H.; Winiwarter, W. Too much of a good thing. Nature 2011, 472, 159–161. [Google Scholar] [CrossRef] [Green Version]
- Botha, P.R. Factors influencing the persistence and production potential of kikuyu (Pennisetum clandestinum) over-sown with different ryegrass and clover species in the southern Cape. Elsenbg. Joernall 2009, 3, 4–9. [Google Scholar]
- Hao, X.; Chang, C.; Carefoot, J.M.; Janzen, H.H.; Ellert, B.H. Nitrous oxide emissions from an irrigated soil as affected by fertilizer and straw management. Nutr. Cycl. Agroecosyst. 2001, 60, 1–8. [Google Scholar] [CrossRef]
- Mosier, A.; Schimel, D.; Valentine, D.; Bronson, K.; Parton, W. Methane and nitrous oxide fluxes in native, fertilized and cultivated grasslands. Nature 1991, 350, 330–332. [Google Scholar] [CrossRef]
- Breitenbeck, G.A.; Blackmer, A.M.; Bremner, J.M. Effects of different nitrogen fertilizers on emission of nitrous-oxide from soil. Geophys. Res. Lett. 1980, 7, 85–88. [Google Scholar] [CrossRef]
- Velthof, G.L.; Oenema, O.; Postma, R.; van Beusichem, M.L. Effects of type and amount of applied nitrogen fertilizer on nitrous oxide fluxes from intensively managed grassland. Nutr. Cycl. Agroecosyst. 1996, 46, 257–267. [Google Scholar] [CrossRef]
- Zanatta, J.A.; Bayer, C.; Vieira, F.C.B.; Gomes, J.; Tomazi, M. Nitrous oxide and methane fluxes in south Brazilian gleysol as affected by nitrogen fertilizers. Rev. Bras. Ciência do Solo 2010, 34, 1653–1665. [Google Scholar] [CrossRef] [Green Version]
- Shcherbak, I.; Millar, N.; Robertson, G.P. Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc. Natl. Acad. Sci. USA 2014, 111, 9199–9204. [Google Scholar] [CrossRef] [Green Version]
- Viljoen, C.; van der Colf, J.; Swanepoel, P.A. Benefits are Limited with High Nitrogen Fertiliser Rates in Kikuyu-Ryegrass Pasture Systems. Land 2020, 9, 173. [Google Scholar] [CrossRef]
- Swanepoel, P.A.; du Preez, C.C.; Botha, P.R.; Snyman, H.A. A critical view on the soil fertility status of minimum-till kikuyu–ryegrass pastures in South Africa. Afr. J. Range For. Sci. 2015, 32, 113–124. [Google Scholar] [CrossRef]
- Sutton, M.A.; Howard, C.M.; Brownlie, W.J.; Skiba, U.; Hicks, W.K.; Winiwarter, W.; van Grinsven, H.; Bleeker, A.; Westhoek, H.; Oenema, O.; et al. The European Nitrogen Assessment 6 years after: What was the outcome and what are the future research challenges? Proc. Int. Conf. Innov. Solut. Sustain. Manag. Nitrogen 2017, 40–49. [Google Scholar]
- Roy, P.; Nei, D.; Orikasa, T.; Xu, Q.; Okadome, H.; Nakamura, N.; Shiina, T. A review of life cycle assessment (LCA) on some food products. J. Food Eng. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Swanepoel, P.A.; du Preez, C.C.; Botha, P.R.; Snyman, H.A.; Habig, J. Soil quality characteristics of kikuyu-ryegrass pastures in South Africa. Geoderma 2014, 232–234, 589–599. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods, 14th ed.; John Wiley & Sons.: Madison, WI, USA, 2020. [Google Scholar]
- Hutchinson, G.L.; Mosier, A.R. Improved Soil Cover Method for Field Measurement of Nitrous Oxide Fluxes. Soil Sci. Soc. Am. J. 1981, 45, 311–316. [Google Scholar] [CrossRef]
- Rochette, P. Towards a standard non-steady-state chamber methodology for measuring soil N2O emissions. Anim. Feed Sci. Technol. 2011, 166–167, 141–146. [Google Scholar] [CrossRef]
- Beetz, S.; Liebersbach, H.; Glatzel, S.; Jurasinski, G.; Buczko, U.; Höper, H. Effects of land use intensity on the full greenhouse gas balance in an Atlantic peat bog. Biogeosciences 2013, 10, 1067–1082. [Google Scholar] [CrossRef] [Green Version]
- Nennich, T.D.; Harrison, J.H.; VanWieringen, L.M.; Meyer, D.; Heinrichs, A.J.; Weiss, W.P.; St-Pierre, N.R.; Kincaid, R.L.; Davidson, D.L.; Block, E. Prediction of manure and nutrient excretion from dairy cattle. J. Dairy Sci. 2005, 88, 3721–3733. [Google Scholar] [CrossRef] [Green Version]
- Loague, K.; Green, R.E. Statistical and graphical methods for evaluating solute transport models: Overview and application. J. Contam. Hydrol. 1991, 7, 51–73. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis Part 2 (Agronomy Monographs 9); Wiley: Madison, WI, USA, 1982. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-147. 2020. Available online: https://CRAN.R-project.org/package=nlme (accessed on 11 March 2020).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Laird, N.M.; Ware, J.H. Random-effects models for longitudinal data. Biometrics 1982, 38, 963–974. [Google Scholar] [CrossRef]
- Verbeke, G.; Molenberghs, G. Linear Mixed Models for Longitudinal Data. In Springer Series in Statistics; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Kozak, M.; Piepho, H.P. What’s normal anyway? Residual plots are more telling than significance tests when checking ANOVA assumptions. J. Agron. Crop Sci. 2018, 204, 86–98. [Google Scholar] [CrossRef]
- Bretz, F.; Hothorn, T.; Westfall, P. Multiple Comparisons Using R, 1st ed.; Chapman and Hall/CRC: London, UK, 2011. [Google Scholar]
- Botha, P.R.; Meeske, R.; Snyman, H.A. Kikuyu over-sown with ryegrass and clover: Grazing capacity, milk production and milk composition. Afr. J. Range Forage Sci. 2008, 25, 103–110. [Google Scholar] [CrossRef]
- Van der Colf, J.; Botha, P.R.; Meeske, R.; Truter, W.F. Seasonal dry matter production, botanical composition and forage quality of kikuyu over-sown with annual or perennial ryegrass. Afr. J. Range Forage Sci. 2015, 32, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Rafique, R.; Hennessy, D.; Kiely, G. Nitrous Oxide Emission from Grazed Grassland Under Different Management Systems. Ecosystems 2011, 14, 563–582. [Google Scholar] [CrossRef]
- Frank, B.; Swensson, C. Relationship between content of crude protein in rations for dairy cows and milk yield, concentration of urea in milk and ammonia emissions. J. Dairy Sci. 2002, 85, 1829–1838. [Google Scholar] [CrossRef] [Green Version]
- Maharjan, B.; Venterea, R.T.; Rosen, C. Fertilizer and irrigation management effects on nitrous oxide emissions and nitrate leaching. Agron. J. 2014, 106, 703–714. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.G.; Hernandez-Ramirez, G.; Giltrap, D. Linear and nonlinear dependency of direct nitrous oxide emissions on fertilizer nitrogen input: A meta-analysis. Agric. Ecosyst. Environ. 2013, 168, 53–65. [Google Scholar] [CrossRef]
- Van der Weerden, T.J.; Cox, N.; Luo, J.; Di, H.J.; Podolyan, A.; Phillips, R.L.; Saggar, S.; de Klein, C.A.M.; Ettema, P.; Rys, G. Refining the New Zealand nitrous oxide emission factor for urea fertiliser and farm dairy effluent. Agric. Ecosyst. Environ. 2016, 222, 133–137. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Glob. Biogeochem. Cycles 2002, 16, 1058. [Google Scholar] [CrossRef]
- Bergstrom, D.W.; Tenuta, M.; Beauchamp, E.G. Nitrous oxide production and flux from soil under sod following application of different nitrogen fertilizers. Commun. Soil Sci. Plant Anal. 2001, 32, 553–570. [Google Scholar] [CrossRef]
- Liu, X.J.; Mosier, A.R.; Halvorson, A.D.; Zhang, F.S. Tillage and nitrogen application effects on nitrous and nitric oxide emissions from irrigated corn fields. Plant Soil 2005, 276, 235–249. [Google Scholar] [CrossRef]
- Liu, X.J.; Mosier, A.R.; Halvorson, A.D.; Zhang, F.S. The impact of nitrogen placement and tillage on NO, N2O, CH4 and CO2 fluxes from a clay loam soil. Plant Soil 2006, 280, 177–188. [Google Scholar] [CrossRef]
- Schils, R.L.M.; Van Groenigen, J.W.; Velthof, G.L.; Kuikman, P.J. Nitrous oxide emissions from multiple combined applications of fertiliser and cattle slurry to grassland. Plant Soil 2008, 310, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A.; Swank, W.T. Environmental parameters regulating gaseous nitrogen losses from two forested ecosystems via nitrification and denitrification. Appl. Environ. Microbiol. 1986, 52, 1287–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobbie, K.E.; Smith, K.A. The effects of temperature, water-filled pore space and land use on N2O emissions from an imperfectly drained gleysol. Eur. J. Soil Sci. 2001, 52, 667–673. [Google Scholar] [CrossRef]
- Firestone, M.K.; Davidson, E.A. Microbiologial Basis of NO and N2O production and consumption in soil. Exch. Trace Gases Terr. Ecosyst. Atmos. 1989, 47, 7–21. [Google Scholar]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Chemie der Erde 2016, 76, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Ju, X.; Su, F.; Meng, Q.; Oenema, O.; Christie, P.; Chen, X.; Zhang, F. Nitrous oxide and methane emissions from optimized and alternative cereal cropping systems on the North China Plain: A two-year field study. Sci. Total Environ. 2014, 472, 112–124. [Google Scholar] [CrossRef]
- Akiyama, H.; Tsuruta, H.; Watanabe, T. N2O and NO emissions from soils after the application of different chemical fertilizers. Chemosph. Glob. Chang. Sci. 2000, 2, 313–320. [Google Scholar] [CrossRef]
- Brentrup, F.; Küsters, J.; Lammel, J.; Kuhlmann, H. Methods to estimate on-field nitrogen emissions from crop production as an input to LCA studies in the agricultural sector. Int. J. Life Cycle Assess. 2000, 5, 349–357. [Google Scholar] [CrossRef]
- McAuliffe, G.A.; López-Aizpún, M.; Blackwell, M.S.A.; Castellano-Hinojosa, A.; Darch, T.; Evans, J.; Horrocks, C.; le Cocq, K.; Takahashi, T.; Harris, P.; et al. Elucidating three-way interactions between soil, pasture and animals that regulate nitrous oxide emissions from temperate grazing systems. Agric. Ecosyst. Environ. 2020, 300, 106978. [Google Scholar] [CrossRef]
- Kristensen, H.L.; McCarty, G.W.; Meisinger, J.J. Effects of Soil Structure Disturbance on Mineralization of Organic Soil Nitrogen. Soil Sci. Soc. Am. J. 2000, 64, 371–378. [Google Scholar] [CrossRef]
- Fang, S.; Xie, B.; Zhang, H. Nitrogen dynamics and mineralization in degraded agricultural soil mulched with fresh grass. Plant Soil 2007, 300, 269–280. [Google Scholar] [CrossRef]
- Van Beek, C.L.; Pleijter, M.; Jacobs, C.M.J.; Velthof, G.L.; van Groenigen, J.W.; Kuikman, P.J. Emissions of N2O from fertilized and grazed grassland on organic soil in relation to groundwater level. Nutr. Cycl. Agroecosyst. 2010, 86, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Flechard, C.R.; Ambus, P.; Skiba, U.; Rees, R.M.; Hensen, A.; van Amstel, A.; van den Pol-van Dasselaar, A.; Soussana, J.F.; Jones, M.; Clifton-Brown, J.; et al. Effects of climate and management intensity on nitrous oxide emissions in grassland systems across Europe. Agric. Ecosyst. Environ. 2007, 121, 135–152. [Google Scholar] [CrossRef]
- Hong, N.; Scharf, P.C.; Davis, J.G.; Kitchen, N.R.; Sudduth, K.A. Economically Optimal Nitrogen Rate Reduces Soil Residual Nitrate. J. Environ. Qual. 2007, 36, 354–362. [Google Scholar] [CrossRef] [Green Version]
- McSwiney, C.P.; Robertson, G.P. Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system. Glob. Chang. Biol. 2005, 11, 1712–1719. [Google Scholar] [CrossRef]
- Saggar, S.; Andrew, R.M.; Tate, K.R.; Hedley, C.B.; Rodda, N.J.; Townsend, J.A. Modelling nitrous oxide emissions from dairy-grazed pastures. Nutr. Cycl. Agroecosyst. 2004, 68, 243–255. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Velthof, G.L.; Oenema, O.; van Groenigen, K.J.; Van Kessel, C. Towards an agronomic assessment of N2O emissions: A case study for arable crops. Eur. J. Soil Sci. 2010, 61, 903–913. [Google Scholar] [CrossRef]
- Luo, J.; Balvert, S.F.; Wise, B.; Welten, B.; Ledgard, S.F.; de Klein, C.A.M.; Lindsey, S.; Judge, A. Using alternative forage species to reduce emissions of the greenhouse gas nitrous oxide from cattle urine deposited onto soil. Sci. Total Environ. 2018, 610–611, 1271–1280. [Google Scholar] [CrossRef]
- Chadwick, D.R.; Cardenas, L.M.; Dhanoa, M.S.; Donovan, N.; Misselbrook, T.; Williams, J.R.; Thorman, R.E.; McGeough, K.L.; Watson, C.J.; Bell, M.; et al. The contribution of cattle urine and dung to nitrous oxide emissions: Quantification of country specific emission factors and implications for national inventories. Sci. Total Environ. 2018, 635, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, F.M.; Cox, N.; van der Weerden, T.J.; de Klein, C.A.M.; Luo, J.; Cameron, K.C.; Di, H.J.; Giltrap, D.; Rys, G. Statistical analysis of nitrous oxide emission factors from pastoral agriculture field trials conducted in New Zealand. Environ. Pollut. 2014, 186, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Rochette, P.; Chantigny, M.H.; Ziadi, N.; Angers, D.A.; Bélanger, G.; Charbonneau, É.; Pellerin, D.; Liang, C.; Bertrand, N. Soil Nitrous Oxide Emissions after Deposition of Dairy Cow Excreta in Eastern Canada. J. Environ. Qual. 2014, 43, 829–841. [Google Scholar] [CrossRef]
- Wang, W.; Robertson, G.P.; Parton, W.J.; Dalal, R.C. Nitrous oxide emission from Australian agricultural lands and mitigation options: A review. Aust. J. Soil Res. 2003, 41, 165–195. [Google Scholar]
- Reinsch, T.; Malisch, C.; Loges, R.; Taube, F. Nitrous oxide emissions from grass–clover swards as influenced by sward age and biological nitrogen fixation. Grass Forage Sci. 2020. [Google Scholar] [CrossRef]
- De Klein, C.A.M.; van der Weerden, T.J.; Luo, J.; Cameron, K.C.; Di, H.J. A review of plant options for mitigating nitrous oxide emissions from pasture-based systems. N. Z. J. Agric. Res. 2020, 63, 29–43. [Google Scholar] [CrossRef]
- Jamali, H.; Quayle, W.; Scheer, C.; Baldock, J. Mitigation of N2O emissions from surface-irrigated cropping systems using water management and the nitrification inhibitor DMPP. Soil Res. 2016, 54, 481–493. [Google Scholar] [CrossRef]
- Jamali, H.; Quayle, W.C.; Baldock, J. Reducing nitrous oxide emissions and nitrogen leaching losses from irrigated arable cropping in Australia through optimized irrigation scheduling. Agric. For. Meteorol. 2015, 208, 32–39. [Google Scholar] [CrossRef]
- Deng, J.; Guo, L.; Salas, W.; Ingraham, P.; Charrier-Klobas, J.G.; Frolking, S.; Li, C. Changes in Irrigation Practices Likely Mitigate Nitrous Oxide Emissions From California Cropland. Glob. Biogeochem. Cycles 2018, 32, 1514–1527. [Google Scholar] [CrossRef]



| Soil Depth | pH | R | Acidity | CEC | Ca | Mg | Na | K | P | S | Cu | Zn | Mn | B | Organic C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (KCl) | (Ohm) | (cmol kg−1) | (mg kg−1) | (%) | ||||||||||
| 0–10 | 5.0 | 145 | 1.0 | 8.5 | 442 | 235 | 153 | 42 | 30 | 19.18 | 1.85 | 7.03 | 9.86 | 0.42 | 1.59 |
| 10–20 | 5.4 | 385 | 0.5 | 5.2 | 291 | 144 | 124 | 13 | 13 | 6.55 | 0.65 | 1.73 | 3.10 | 0.30 | 1.14 |
| 20–30 | 5.3 | 325 | 0.7 | 5.4 | 279 | 124 | 153 | 16 | 11 | 5.89 | 1.11 | 1.45 | 2.81 | 0.31 | 1.28 |
| Variable | Depth (cm) | N0 | N20 | N40 | N60 | N80 |
|---|---|---|---|---|---|---|
| Nmin (kg ha−1) | 0–10 | 26.95 a ± 0.67 | 27.48 a ± 1.61 | 28.86 a ± 1.98 | 44.12 b ± 1.68 | 60.02 b ± 7.53 |
| 10–20 | 26.74 a ± 2.51 | 26.74 a ± 2.94 | 25.93 a ± 0.55 | 28.23 a ± 1.47 | 38.73 a ± 6.82 | |
| 20–30 | 24.90 ab ± 2.35 | 23.06 a ± 1.19 | 23.95 a ± 1.59 | 25.39 a ± 0.74 | 31.47 b ± 1.39 | |
| NH4+-N (kg ha−1) | 0–10 | 20.40 a ± 0.72 | 20.15 ab ± 1.47 | 19.90 ab ± 1.07 | 21.66 b ± 0.48 | 23.99 ab ± 1.93 |
| 10–20 | 18.19 a ± 0.40 | 18.01 a ± 0.99 | 18.91 a ± 0.54 | 17.67 a ± 0.59 | 19.47 a ± 1.96 | |
| 20–30 | 16.43 a ± 0.78 | 17.40 a ± 1.41 | 17.79 a ± 1.23 | 17.73 a ± 1.05 | 16.42 a ± 0.87 | |
| NO3−-N (kg ha−1) | 0–10 | 6.55 a ± 1.08 | 7.33 a ± 1.38 | 8.95 a ± 1.23 | 22.47 b ± 1.23 | 36.03 b ± 6.17 |
| 10–20 | 8.55 a ± 2.39 | 8.73 a ± 3.23 | 7.02 a ± 0.27 | 10.56 a ± 1.70 | 19.27 a ± 6.38 | |
| 20–30 | 8.47 ab ± 2.13 | 5.66 a ± 0.73 | 6.16 a ± 0.70 | 7.66 a ± 1.57 | 15.05 b ± 1.59 | |
| WFPS (%) | 0–10 | 53.48 a ± 1.21 | 51.19 a ± 0.41 | 51.72 a ± 0.92 | 50.02 a ± 1.15 | 49.17 a ± 1.32 |
| 10–20 | 54.61 a ± 2.65 | 50.48 a ± 2.05 | 50.48 a ± 2.26 | 48.69 a ± 2.30 | 50.44 a ± 2.50 | |
| 20–30 | 46.52 a ± 3.54 | 41.34 a ± 1.01 | 47.06 a ± 3.26 | 43.03 a ± 3.74 | 40.4 a ± 2.11 |
| Unit | Variable | N0 | N20 | N40 | N60 | N80 |
|---|---|---|---|---|---|---|
| (t ha−1 year−1) | Herbage Yield | 18.7 a ± 1.10 | 20.6 ab ± 0.65 | 20.3 a ± 0.40 | 20.6 ab ± 0.47 | 21.5 b ± 0.70 |
| (kg ha−1 year−1) | N yield | 582 a ± 38.90 | 645 a ± 17.70 | 707 b ± 13.50 | 797 c ± 13.00 | 900 d ± 35.30 |
| Ninput | 450 | 670 | 890 | 1110 | 1330 | |
| Nbalance | −131.97 ± 38.93 | 25.06 ± 17.66 | 182.93 ± 13.43 | 312.58 ± 13.01 | 430.36 ± 35.28 | |
| N2O-N | 2.45 a ± 0.86 | 3.85 b ± 0.54 | 5.79 bc ± 0.63 | 6.5 c ± 1.28 | 15.5 d ± 2.29 |
| Treatment | N0 | N20 | N40 | N60 | N80 | R2 | RMSE | NSE |
|---|---|---|---|---|---|---|---|---|
| IPCCmin | 0.0 | 2.2 | 4.4 | 6.6 | 8.8 | 0.59 | 3.15 | 0.30 |
| IPCCmin+exc | 9.00 | 11.2 | 13.4 | 15.6 | 17.8 | 0.62 | 3.05 | −1.07 |
| EFdata | 0.0 | 1.9 | 3.8 | 5.8 | 7.7 | 0.59 | 3.15 | 0.11 |
| E1 | 1.1 | 4.0 | 6.9 | 9.7 | 12.6 | 0.62 | 3.05 | 0.64 |
| E2 | 1.2 | 4.2 | 7.2 | 9.6 | 11.9 | 0.56 | 3.30 | 0.58 |
| E3 | 3.3 | 3.6 | 4.5 | 7.3 | 15.3 | 0.77 | 2.36 | 0.72 |
| E4 | 2.7 | 3.6 | 5.4 | 8.4 | 13.3 | 0.71 | 2.65 | 0.63 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smit, H.P.J.; Reinsch, T.; Swanepoel, P.A.; Kluß, C.; Taube, F. Grazing under Irrigation Affects N2O-Emissions Substantially in South Africa. Atmosphere 2020, 11, 925. https://doi.org/10.3390/atmos11090925
Smit HPJ, Reinsch T, Swanepoel PA, Kluß C, Taube F. Grazing under Irrigation Affects N2O-Emissions Substantially in South Africa. Atmosphere. 2020; 11(9):925. https://doi.org/10.3390/atmos11090925
Chicago/Turabian StyleSmit, Hendrik P. J., Thorsten Reinsch, Pieter A. Swanepoel, Christof Kluß, and Friedhelm Taube. 2020. "Grazing under Irrigation Affects N2O-Emissions Substantially in South Africa" Atmosphere 11, no. 9: 925. https://doi.org/10.3390/atmos11090925
APA StyleSmit, H. P. J., Reinsch, T., Swanepoel, P. A., Kluß, C., & Taube, F. (2020). Grazing under Irrigation Affects N2O-Emissions Substantially in South Africa. Atmosphere, 11(9), 925. https://doi.org/10.3390/atmos11090925








