Characteristics of PM2.5 Pollution with Comparative Analysis of O3 in Autumn–Winter Seasons of Xingtai, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling of PM2.5
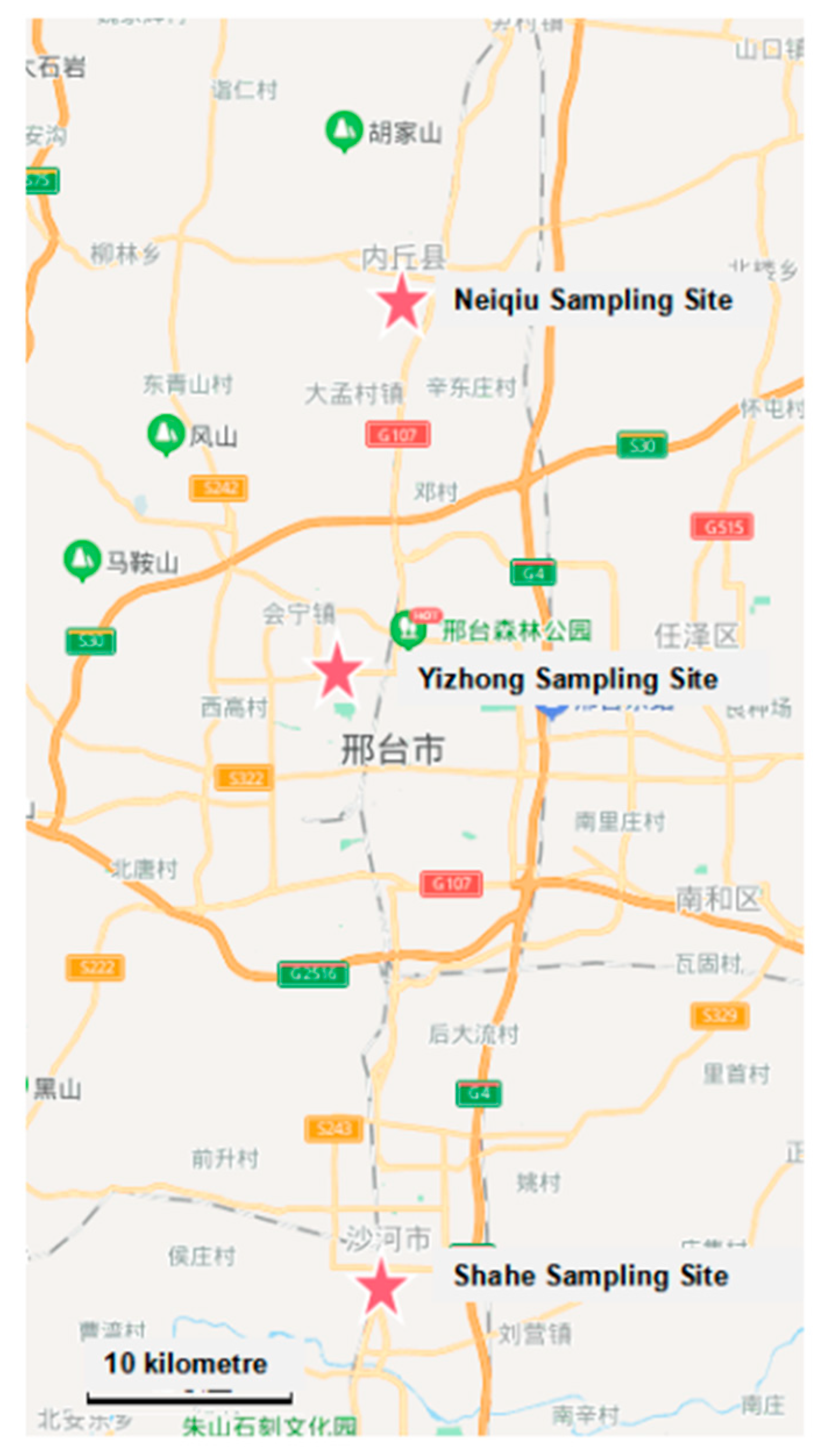
2.1.1. Sampling Position, Period, and Samples Collection
2.1.2. Quality Assurance and Quality Control of Sampling
2.2. Chemical Components Analysis
2.2.1. Water Soluble Ions
2.2.2. Carbonaceous Species
2.2.3. Inorganic Element
2.3. Data Analysis Method
2.3.1. Online Data Source
2.3.2. Analysis of Secondary Pollution
2.3.3. Back Trajectory and Clustering Analysis
3. Results and Discussion
3.1. Mass Concentrations and Chemical Compositions of PM2.5
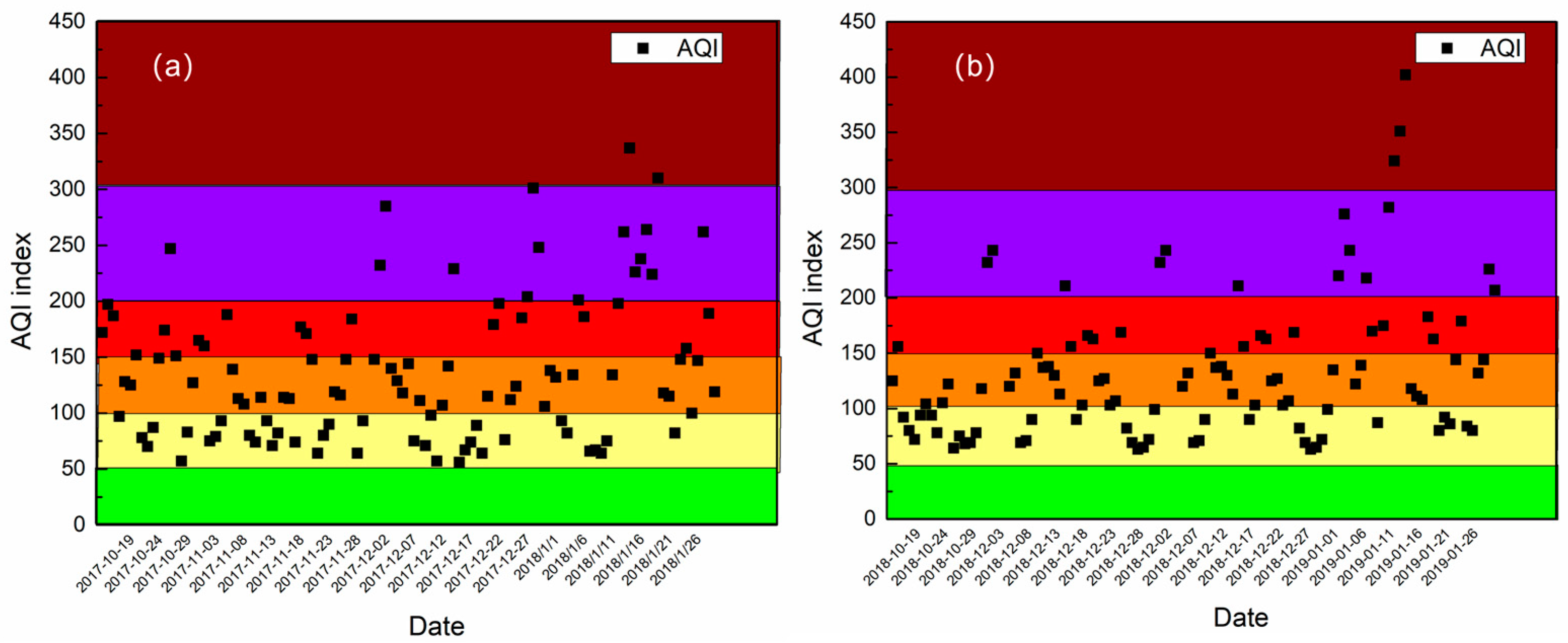
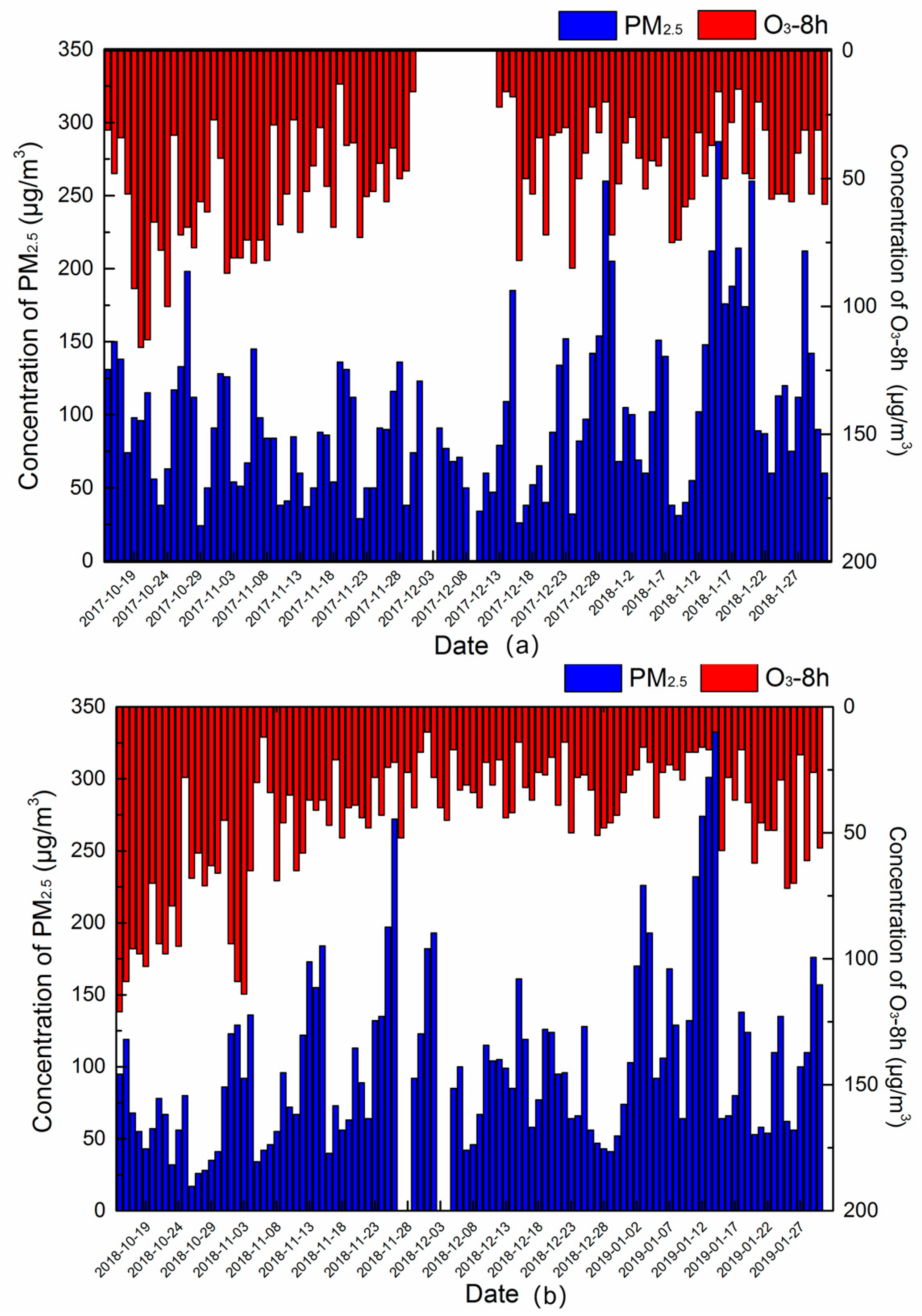
3.1.1. The Mean Values of Pollutants in the Two Autumn–Winter Seasons
3.1.2. The Mean Values of the Three Sampling Sites in the Autumn–Winter Seasons
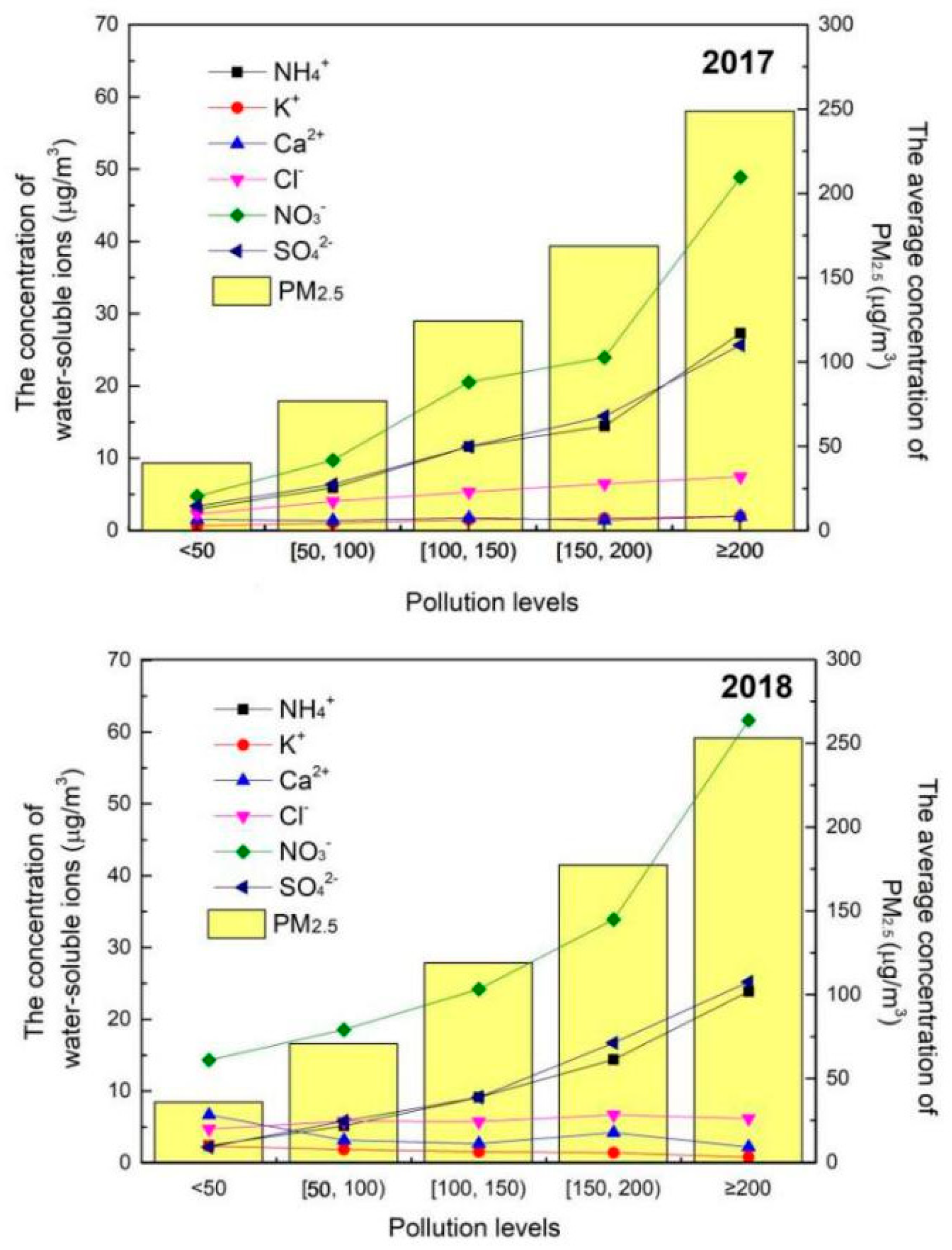
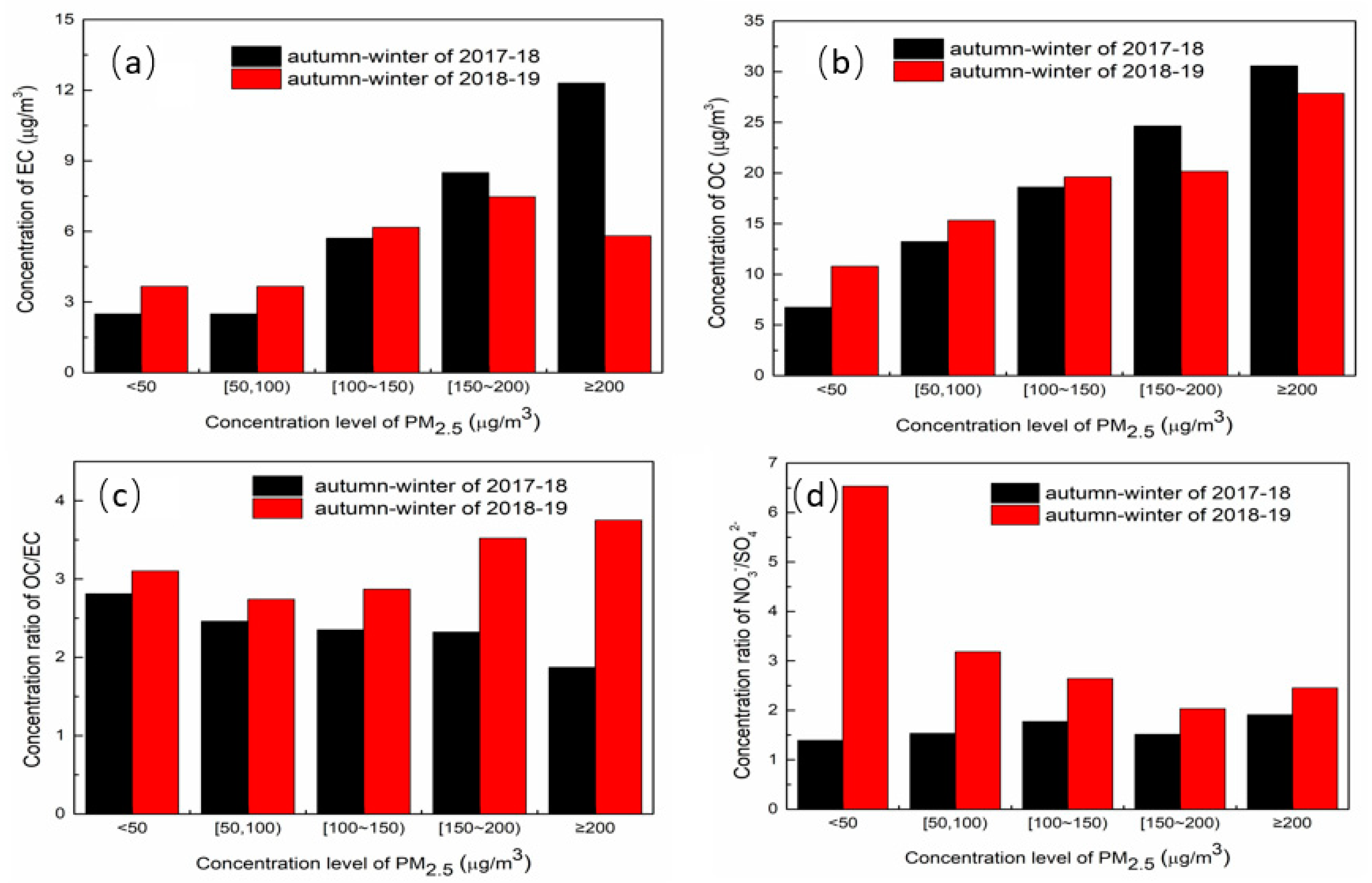
3.2. Chemical Components Analysis at Different PM2.5 Concentration Levels
3.3. Impacts of Meteorological Parameters and Secondary Formation
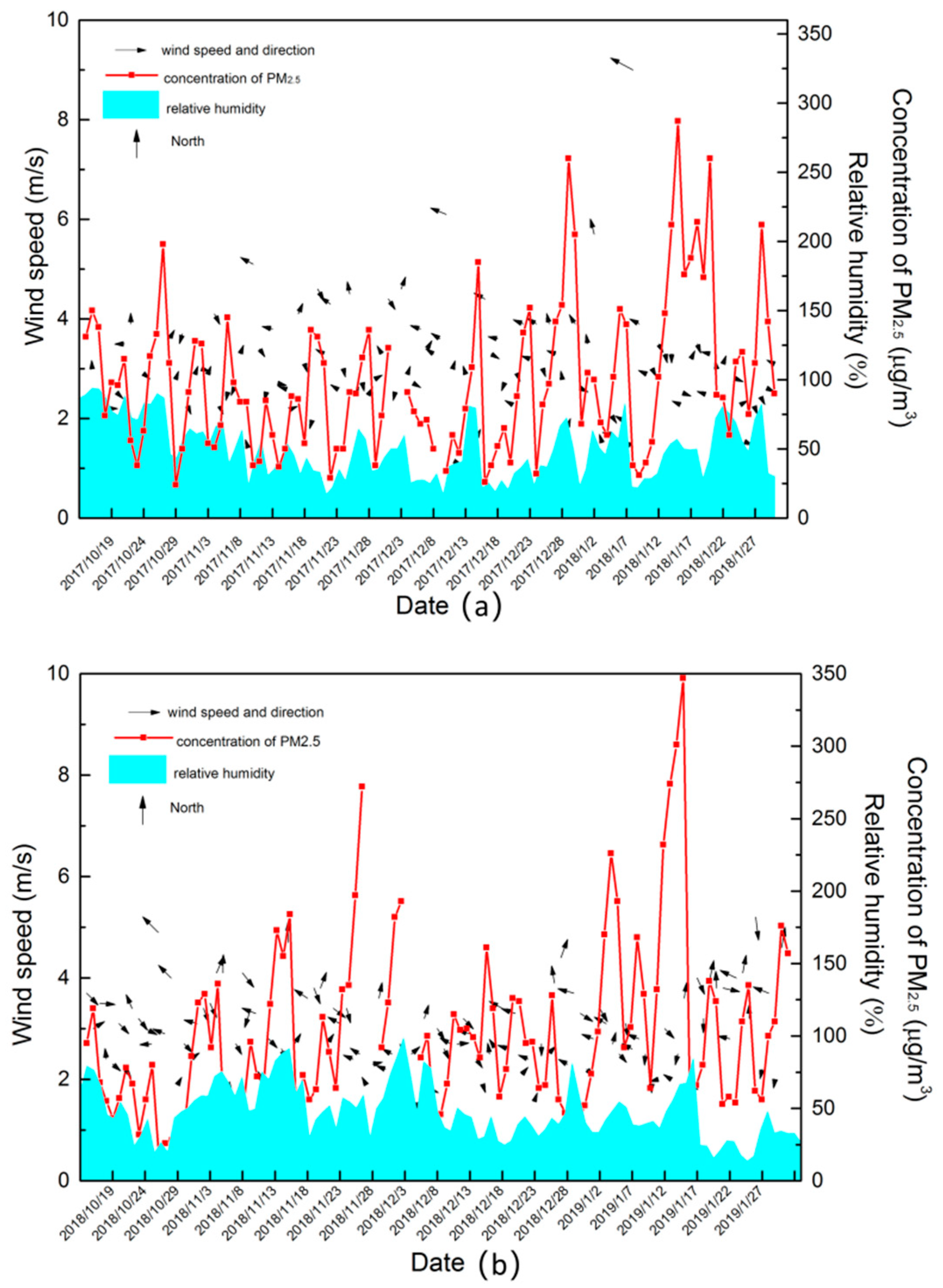
3.3.1. Impacts of Meteorological Parameters on the Formation of PM2.5 and O3
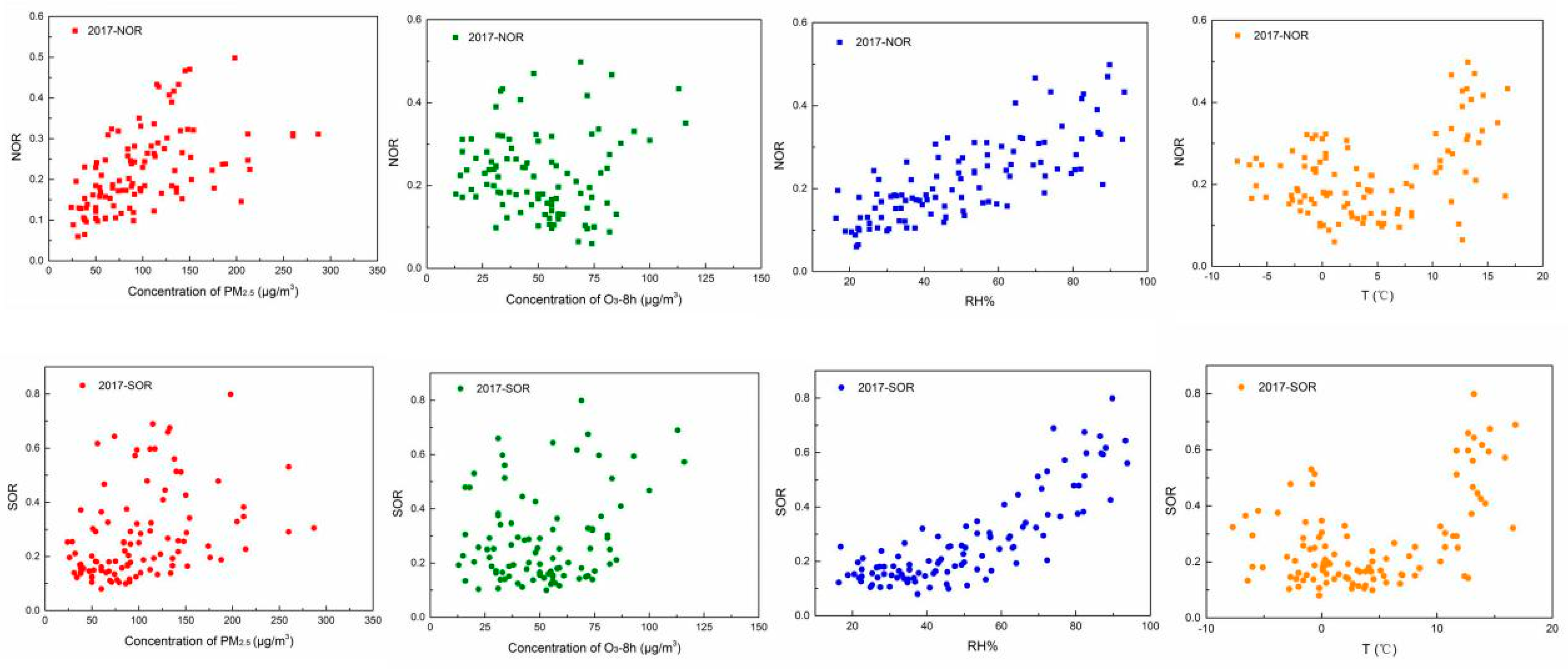
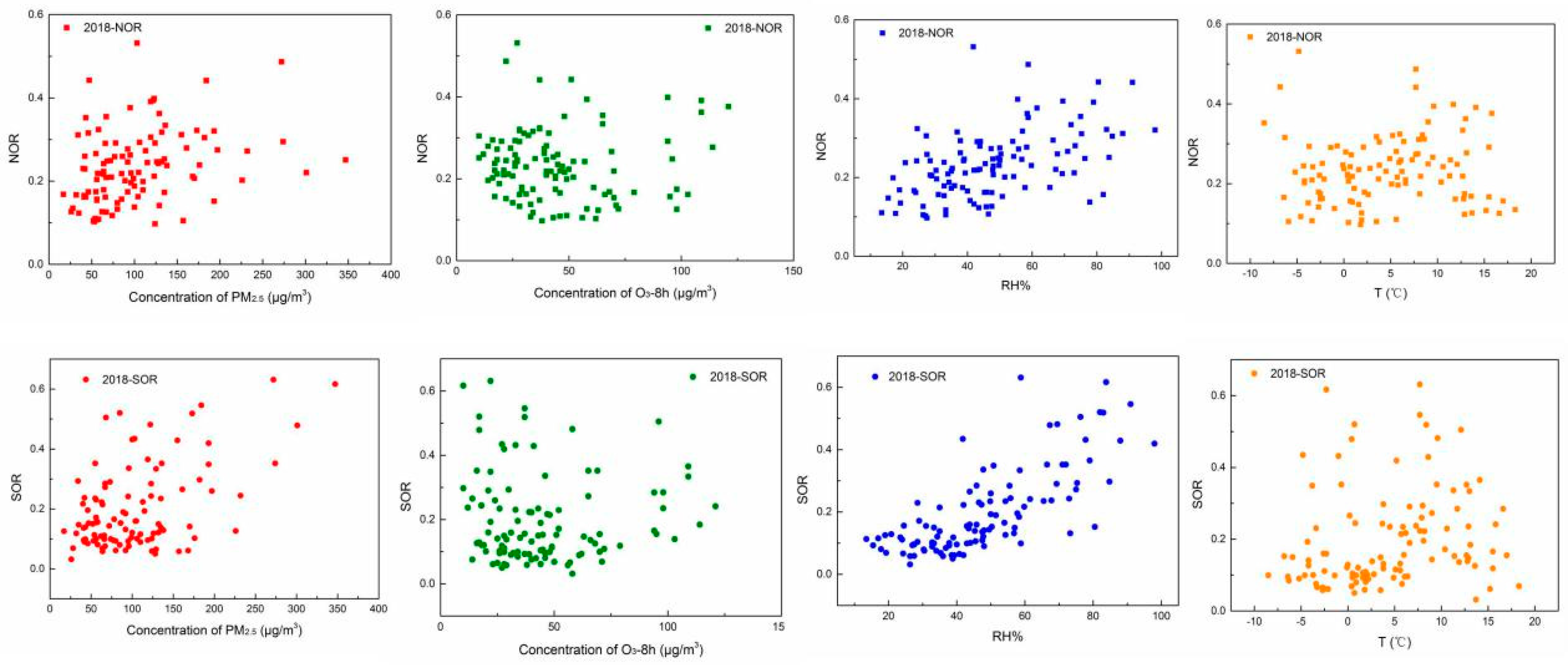
3.3.2. The Influence Factor of Secondary Aerosols in the Autumn–Winter Seasons
3.4. Identification of Potential Transported Sources
4. Conclusions
- PM2.5 concentration had a slight decrease in the autumn–winter season of 2018–2019 compared to that of 2017–2018, the concentration of EC, SO42−, NH4+, Cl−, and K+ all decreased, which could attribute to carrying out the central and clean heating policies and control of biomass burning policy at Xingtai in 2018–2019. However, the secondary formation rates increased obviously in the autumn–winter season and the concentration of NO3− kept steady. In addition, the average concentration of NO2 also increased. Thus, NOx control was an important pollutant control work in the future.
- The formation of O3 and PM2.5 all affected by the secondary formation and meteorological conditions, but the impact effects of the factors were not the same. With the increase in PM2.5 pollution levels, the secondary formation increased, and O3 was consumed. Furthermore, the study of correlation suggested that the concentrations of O3 and PM2.5 were easily affected by the temperature and relative humidity, respectively.
- The increase in the relative humidity benefited the formation of SOR and NOR. The relationships of NOR and SOR against temperature present a “U” shape. The values of NOR and SOR showed small values at a temperature of around 4 °C because gas-phase oxidation and gas-solid-phase oxidation were also not promoted at this temperature, thus decreased the secondary formation.
- The cluster of air trajectories and concentrations of PM2.5 and O3 showed that the pollution of Xingtai is mainly affected by the east cities located in the Shandong and Hebei province. The east route was the main transport route for O3 and PM2.5. However, the concentration of PM2.5 was also affected by the airflow coming from the northwest.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuling, H.; Shigong, W. Formation mechanism of a severe air pollution event: A case study in the Sichuan Basin, Southwest China. Atmos. Environ. 2021, 246, 118135. [Google Scholar] [CrossRef]
- Jianjun, H.; Sunling, G.; Ye, Y.; Lijuan, Y.; Lin, W.; Hongjun, M.; Congbo, S.; Suping, Z.; Hongli, L.; Xiaoyu, L.; et al. Air pollution characteristics and their relation to meteorological conditions during 2014–2015 in major Chinese cities. Environ. Pollut. 2017, 223, 484–496. [Google Scholar]
- Chen, Y.; Xie, S. Spatiotemporal pattern and regional characteristics of visibility in China during 1976–2010. Chin. Sci. Bull. 2014, 59, 3054–3065. [Google Scholar] [CrossRef]
- Fanglin, C.; Zhongfei, C. Cost of economic growth: Air pollution and health expenditure. Sci. Total. Environ. 2021, 755, 142543. [Google Scholar] [CrossRef]
- Hawawu, H.; Mansour, S.; Masud, Y.; Mohammad, S.H.; Tanko, M.; Akbar, F. Fuel type use and risk of respiratory symptoms: A cohort study of infants in the Northern region of Ghana. Sci. Total. Environ. 2021, 755, 142501. [Google Scholar] [CrossRef]
- Wenxing, W.; Tao, W. On acid rain formation in China. Atmos. Environ. 1996, 30, 4091–4093. [Google Scholar] [CrossRef]
- Qingqing, H.; Ming, Z.; Yimeng, S.; Bo, H. Spatiotemporal assessment of PM2.5 concentrations and exposure in China from 2013 to 2017 using satellite-derived data. J. Clean. Prod. 2021, 286, 164965. [Google Scholar] [CrossRef]
- Fangyuan, W.; Xionghui, Q.; Jingyuan, C.; Lin, P.; Nannan, Z.; Yulong, Y.; Rumei, L. Policy-driven changes in the health risk of PM2.5 and O3 exposure in China during 2013–2018. Sci. Total. Environ. 2021, 757, 143775. [Google Scholar] [CrossRef]
- Chong, J.; Reshmita, N.; Lev, L.; Dewang, W. Integrating ecosystem services into effectiveness assessment of ecological restoration program in northern China’s arid areas: Insights from the Beijing-Tianjin Sandstorm Source Region. Land Use Policy 2018, 75, 201–214. [Google Scholar] [CrossRef]
- Qiang, J.; Xinyue, F.; Bo, W.; Aidang, S. Spatio-temporal variations of PM2.5 emission in China from 2005 to 2014. Chemosphere 2017, 183, 429–436. [Google Scholar] [CrossRef]
- Pengfei, W.; Hao, G.; Jian, H.; Sri, H.K.; Qi, Y.; Hongliang, Z. Responses of PM2.5 and O3 concentrations to changes of meteorology and emissions in China. Sic. Total. Environ. 2019, 662, 297–306. [Google Scholar]
- Lei, C.; Jia, Z.; Yang, Y.; Xu, Y. Meteorological influences on PM2.5 and O3 trends and associated health burden since China’s clean air actions. Sci. Total. Environ. 2020, 744, 140837. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Liu, Z.; Wang, L.; Xia, X.; Tao, M.; Zhu, L. Modeling the feedback between aerosol and meteorological variables in the atmospheric boundary layer during a severe fog–haze event over the North China Plain. Atmos. Chem. Phys. 2015, 15, 4279–4295. [Google Scholar] [CrossRef] [Green Version]
- Ziyue, C.; Danlu, C.; Mei, P.K.; Bin, C.; Bingbo, G.; Yan, Z.; Ruiyuan, L.; Bing, X. The control of anthropogenic emissions contributed to 80% of the decrease in PM2.5 concentrations in Beijing from 2013 to 2017. Atmos. Chem. Phys. 2019, 19, 13519–13533. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Lynn, M.R.; Sijia, L.; Hong, L.; Jianping, G.; Ying, L.; Balwinder, S.; Steven, J.G. Dust-wind interactions can intensify aerosol pollution over eastern China. Nat. Commun. 2017, 8, 15333. [Google Scholar] [CrossRef] [PubMed]
- Zhengtai, Z.; Kaicun, W. Stilling and recovery of the surface wind speed based on observation, reanalysis, and geostrophic wind theory over China from 1960 to 2017. J. Clim. 2020, 33, 3989–4008. [Google Scholar] [CrossRef] [Green Version]
- Nadine, U.; Drew, T.S.; Dorothy, K.; Markus, A.; Janusz, C.; David, G.S. Influences of man-made emissions and climate changes on tropospheric ozone, methane, and sulfate at 2030 from a broad range of possible futures. J. Geophy. Res. 2006, 111, D12. [Google Scholar] [CrossRef]
- Isaksen, I.S.A.; Granier, C.; Myhre, G.; Berntsen, T.K.; Dalsøren, S.B.; Gauss, M.; Klimont, Z.; Benestad, R.; Bousquet, P.; Colins, W.; et al. Increase of ozone concentrations, its temperature sensitivity and the precursor factor in South China. Atmos. Environ. 2009, 43, 5138–5192. [Google Scholar] [CrossRef]
- Pavan, N.R.; Peter, J.A. Sensitivity of global tropospheric ozone and fine particulate matter concentrations to climate change. J. Geophys. Res. 2006, 111, D24103. [Google Scholar] [CrossRef]
- Xiaoyan, W.; Robert, E.D.; Liangyuan, S.; Chunlüe, Z.; Kaicun, W. PM2.5 pollution in China and how it has been exacerbated by terrain and meteorological conditions. Bull. Am. Meteorol. Soc. 2018, 99, 105–119. [Google Scholar] [CrossRef]
- Yuzhu, X.; Zirui, L.; Tianxue, W.; Xiaojuan, H.; Jingyuan, L.; Guiqian, T.; Yang, Y.; Xingru, L.; Rongrong, S.; Bo, H.; et al. Characteristics of chemical composition and seasonal variations of PM2.5 in Shijiazhuang, China: Impact of primary emissions and secondary formation. Sci. Total Environ. 2019, 677, 215–229. [Google Scholar] [CrossRef]
- Yuesi, W.; Li, Y.; Lili, W.; Zirui, L.; Dongsheng, J.; Guiqian, T.; Junke, Z.; Yang, S.; Bo, H.; Jinyuan, X. Mechanism for the formation of the January 2013 heavy haze pollution episode over central and eastern China. Sci. China Earth Sci. 2013, 57, 14–25. [Google Scholar] [CrossRef]
- Zhaofeng, T.; Keding, L.; Meiqing, J.; Rong, S.; Huabin, D.; Limin, Z.; Shaodong, X.; Qinwen, T.; Yuanhang, Z. Exploring ozone pollution in Chengdu, southwestern China: A case study from radical chemistry to O3-VOC-NOx sensitivity. Sci. Total Environ. 2018, 636, 775–786. [Google Scholar] [CrossRef]
- Renyi, Z.; Gehui, W.; Song, G.; Misti, L.Z.; Qi, Y.; Yun, L.; Weigang, W.; Min, H.; Yuan, W. Formation of urban fine particulate matter. Chem. Rev. 2015, 115, 3803–3855. [Google Scholar] [CrossRef]
- Huijia, Z.; Huizheng, C.; Lei, Z.; Ke, G.; Yanjun, M.; Yaqiang, W.; Hong, W.; Yu, Z.; Xiaoye, Z. How aerosol transport from the North China plain contributes to air quality in northeast China. Sci. Total Environ. 2020, 738, 139555. [Google Scholar] [CrossRef]
- Charles, L.B.; Philip, M.R.; Shelley, J.T.; Steve, D.Z.; John, H.S. The use of ambient measurements to identify which precursor species limit aerosol nitrate formation. J. Air Waste Manag. Assoc. 2011, 50, 2073–2084. [Google Scholar] [CrossRef] [Green Version]
- Yanlin, Z.; Jun, L.; Gan, Z.; Peter, Z.; Rujin, H.; Jianhui, T.; Lukas, W.; André, S.H.P.; Sönke, S. Radiocarbon-based source apportionment of carbonaceous aerosols at a regional background site on Hainan Island, South China. Environ. Sci. Technol. 2014, 48, 2651–2659. [Google Scholar] [CrossRef]
- Chen, Y.; Zhi, G.; Feng, Y.; Fu, J.; Feng, J.; Sheng, G.; Simoneit, B.R.T. Measurements of emission factors for primary carbonaceous particles from residential raw-coal combustion in China. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Baoqing, W.; Zhenzhen, T.; Ningning, C.; Honghong, N. The characteristics and sources apportionment of water-soluble ions of PM2.5 in suburb Tangshan, China. Urban Clim. 2021, 35, 100742. [Google Scholar] [CrossRef]
- Dan, W.; Shao-Long, L.; Huan-Qiang, Y.; Rong-Guang, D.; Jun-Rong, X.; Bing, Q.; Gang, L.; Feng-Ying, L.; Meng, Y.; Xin-Lei, G. Pollution characteristics and light extinction contribution of water-soluble ions of PM2.5 in Hangzhou. Environ. Sci. 2017, 38, 2656–2666. [Google Scholar] [CrossRef]
- Mo, D.; Guoshun, Z.; Xinxin, L.; Hairong, T.; Yahui, Z. The characteristics of carbonaceous species and their sources in PM2.5 in Beijing. Atmos. Environ. 2004, 38, 3443–3452. [Google Scholar] [CrossRef]
- Di, Y.; Qi, Z.; Changtan, J.; Jun, C.; Xiaoxing, M. Characteristics of elemental carbon and organic carbon in PM10 during spring and autumn in Chongqing, China. China Part. 2007, 5, 255–260. [Google Scholar] [CrossRef]
- Meng, C.C.; Wang, L.T.; Zhang, F.F.; Wei, Z.; Ma, S.M.; Ma, X.; Yang, J. Characteristics of concentrations and water-soluble inorganic ions in PM2.5 in Handan City, Hebei province, China. Atmos. Res. 2016, 171, 133–146. [Google Scholar] [CrossRef]
- Yuanxun, Z.; Min, S.; Yuanhang, Z.; Limin, Z.; Lingyan, H.; Bin, Z.; Yongjie, W.; Xianlei, Z. Source profiles of particulate organic matters emitted from cereal straw burnings. J. Environ. Sci. 2007, 19, 167–175. [Google Scholar] [CrossRef]
- Jay, R.O.; Thorsten, H.; Fran, B.; Don, C.; Richard, C.F.; John, H.S. Gas/particle partitioning and secondary organic aerosol yields. Environ. Sci. Technol. 1996, 30, 2580–2585. [Google Scholar] [CrossRef]
- Pandis, S.N.; Wexler, A.S.; Seinfeld, J.H. Secondary organic aerosol formation and transport—II. Predicting the ambient secondary organic aerosol size distribution. Atmos. Environ. A Gen. Top. 1993, 27, 2403–2416. [Google Scholar] [CrossRef]
- Duan, F.; Liu, X.; Yu, T.; Cachier, H. Identification and estimate of biomass burning contribution to the urban aerosol organic carbon concentrations in Beijing. Atmos. Environ. 2004, 38, 1275–1282. [Google Scholar] [CrossRef]
- Mei, Z.; Yanjun, Z.; Caiqing, Y.; Xianlei, Z.; James, J.S.; Yuanhang, Z. Review of PM2.5 source apportionment methods in China. Acta Sci. Nauralium Univ. Pekin. 2014, 50, 1141–1154. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Tang, A.; Yuan, H.; Sun, Y.; Chen, S.; Zheng, A. The ion chemistry and the source of PM2.5 aerosol in Beijing. Atmos. Environ. 2005, 39, 3771–3784. [Google Scholar] [CrossRef]
- Tie, X.; Geng, F.; Guenther, A.; Cao, J.; Greenberg, J.; Zhang, R.; Apel, E.; Li, G.; Weinheimer, A.; Chen, J.; et al. Megacity impacts on regional ozone formation: Observations and WRF-Chem modeling for the MIRAGE-Shanghai field campaign. Atmos. Chem. Phys. 2013, 13, 5655–5669. [Google Scholar] [CrossRef] [Green Version]
- Wofsy, S.C. Atmospheric Chemistry and Global Change; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Cai, Y.; Mei, Z.; Amy, P.S.; Carme, B.; Yury, D.; August, A.; Xiaoying, L.; Xiaoshuang, G.; Tian, Z.; Örjan, G.; et al. Chemical characteristics and light-absorbing property of water-soluble organic carbon in Beijing: Biomass burning contributions. Atmos. Environ. 2015, 121, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Qiang, Z.; Jiannong, Q.; Xuexi, T.; Xia, L.; Quan, L.; Yang, G.; Delong, Z. Effects of meteorology and secondary particle formation on visibility during heavy haze events in Beijing, China. Sci. Total Environ. 2015, 502, 578–584. [Google Scholar] [CrossRef]
- Junyang, Z.; Luying, Z.; Chuanhe, Y.; Tingting, X.; Lanfang, R.; Hui, L.; Xinchun, L.; Qingyue, W.; Senlin, L. Relationships between chemical elements of PM2.5 and O3 in Shanghai atmosphere based on the 1-year monitoring observation. J. Environ. Sci. 2020, 95, 49–57. [Google Scholar] [CrossRef]
- Suping, Z.; Daiying, Y.; Ye, Y.; Shichang, K.; Dahe, Q.; Longxiang, D. PM2.5 and O3 pollution during 2015-2019 over 367 Chinese cities: Spatiotemporal variations, meteorological and topographical impacts. Environ. Pollut. 2020, 264, 114694. [Google Scholar] [CrossRef]
- Hui, L.; Yongliang, M.; Fengkui, D.; Lidan, Z.; Tao, M.; Shuo, Y.; Yunzhi, X.; Fan, L.; Tao, H.; Takashi, K.; et al. Stronger secondary pollution processes despite decrease in gaseous precursors: A comparative analysis of summer 2020 and 2019 in Beijing. Environ. Pollut. 2021, 279, 116923. [Google Scholar] [CrossRef]


| 2017–2018 | 2018–2019 | |||||||
|---|---|---|---|---|---|---|---|---|
| YZ | NQ | SH | Average | YZ | NQ | SH | Average | |
| NO3− | 21.8 | 19.3 | 20.9 | 20.7 | 18.8 | 20.6 | 20.4 | 20.0 |
| SO42− | 11.8 | 10.9 | 12.8 | 11.8 | 9.0 | 10.6 | 9.3 | 9.6 |
| NH4+ | 11.7 | 11.0 | 12.6 | 11.8 | 8.7 | 9.0 | 9.7 | 9.1 |
| K+ | 1.2 | 1.3 | 1.5 | 1.3 | 0.7 | 1.0 | 0.7 | 0.8 |
| Ca2+ | 1.9 | 1.4 | 1.6 | 1.6 | 1.5 | 1.5 | 1.7 | 1.6 |
| Cl− | 4.4 | 4.3 | 6.3 | 5.0 | 3.5 | 4.7 | 3.7 | 4.0 |
| OC | 17.5 | 16.3 | 20.4 | 18.1 | 15.3 | 21.8 | 20.0 | 19.0 |
| EC | 7.5 | 8.5 | 9.8 | 8.6 | 6.9 | 8.4 | 7.1 | 7.5 |
| PM2.5 | 121.8 | 117.1 | 136.2 | 125.0 | 105.1 | 112.1 | 119.1 | 112.1 |
| NO2 1 | 60 | 64 | ||||||
| SO2 1 | 32 | 31 | ||||||
| O3 1 | 51 | 40 | ||||||
| PM2.5 Concentration Levels | PM2.5/CO | SO2/CO | NO2/CO | O3/CO | ||||
|---|---|---|---|---|---|---|---|---|
| 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | |
| <50 | 54 | 60 | 15 | 17 | 34 | 38 | 30 | 33 |
| (50,100) | 45 | 54 | 16 | 19 | 31 | 43 | 32 | 38 |
| (100,150) | 45 | 52 | 15 | 19 | 30 | 40 | 22 | 25 |
| (150,200) | 41 | 47 | 15 | 18 | 25 | 36 | 26 | 22 |
| >200 | 57 | 44 | 19 | 21 | 29 | 35 | 25 | 24 |
| Year | Pollutant | Average Air Pressure | Average Wind Speed of 2 Min | Average Temperature | Average Relative Humidity |
|---|---|---|---|---|---|
| 2017–2018 | PM2.5 | −0.137 | −0.163 | −0.097 | 0.165 |
| O3 | −0.075 | 0.135 | 0.340 | −0.027 | |
| 2018–2019 | PM2.5 | 0.018 | −0.123 | −0.229 | 0.221 |
| O3 | −0.210 | 0.267 | 0.487 | −0.078 |
| PM2.5 | O3 | Average Relative Humidity | Average Temperature | ||
|---|---|---|---|---|---|
| 2017–2018 | NOR | 0.440 | −0.101 | 0.579 | 0.139 |
| SOR | 0.295 | 0.082 | 0.593 | 0.214 | |
| 2018–2019 | NOR | 0.252 | −0.076 | 0.372 | 0.090 |
| SOR | 0.205 | −0.032 | 0.540 | 0.209 |
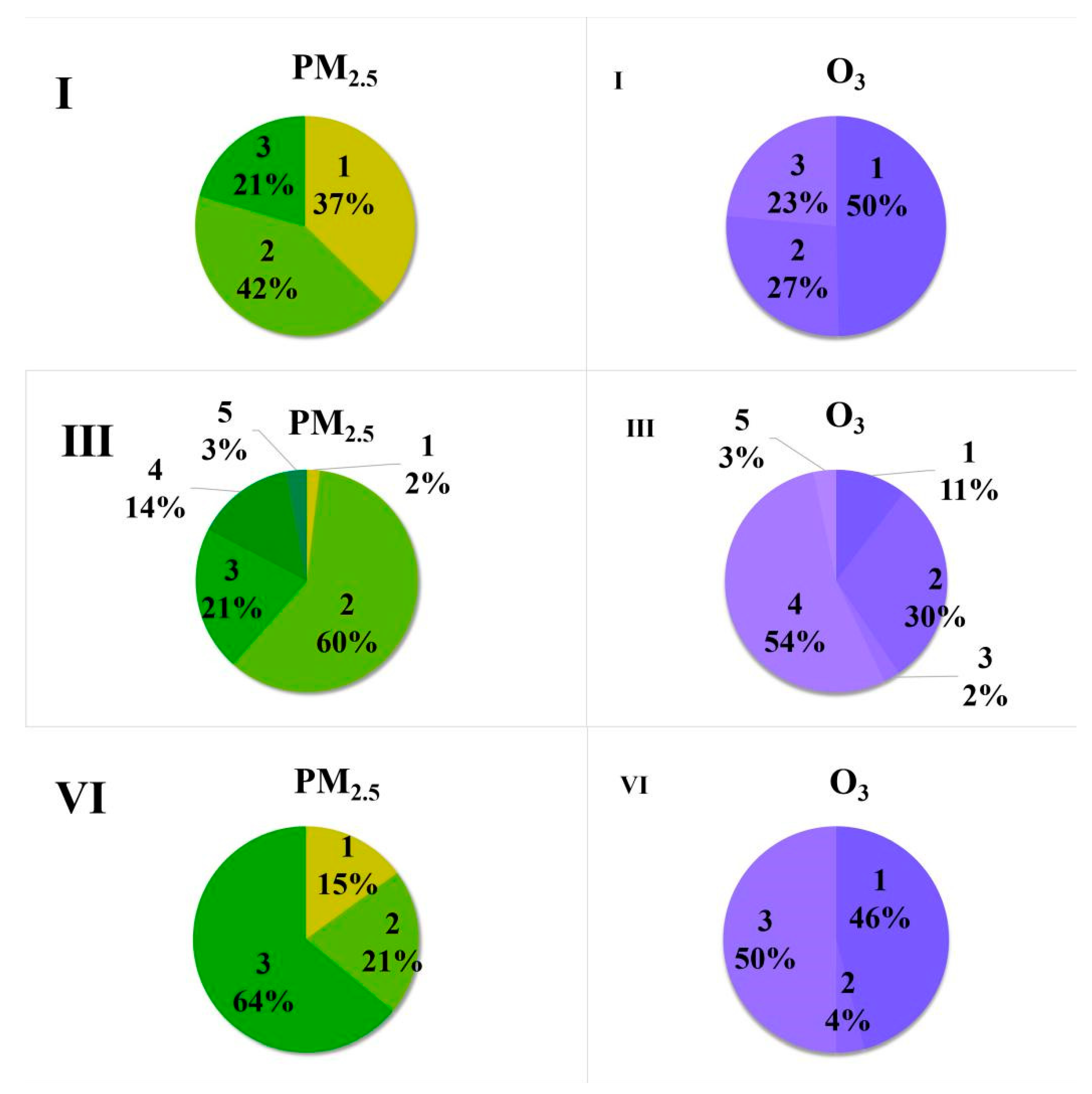
| No. | Pollution Times (Month/Day) | Duration Times | Concentration of PM2.5(μg/m3) | Pollution Level |
|---|---|---|---|---|
| I | 10/25–10/28 | 4 days | Average value: 140.3 μg/m3 Peak value: 198 μg/m3 | medium pollution: 2 days heavy pollution: 1day light pollution: 1day |
| II | 12/1–12/5 | 5 days | Average value:143 μg/m3 Peak value: 214 μg/m3 | medium pollution: 2 days heavy pollution: 2day light pollution: 1day |
| III | 12/12–12/15 | 3 days | Average value:122 μg/m3 Peak value: 179 μg/m3 | heavy pollution: 1day light pollution: 1day |
| IV | 12/25–1/2 | 9 days | Average value:132 μg/m3 Peak value: 251 μg/m3 | light pollution: 5 days heavy pollution: 2 days sever pollution: 1 day |
| V | 1/12–1/22 | 10 days | Average value:176 μg/m3 Peak value: 286 μg/m3 | light pollution: 3 days medium pollution: 1 day heavy pollution: 4 days sever pollution: 2 days |
| VI | 1/27–1/30 | 4 days | Average value:135 μg/m3 Peak value: 209 μg/m3 | light pollution: 2 days medium pollution: 1 day heavy pollution: 2 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, S.; Zhang, J.; Li, H. Characteristics of PM2.5 Pollution with Comparative Analysis of O3 in Autumn–Winter Seasons of Xingtai, China. Atmosphere 2021, 12, 569. https://doi.org/10.3390/atmos12050569
Wang H, Wang S, Zhang J, Li H. Characteristics of PM2.5 Pollution with Comparative Analysis of O3 in Autumn–Winter Seasons of Xingtai, China. Atmosphere. 2021; 12(5):569. https://doi.org/10.3390/atmos12050569
Chicago/Turabian StyleWang, Han, Shulan Wang, Jingqiao Zhang, and Hui Li. 2021. "Characteristics of PM2.5 Pollution with Comparative Analysis of O3 in Autumn–Winter Seasons of Xingtai, China" Atmosphere 12, no. 5: 569. https://doi.org/10.3390/atmos12050569





