Secondary Organic Aerosol Formation from Isoprene: Selected Research, Historic Account and State of the Art
Abstract
:1. Introduction
2. Early Research (2004–2014): First Observations, Laboratory Studies, Field Monitoring Studies, Quantitation, and Mechanistic Insights
2.1. First Observations
2.2. Laboratory Studies
2.3. Field Monitoring Studies
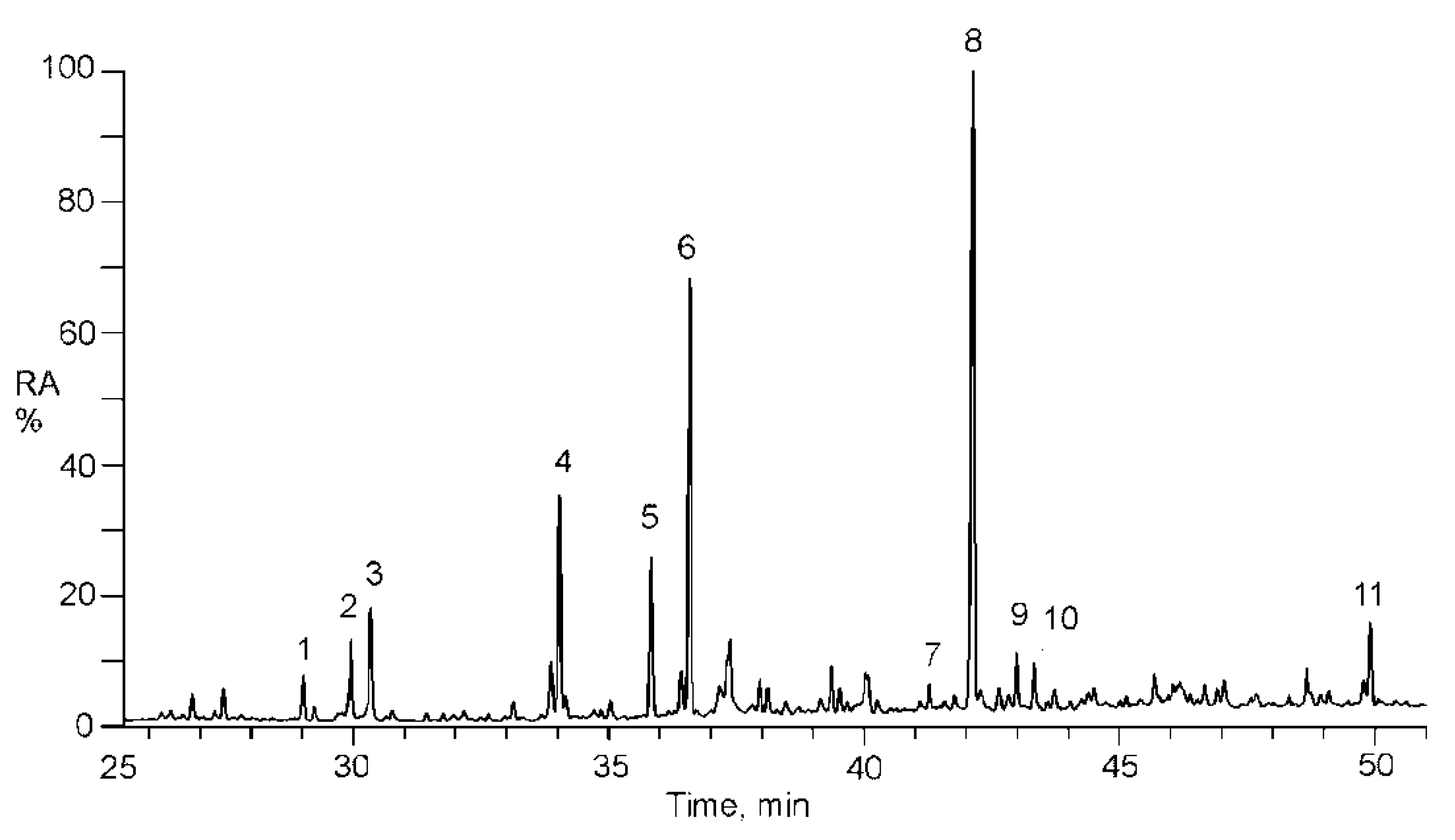
2.4. Quantitation of Isoprene SOA Markers
2.5. Mechanistic Insights into the Formation of the 2-methyltetrols, 2-methylglyceric acid, and Derivatives
3. Isoprene SOA-Related Organosulfates: Detection, Structural Characterization, Formation Mechanisms, Analytical Challenges, and Degradation
3.1. Detection and Structural Characterization
3.2. Formation Mechanisms
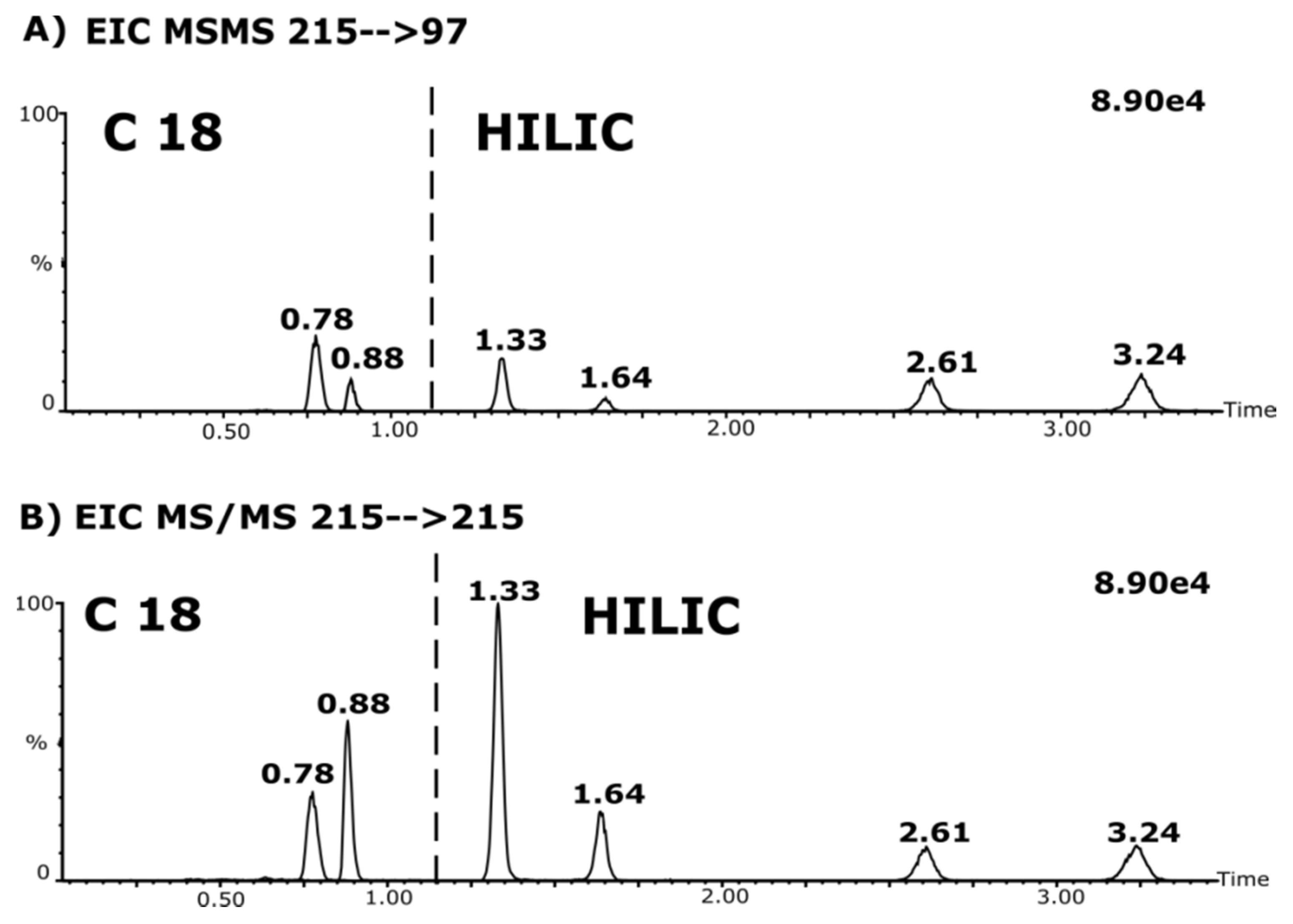
3.3. Analytical Challenges and Quantitation
3.4. Degradation of Organosulfates
4. Markers for Isoprene SOA Aging
5. Source Apportionment
5.1. The Organic Marker-Based Approach
5.2. Positive Matrix Factorization
6. Overview of Molecular Markers
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Claeys, M.; Graham, B.; Vas, G.; Wang, W.; Vermeylen, R.; Pashynska, V.; Cafmeyer, J.; Guyon, P.; Andreae, M.O.; Artaxo, P.; et al. Formation of secondary organic aerosols through photooxidation of isoprene. Science 2004, 303, 1173. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, P.A. Atmospheric tracers and the study of the general circulation of the atmosphere. Rep. Prog. Phys. 1963, 26, 213. [Google Scholar] [CrossRef]
- Carlton, A.G.; Wiedinmyer, C.; Kroll, J.H. A review of secondary organic aerosol (SOA) formation from isoprene. Atmos. Chem. Phys. 2009, 9, 4987. [Google Scholar] [CrossRef] [Green Version]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155. [Google Scholar] [CrossRef] [Green Version]
- Nozière, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgic, I.; Hamilton, J.F.; et al. The molecular identification of organic compounds in the atmosphere: State of the art and challenges. Chem. Rev. 2015, 115, 3919. [Google Scholar] [CrossRef] [PubMed]
- Surratt, J.D.; Chan, A.W.H.; Eddingsaas, N.C.; Chan, M.N.; Loza, C.L.; Kwan, A.J.; Hersey, S.P.; Flagan, R.C.; Wennberg, P.O.; Seinfeld, J.H. Reactive intermediates revealed in secondary organic aerosol formation from isoprene. Proc. Natl. Acad. Sci. USA 2010, 107, 6640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K. Detection of nitrooxypolyols in secondary organic aerosol formed from the oxidation of conjugated dienes under high-NOx conditions. Atmos. Environ. 2008, 42, 6851. [Google Scholar] [CrossRef]
- Lin, Y.H.; Zhang, H.F.; Pye, H.O.T.; Zhang, Z.F.; Marth, W.J.; Park, S.; Arashiro, M.; Cui, T.Q.; Budisulistiorini, H.; Sexton, K.G.; et al. Epoxide as a precursor to secondary organic aerosol formation from isoprene photooxidation in the presence of nitrogen oxides. Proc. Natl. Acad. Sci. USA 2013, 110, 6718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys. 2006, 6, 3181. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Vas, G.; Dommisse, R.; Loones, K.; Claeys, M. Fragmentation study of diastereoisomeric 2-methyltetrols, oxidation products of isoprene, as their trimethylsilyl ethers, using gas chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1787. [Google Scholar] [CrossRef]
- Paulot, F.; Crounse, J.D.; Kjaergaard, H.G.; Kürten, A.; Clair, J.M.; Seinfeld, J.H.; Wennberg, P.O. Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science 2009, 325, 730. [Google Scholar] [CrossRef] [Green Version]
- Surratt, J.D.; Kroll, J.H.; Kleindienst, T.E.; Edney, E.O.; Claeys, M.; Sorooshian, A.; Ng, N.L.; Offenberg, J.H.; Lewandowski, M.; Jaoui, M.; et al. Evidence for organosulfates in secondary organic aerosol. Environ. Sci. Technol. 2007, 41, 517. [Google Scholar] [CrossRef]
- Kroll, J.H.; Ng, N.L.; Murphy, S.M.; Flagan, R.C.; Seinfeld, J.H. Secondary organic aerosol formation from isoprene photooxidation under high-NOx conditions. Geophys. Res. Lett. 2005, 32, L18808. [Google Scholar] [CrossRef] [Green Version]
- Kroll, J.H.; Ng, N.L.; Murphy, S.M.; Flagan, R.C.; Seinfeld, J.H. Secondary organic aerosol formation from isoprene photooxidation. Environ. Sci. Technol. 2006, 40, 1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surratt, J.D.; Lewandowski, M.; Offenberg, J.H.; Jaoui, M.; Kleindienst, T.E.; Edney, E.O.; Seinfeld, J.H. Effect of acidity on secondary organic aerosol formation from isoprene. Environ. Sci. Technol. 2007, 41, 5363. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, M.; Jaoui, M.; Offenberg, J.H.; Krug, J.D.; Kleindienst, T.E. Atmospheric oxidation of isoprene and 1,3-butadiene: Influence of aerosol acidity and relative humidity on secondary organic aerosol. Atmos. Chem. Phys. 2015, 15, 3773. [Google Scholar] [CrossRef] [Green Version]
- Edney, E.O.; Kleindienst, T.E.; Jaoui, M.; Lewandowski, M.; Offenberg, J.H.; Wang, W.; Claeys, M. Formation of 2-methyl tetrols and 2-methylglyceric acid in secondary organic aerosol from laboratory irradiated isoprene/NOx/SO2/air mixtures and their detection in ambient PM2.5 samples collected in the Eastern United States. Atmos. Environ. 2005, 39, 5281. [Google Scholar] [CrossRef]
- Wang, W.; Kourtchev, I.; Graham, B.; Cafmeyer, J.; Maenhaut, W.; Claeys, M. Characterization of oxygenated derivatives of isoprene related to 2-methyltetrols in Amazonian aerosols using trimethylsilylation and gas chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1343. [Google Scholar] [CrossRef]
- Surratt, J.D.; Murphy, S.M.; Kroll, J.H.; Ng, N.L.; Hildebrandt, L.; Sorooshian, A.; Szmigielski, R.; Vermeylen, R.; Maenhaut, W.; Claeys, M.; et al. Chemical composition of secondary organic aerosol formed from the photooxidation of isoprene. J. Phys. Chem. A 2006, 110, 9665. [Google Scholar] [CrossRef] [Green Version]
- Cui, T.Q.; Zeng, Z.X.; dos Santos, E.O.; Zhang, Z.F.; Chen, Y.Z.; Zhang, Y.; Rose, C.A.; Budisulistiorini, S.H.; Collins, L.B.; Bodnar, W.M.; et al. Development of a hydrophilic interaction liquid chromatography (HILIC) method for the chemical characterization of water-soluble isoprene epoxydiol (IEPOX)-derived secondary organic aerosol. Environ. Sci. Process. Impacts 2018, 20, 1524. [Google Scholar] [CrossRef]
- Szmigielski, R.; Surratt, J.D.; Vermeylen, R.; Szmigielska, K.; Kroll, J.H.; Ng, N.L.; Murphy, S.M.; Sorooshian, A.; Seinfeld, J.H.; Claeys, M. Characterization of 2-methylglyceric acid oligomers in secondary organic aerosol formed from the photooxidation of isoprene using trimethylsilylation and gas chromatography/ion trap mass spectrometry. J. Mass Spectrom. 2007, 42, 101. [Google Scholar] [CrossRef]
- Claeys, M.; Wang, W.; Ion, A.C.; Kourtchev, I.; Gelencsér, A.; Maenhaut, W. Formation of secondary organic aerosols from isoprene and its gas-phase oxidation products through reaction with hydrogen peroxide. Atmos. Env. 2004, 38, 4093. [Google Scholar] [CrossRef]
- Claeys, M.; Kourtchev, I.; Pashynska, V.; Vas, G.; Vermeylen, R.; Wang, W.; Cafmeyer, J.; Chi, X.; Artaxo, P.; Andreae, M.O.; et al. Polar organic marker compounds in atmospheric aerosols during the LBA-SMOCC 2002 biomass burning experiment in Rondônia, Brazil: Sources and source processes, time series, diel variations and size distributions. Atmos. Chem. Phys. 2010, 10, 9319. [Google Scholar] [CrossRef] [Green Version]
- Ion, A.C.; Vermeylen, R.; Kourtchev, I.; Cafmeyer, J.; Chi, X.; Gelencsér, A.; Maenhaut, W.; Claeys, M. Polar organic compounds in rural PM2.5 aerosols from K-puszta, Hungary, during a 2003 summer field campaign: Sources and diel variations. Atmos. Chem. Phys. 2005, 5, 1805. [Google Scholar] [CrossRef] [Green Version]
- Kourtchev, I.; Ruuskanen, T.; Maenhaut, W.; Kulmala, M.; Claeys, M. Observation of 2-methyltetrols and related photo-oxidation products of isoprene in boreal forest aerosols from Hyytiälä, Finland. Atmos. Chem. Phys. 2005, 5, 2761. [Google Scholar] [CrossRef] [Green Version]
- Kourtchev, I.; Warnke, J.; Maenhaut, W.; Hoffmann, T.; Claeys, M. Polar organic marker compounds in PM2.5 aerosol from a mixed forest site in Western Germany. Chemosphere 2008, 73, 1308. [Google Scholar] [CrossRef]
- Kourtchev, I.; Ruuskanen, T.M.; Keronen, P.; Sogacheva, L.; Dal Maso, M.; Reissell, A.; Chi, X.; Vermeylen, R.; Kulmala, M.; Maenhaut, W.; et al. Determination of isoprene and α-/β-pinene oxidation products in boreal forest aerosols from Hyytiälä, Finland: Diel variations and possible link with particle formation events. Plant Biol. 2008, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Yeh, S.S. Isoprene emission from plants. Annu. Rev. Plant Phys. 2001, 52, 407. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental implications of hydroxyl radicals (•OH). Chem. Rev. 2015, 115, 13051. [Google Scholar] [CrossRef]
- Kleindienst, T.E.; Lewandowski, M.; Offenberg, J.H.; Jaoui, M.; Edney, E.O. Ozone-isoprene reaction: Re-examination of the formation of secondary organic aerosol. Geophys. Res. Lett. 2007, 34, L01805. [Google Scholar] [CrossRef]
- Clements, A.L.; Seinfeld, J.H. Detection and quantification of 2-methyltetrols in ambient aerosol in the Southeastern United States. Atmos. Environ. 2007, 41, 1825. [Google Scholar] [CrossRef]
- Kristensen, K.; Glasius, M. Organosulfates and oxidation products from biogenic hydrocarbons in fine aerosols from a forest in North West Europe during spring. Atmos. Environ. 2011, 45, 4546. [Google Scholar] [CrossRef]
- Yee, L.D.; Isaacman-VanWertz, G.; Wernis, R.A.; Kreisberg, N.M.; Glasius, M.; Riva, M.; Surratt, J.D.; de Sa, S.S.; Martin, S.T.; Alexander, M.L.; et al. Natural and anthropogenically influenced isoprene oxidation in Southeastern United States and Central Amazon. Environ. Sci. Technol. 2020, 54, 5980. [Google Scholar] [CrossRef] [PubMed]
- Isaacman, G.; Kreisberg, N.M.; Yee, L.D.; Worton, D.R.; Chan, A.W.H.; Moss, J.A.; Hering, S.V.; Goldstein, A.H. Online derivatization for hourly measurements of gas- and particle-phase semi-volatile oxygenated organic compounds by thermal desorption aerosol gas chromatography (SV-TAG). Atmos. Meas. Tech. 2014, 7, 4417. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Zhang, Z.F.; Docherty, K.S.; Zhang, H.F.; Budisulistiorini, S.H.; Rubitschun, C.L.; Shaw, S.L.; Knipping, E.M.; Edgerton, E.S.; Kleindienst, T.E.; et al. Isoprene epoxydiols as precursors to secondary organic aerosol formation: Acid-catalyzed reactive uptake studies with authentic compounds. Environ. Sci. Technol. 2012, 46, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.F.; Lin, Y.H.; Zhang, Z.F.; Zhang, X.L.; Shaw, S.L.; Knipping, E.M.; Weber, R.J.; Gold, A.; Kamens, R.M.; Surratt, J.D. Secondary organic aerosol formation from methacrolein photooxidation: Roles of NOx level, relative humidity and aerosol acidity. Environ. Chem. 2012, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Bruggemann, M.; Xu, R.S.; Tilgner, A.; Kwong, K.C.; Mutzel, A.; Poon, H.Y.; Otto, T.; Schaefer, T.; Poulain, L.; Chan, M.N.; et al. Organosulfates in ambient aerosol: State of knowledge and future research directions on formation, abundance, fate, and importance. Environ. Sci. Technol. 2020, 54, 3782. [Google Scholar] [CrossRef]
- Surratt, J.D.; Gómez-González, Y.; Chan, A.W.H.; Vermeylen, R.; Shahgholi, M.; Kleindienst, T.E.; Edney, E.O.; Offenberg, J.H.; Lewandowski, M.; Jaoui, M.; et al. Organosulfate formation in biogenic secondary organic aerosol. J. Phys. Chem. A 2008, 112, 8345. [Google Scholar] [CrossRef] [Green Version]
- Gómez-González, Y.; Surratt, J.D.; Cuyckens, F. Szmigielski, R.; Vermeylen, R.; Jaoui, M.; Lewandowski, M.; Offenberg, J.H.; Kleindienst, T.E.; Edney, E.O.; et al. Characterization of organosulfates from the photooxidation of isoprene and unsaturated fatty acids in ambient aerosol using liquid chromatography/(–) electrospray ionization mass spectrometry. J. Mass Spectrom. 2008, 43, 371. [Google Scholar] [CrossRef]
- Safi Shalamzari, M.; Ryabtsova, O.; Kahnt, A.; Vermeylen, R.; Herent, M.F.; Quetin-Leclercq, J.; Van der Veken, P.; Maenhaut, W.; Claeys, M. Mass spectrometric characterization of organosulfates related to secondary organic aerosol from isoprene. Rapid Commun. Mass Spectrom. 2013, 27, 784. [Google Scholar] [CrossRef]
- Hettiyadura, A.P.S.; Stone, E.A.; Kundu, S.; Baker, Z.; Geddes, E.; Richards, K.; Humphry, T. Determination of atmospheric organosulfates using HILIC chromatography with MS detection. Atmos. Meas. Tech. 2015, 8, 2347. [Google Scholar] [CrossRef] [Green Version]
- Wach, P.; Spólnik, G.; Surratt, J.D.; Blaziak, K.; Rudzinski, K.J.; Lin, Y.H.; Maenhaut, W.; Danikiewicz, W.; Claeys, M.; Szmigielski, R. Structural characterization of lactone-containing MW 212 organosulfates originating from isoprene oxidation in ambient fine aerosol. Environ. Sci. Technol. 2020, 54, 1415. [Google Scholar] [CrossRef] [PubMed]
- Rudziński, K.J.; Gmachowski, L.; Kuznietsova, I. Reactions of isoprene and sulphoxy radical-anions—A possible source of atmospheric organosulphites and organosulphates. Atmos. Chem. Phys. 2009, 9, 2129. [Google Scholar] [CrossRef] [Green Version]
- Gómez-González, Y.; Wang, W.; Vermeylen, R.; Chi, X.; Neirynck, J.; Janssens, I.A.; Maenhaut, W.; Claeys, M. Chemical characterisation of atmospheric aerosols during a 2007 summer field campaign at Brasschaat, Belgium: Sources and source processes of biogenic secondary organic aerosol. Atmos. Chem. Phys. 2012, 12, 125. [Google Scholar] [CrossRef] [Green Version]
- Szmigielski, R.; Surratt, J.D.; Gómez-González, Y.; Van der Veken, P.; Kourtchev, I.; Vermeylen, R.; Blockhuys, F.; Jaoui, M.; Kleindienst, T.E.; Lewandowski, M.; et al. 3-Methyl-1,2,3-butanetricarboxylic acid: An atmospheric tracer for terpene secondary organic aerosol. Geophys. Res. Lett. 2007, 34, L24811. [Google Scholar] [CrossRef]
- Spólnik, G.; Wach, P.; Rudziński, K.J.; Skotak, K.; Danikiewicz, W.; Szmigielski, R. Improved UHPLC-MS/MS methods for analysis of isoprene-derived organosulfates. Anal. Chem. 2018, 90, 3416. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Zhang, Y.; Lambe, A.T.; Xu, R.S.; Lei, Z.Y.; Olson, N.E.; Zhang, Z.F.; Szalkowski, T.; Cui, T.Q.; Vizuete, W.; et al. Heterogeneous hydroxyl radical oxidation of isoprene-epoxydiol-derived methyltetrol sulfates: Plausible formation mechanisms of previously unexplained organosulfates in ambient fine aerosols. Environ. Sci. Technol. Lett. 2020, 7, 460. [Google Scholar] [CrossRef]
- Olson, C.N.; Galloway, M.M.; Yu, G.; Hedman, C.J.; Lockett, M.R.; Yoon, T.; Stone, E.A.; Smith, L.M.; Keutsch, F.N. Hydroxycarboxylic acid-derived organosulfates: Synthesis, stability, and quantification in ambient aerosol. Environ. Sci. Technol. 2011, 45, 6468. [Google Scholar] [CrossRef]
- Nestorowicz, K.; Jaoui, M.; Rudziński, K.J.; Lewandowski, M.; Kleindienst, T.E.; Spólnik, G.; Danikiewicz, W.; Szmigielski, R. Chemical composition of isoprene SOA under acidic and non-acidic conditions: Effect of relative humidity. Atmos. Chem. Phys. 2018, 18, 18101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockwood, A.L.; Shepson, P.B.; Fiddler, M.N.; Alaghmand, M. Isoprene Nitrates: Preparation, separation, identification, yields, and atmospheric chemistry. Atmos. Chem. Phys. 2010, 10, 6169. [Google Scholar] [CrossRef] [Green Version]
- Jaoui, M.; Corse, E.W.; Lewandowski, M.; Offenberg, J.H.; Kleindienst, T.E.; Edney, E.O. Formation of organic tracers for isoprene SOA under acidic conditions. Atmos. Environ. 2010, 44, 1798. [Google Scholar] [CrossRef]
- Schindelka, J.; Iinuma, Y.; Hoffmann, D.; Herrmann, H. Sulfate radical-initiated formation of isoprene-derived organosulfates in atmospheric aerosols. Faraday Discuss. 2013, 165. [Google Scholar] [CrossRef] [PubMed]
- Schone, L.; Schindelka, J.; Szeremeta, E.; Schaefer, T.; Hoffmann, D.; Rudziński, K.J.; Szmigielski, R.; Herrmann, H. Atmospheric aqueous phase radical chemistry of the isoprene oxidation products methacrolein, methyl vinyl ketone, methacrylic acid and acrylic acid—Kinetics and product studies. Phys. Chem. Chem. Phys. 2014, 16, 6272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szmigielski, R. Evidence for C5 organosulfur secondary organic aerosol components from in-cloud processing of isoprene: Role of reactive SO4 and SO3 radicals. Atmos. Environ. 2016, 130, 14. [Google Scholar] [CrossRef]
- Wach, P.; Spólnik, G.; Rudziński, K.J.; Skotak, K.; Claeys, M.; Danikiewicz, W.; Szmigielski, R. Radical oxidation of methyl vinyl ketone and methacrolein in aqueous droplets: Characterization of organosulfates and atmospheric implications. Chemosphere 2019, 214, 1. [Google Scholar] [CrossRef] [PubMed]
- Shalamzari, M.S.; Kahnt, A.; Vermeylen, R.; Kleindienst, T.E.; Lewandowski, M.; Cuyckens, F.; Maenhaut, W.; Claeys, M. Characterization of polar organosulfates in secondary organic aerosol from the green leaf volatile 3-Z-hexenal. Environ. Sci. Technol. 2014, 48, 12671. [Google Scholar] [CrossRef]
- Shalamzari, M.S.; Vermeylen, R.; Blockhuys, F.; Kleindienst, T.E.; Lewandowski, M.; Szmigielski, R.; Rudziński, K.J.; Spólnik, G.; Danikiewicz, W.; Maenhaut, W.; et al. Characterization of polar organosulfates in secondary organic aerosol from the unsaturated aldehydes 2-E-pentenal, 2-E-hexenal, and 3-Z-hexenal. Atmos. Chem. Phys. 2016, 16, 7135. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.F.; Worton, D.R.; Lewandowski, M.; Ortega, J.; Rubitschun, C.L.; Park, J.H.; Kristensen, K.; Campuzano-Jost, P.; Day, D.A.; Jimenez, J.L.; et al. Organosulfates as tracers for secondary organic aerosol (SOA) formation from 2-methyl-3-buten-2-ol (MBO) in the atmosphere. Environ. Sci. Technol. 2012, 46, 9437. [Google Scholar] [CrossRef]
- Barbosa, T.S.; Riva, M.; Chen, Y.Z.; da Silva, C.M.; Ameida, J.C.S.; Zhang, Z.; Gold, A.; Arbilla, G.; Bauerfeldt, G.F.; Surratt, J.D. Chemical characterization of organosulfates from the hydroxyl radical-initiated oxidation and ozonolysis of cis-3-hexen-1-ol. Atmos. Environ. 2017, 162, 141. [Google Scholar] [CrossRef]
- Hettiyadura, A.P.S.; Jayarathne, T.; Baumann, K.; Goldstein, A.H.; de Gouw, J.A.; Koss, A.; Keutsch, F.N.; Skog, K.; Stone, E.A. Qualitative and quantitative analysis of atmospheric organosulfates in Centreville, Alabama. Atmos. Chem. Phys. 2017, 17, 1343. [Google Scholar] [CrossRef] [Green Version]
- Hettiyadura, A.P.S.; Al-Naiema, I.M.; Hughes, D.D.; Fang, T.; Stone, E.A. Organosulfates in Atlanta, Georgia: Anthropogenic influences on biogenic secondary organic aerosol formation. Atmos. Chem. Phys. 2019, 19, 3191. [Google Scholar] [CrossRef] [Green Version]
- Bondy, A.L.; Craig, R.L.; Zhang, Z.; Gold, A.; Surratt, J.D.; Ault, A.P. Isoprene-derived organosulfates: Vibrational mode analysis by Raman spectroscopy, acidity-dependent spectral modes, and observation in individual atmospheric particles. J. Phys. Chem. A 2018, 122, 303. [Google Scholar] [CrossRef] [PubMed]
- Glasius, M.; Bering, M.S.; Yee, L.D.; de Sá, S.S.; Isaacman-VanWertz, G.; Wernis, R.A.; Barbosa, H.M.J.; Alexander, M.L.; Palm, B.B.; Hu, W.; et al. Organosulfates in aerosols downwind of an urban region in Central Amazon. Environ. Sci. Process. Impacts 2018, 20, 1546. [Google Scholar] [CrossRef]
- Stone, E.A.; Yang, L.M.; Yu, L.Y.E.; Rupakheti, M. Characterization of organosulfates in atmospheric aerosols at four Asian locations. Atmos. Environ. 2012, 47, 323. [Google Scholar] [CrossRef]
- Worton, D.R.; Goldstein, A.H.; Farmer, D.K.; Docherty, K.S.; Jimenez, J.-L.; Gilman, J.B.; Kuster, W.C.; de Gouw, J.; Williams, B.J.; Kreisberg, N.M.; et al. Origins and composition of fine atmospheric carbonaceous aerosol in the Sierra Nevada Mountains, California. Atmos. Chem. Phys. Discuss. 2011, 11, 17071. [Google Scholar] [CrossRef] [Green Version]
- Kourtchev, I.; Fuller, S.; Aalto, J.; Ruuskanen, T.M.; McLeod, M.W.; Maenhaut, W.; Jones, R.; Kulmala, M.; Kalberer, M. Molecular composition of boreal forest aerosol from Hyytiälä, Finland, using ultrahigh resolution mass spectrometry. Environ. Sci. Technol. 2013, 47, 4069. [Google Scholar] [CrossRef]
- Kourtchev, I.; Godoi, R.H.M.; Connors, S.; Levine, J.G.; Archibald, A.T.; Godoi, A.F.L.; Paralovo, S.L.; Barbosa, C.G.G.; Souza, R.A.F.; Manzi, A.O.; et al. Molecular composition of organic aerosols in Central Amazonia: An ultra-high-resolution mass spectrometry study. Atmos. Chem. Phys. 2016, 16, 11899. [Google Scholar] [CrossRef] [Green Version]
- Jaoui, M.; Szmigielski, R.; Nestorowicz, K.; Kolodziejczyk, A.; Sarang, K.; Rudziński, K.J.; Konopka, A.; Bulska, E.; Lewandowski, M.; Kleindienst, T.E. Organic hydroxy acids as highly oxygenated molecular (HOM) tracers for aged isoprene aerosol. Environ. Sci. Technol. 2019, 53, 14516. [Google Scholar] [CrossRef]
- Kleindienst, T.E.; Jaoui, M.; Lewandowski, M.; Offenberg, J.H.; Lewis, C.W.; Bhave, P.V.; Edney, E.O. Estimates of the contributions of biogenic and anthropogenic hydrocarbons to secondary organic aerosol at a Southeastern US location. Atmos. Environ. 2007, 41, 8288. [Google Scholar] [CrossRef]
- Chan, M.N.; Surratt, J.D.; Claeys, M.; Edgerton, E.S.; Tanner, R.L.; Shaw, S.L.; Zheng, M.; Knipping, E.M.; Eddingsaas, N.C.; Wennberg, P.O.; et al. Characterization and quantification of isoprene-derived epoxydiols in ambient aerosol in the Southeastern United States. Environ. Sci. Technol. 2010, 44, 4590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Wang, X.M.; Gao, B.; Fu, X.X.; He, Q.F.; Zhao, X.Y.; Yu, J.Z.; Zheng, M. Tracer-based estimation of secondary organic carbon in the Pearl River Delta, South China. J. Geophys. Res. Atmos. 2012, 117, D05313. [Google Scholar] [CrossRef]
- Guo, S.; Hu, M.; Guo, Q.F.; Zhang, X.; Zheng, M.; Zheng, J.; Chang, C.C.; Schauer, J.J.; Zhang, R.Y. Primary sources and secondary formation of organic aerosols in Beijing, China. Environ. Sci. Technol. 2012, 46, 9846. [Google Scholar] [CrossRef]
- Hu, D.; Bian, Q.; Li, T.W.Y.; Lau, A.K.H.; Yu, J.Z. Contributions of isoprene, monoterpenes, beta-caryophyllene, and toluene to secondary organic aerosols in Hong Kong during the summer of 2006. J. Geophys. Res. Atmos. 2008, 113, D22206. [Google Scholar] [CrossRef]
- Kleindienst, T.E.; Lewandowski, M.; Offenberg, J.H.; Edney, E.O.; Jaoui, M.; Zheng, M.; Ding, X.A.; Edgerton, E.S. Contribution of primary and secondary sources to organic aerosol and PM2.5 at SEARCH network sites. J. Air Waste Manag. 2010, 60, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowski, M.; Jaoui, M.; Offenberg, J.H.; Kleindienst, T.E.; Edney, E.O.; Sheesley, R.J.; Schauer, J.J. Primary and secondary contributions to ambient PM in the Midwestern United States. Environ. Sci. Technol. 2008, 42, 3303. [Google Scholar] [CrossRef]
- Kourtchev, I.; Copolovici, L.; Claeys, M.; Maenhaut, W. Characterization of atmospheric aerosols at a forested site in Central Europe. Environ. Sci. Technol. 2009, 43, 4665. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, M.; Piletic, I.R.; Kleindienst, T.E.; Offenberg, J.H.; Beaver, M.R.; Jaoui, M.; Docherty, K.S.; Edney, E.O. Secondary organic aerosol characterisation at field sites across the United States during the spring-summer period. Int. J. Environ. Anal. Chem. 2013, 93, 1084. [Google Scholar] [CrossRef]
- Fu, P.Q.; Kawamura, K.; Chen, J.; Li, J.; Sun, Y.L.; Liu, Y.; Tachibana, E.; Aggarwal, S.G.; Okuzawa, K.; Tanimoto, H.; et al. Diurnal variations of organic molecular tracers and stable carbon isotopic composition in atmospheric aerosols over Mt. Tai in the North China Plain: An influence of biomass burning. Atmos. Chem. Phys. 2012, 12, 8359. [Google Scholar] [CrossRef] [Green Version]
- Offenberg, J.H.; Lewandowski, M.; Jaoui, M.; Kleindienst, T.E. Contributions of biogenic and anthropogenic hydrocarbons to secondary organic aerosol during 2006 in Research Triangle Park, NC. Aerosol Air Qual. Res. 2011, 11, 99–108. [Google Scholar] [CrossRef]
- Wang, W.; Wu, M.H.; Li, L.; Zhang, T.; Liu, X.D.; Feng, J.L.; Li, H.J.; Wang, Y.J.; Sheng, G.Y.; Claeys, M.; et al. Polar organic tracers in PM2.5 aerosols from forests in Eastern China. Atmos. Chem. Phys. 2008, 8, 7507. [Google Scholar] [CrossRef] [Green Version]
- Hopke, P.K. Review of receptor modeling methods for source apportionment. J. Air Waste Manag. 2016, 66, 237. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheesley, R.J.; Schauer, J.J.; Lewandowski, M.; Jaoui, M.; Offenberg, J.H.; Kleindienst, T.E.; Edney, E.O. Source apportionment of primary and secondary organic aerosols using positive matrix factorization (PMF) of molecular markers. Atmos. Environ. 2009, 43, 5567. [Google Scholar] [CrossRef]
- Wang, Q.Q.; He, X.; Huang, X.H.H.; Griffith, S.M.; Feng, Y.M.; Zhang, T.; Zhang, Q.Y.; Wu, D.; Yu, J.Z. Impact of secondary organic aerosol tracers on tracer-based source apportionment of organic carbon and PM2.5: A case study in the Pearl River Delta, China. ACS Earth Space Chem. 2017, 1, 562. [Google Scholar] [CrossRef]
- Li, R.; Wang, Q.Q.; He, X.; Zhu, S.H.; Zhang, K.; Duan, Y.S.; Fu, Q.Y.; Qiao, L.P.; Wang, Y.J.; Huang, L.; et al. Source apportionment of PM2.5 in Shanghai based on hourly organic molecular markers and other source tracers. Atmos. Chem. Phys. 2020, 20, 12047. [Google Scholar] [CrossRef]
- Lanzafame, G.M.; Srivastava, D.; Favez, O.; Bandowe, B.A.M.; Shahpoury, P.; Lammel, G.; Bonnaire, N.; Alleman, L.Y.; Couvidat, F.; Bessagnet, B.; et al. One-year measurements of secondary organic aerosol (SOA) markers in the Paris Region (France): Concentrations, gas/particle partitioning and SOA source apportionment. Sci. Total Environ. 2021, 757, 143921. [Google Scholar] [CrossRef]
- Hu, D.; Bian, Q.J.; Lau, A.K.H.; Yu, J.Z. Source apportioning of primary and secondary organic carbon in summer PM2.5 in Hong Kong using positive matrix factorization of secondary and primary organic tracer data. J. Geophys. Res. Atmos. 2010, 115, D16204. [Google Scholar] [CrossRef]
- Hettiyadura, A.P.; Xu, L.; Jayarathne, T.; Skog, K.; Guo, H.; Weber, R.J.; Nenes, A.; Keutsch, F.N.; Ng, N.L.; Stone, E.A. Source apportionment of organic carbon in Centreville, AL using organosulfates in organic tracer-based positive matrix factorization. Atmos. Environ. 2018, 186, 74. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.W.; Campuzano-Jost, P.; Palm, B.B.; Day, D.A.; Ortega, A.M.; Hayes, P.L.; Krechmer, J.E.; Chen, Q.; Kuwata, M.; Liu, Y.J.; et al. Characterization of a real-time tracer for isoprene epoxydiols-derived secondary organic aerosol (IEPOX-SOA) from aerosol mass spectrometer measurements. Atmos. Chem. Phys. 2015, 15, 11807. [Google Scholar] [CrossRef]
- De Sa, S.S.; Palm, B.B.; Campuzano-Jost, P.; Day, D.A.; Newburn, M.K.; Hu, W.W.; Isaacman-VanWertz, G.; Yee, L.D.; Thalman, R.; Brito, J.; et al. Influence of urban pollution on the production of organic particulate matter from isoprene epoxydiols in Central Amazonia. Atmos. Chem. Phys. 2017, 17, 6611. [Google Scholar] [CrossRef] [Green Version]
- Freney, E.; Sellegri, K.; Chrit, M.; Adachi, K.; Brito, J.; Waked, A.; Borbon, A.; Colomb, A.; Dupuy, R.; Pichon, J.M.; et al. Aerosol composition and the contribution of SOA formation over Mediterranean forests. Atmos. Chem. Phys. 2018, 18, 7041. [Google Scholar] [CrossRef] [Green Version]
- Budisulistiorini, S.H.; Li, X.; Bairai, S.T.; Renfro, J.; Liu, Y.; Liu, Y.J.; McKinney, K.A.; Martin, S.T.; McNeill, V.F.; Pye, H.O.T.; et al. Examining the effects of anthropogenic emissions on isoprene-derived secondary organic aerosol formation during the 2013 Southern Oxidant and Aerosol Study (SOAS) at the Look Rock, Tennessee ground site. Atmos. Chem. Phys. 2015, 15, 8871. [Google Scholar] [CrossRef] [Green Version]
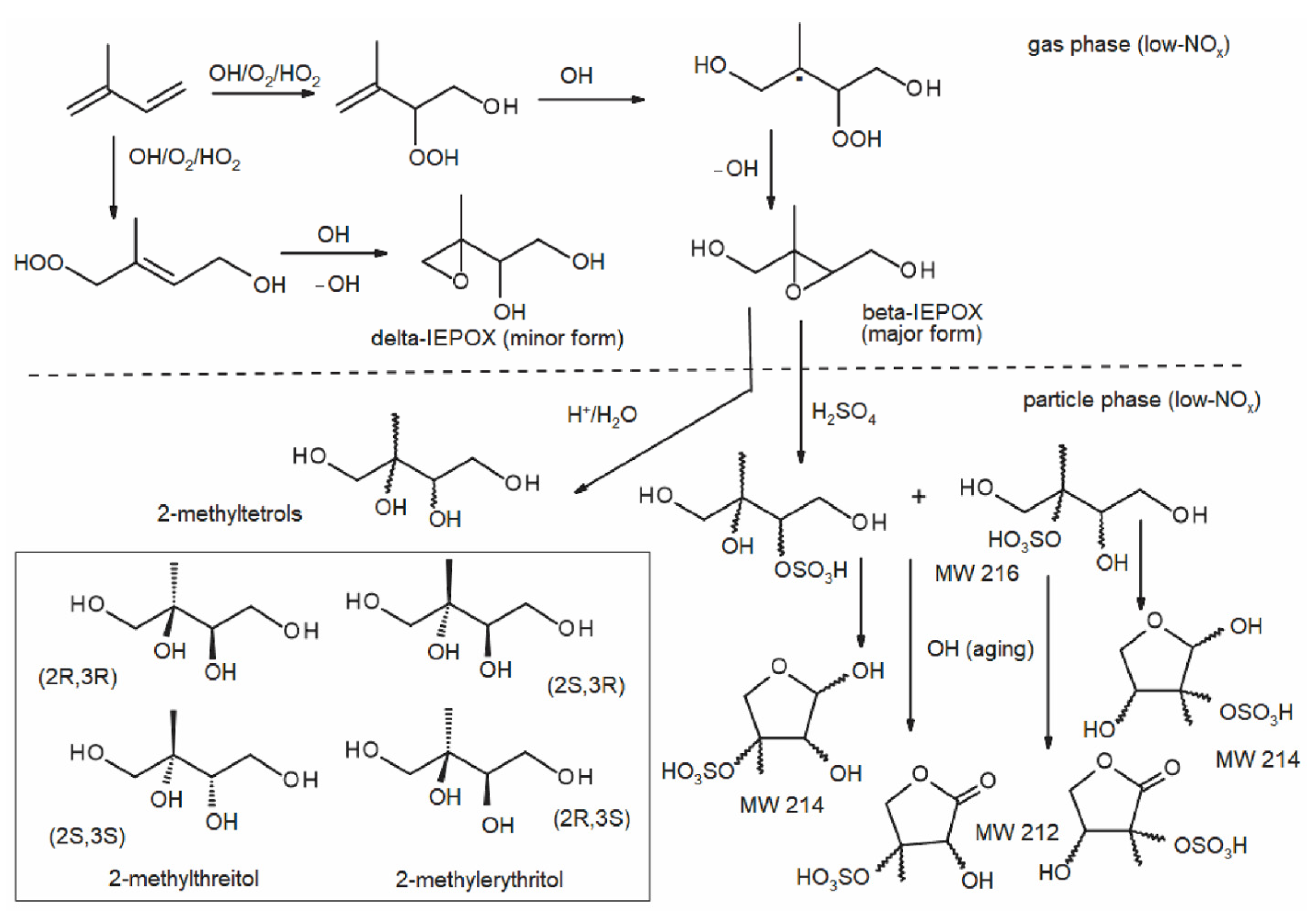

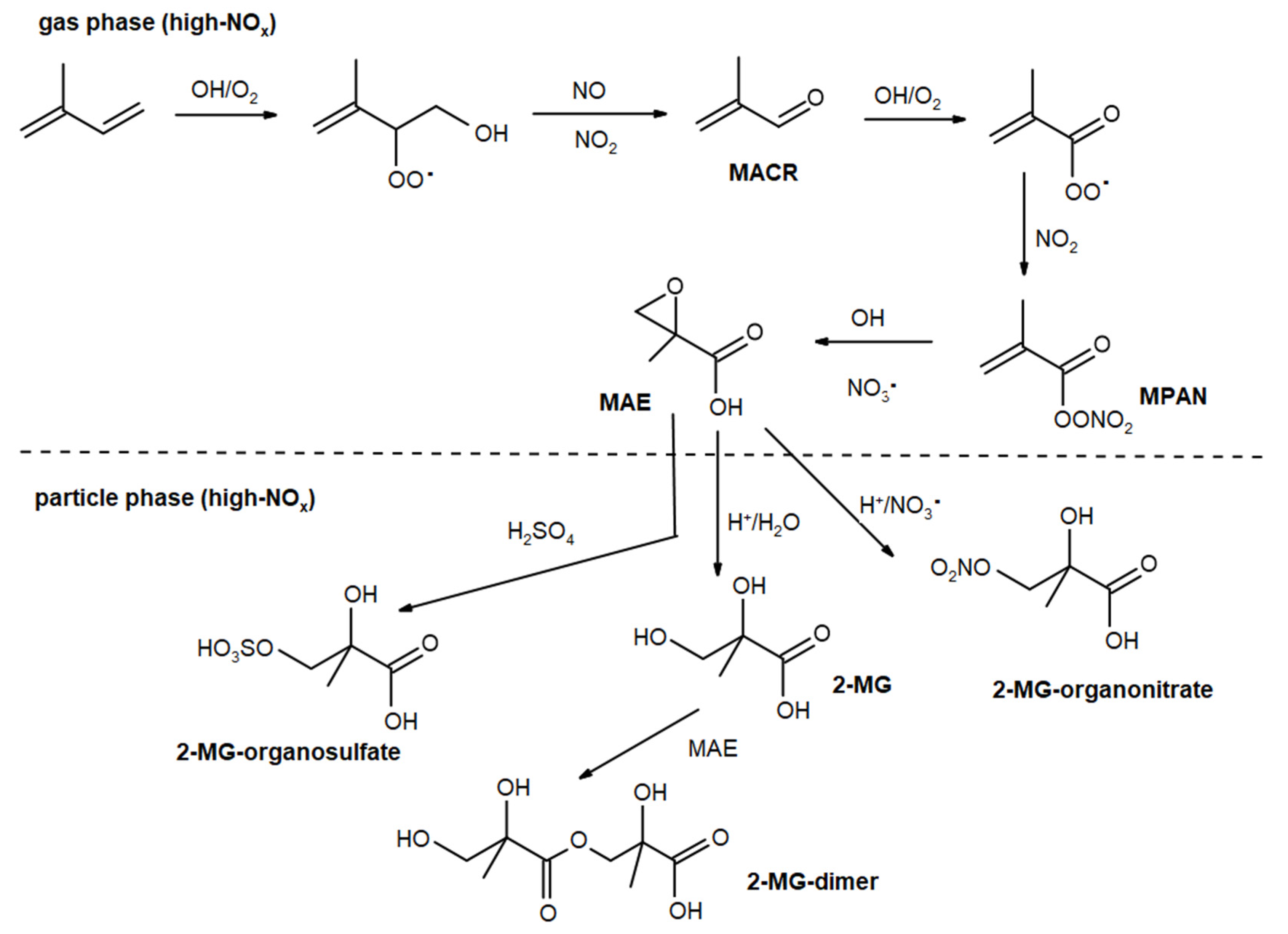
| Molecular Structure + Chemical Name (Trivial Name) | Chemical Formula MW | Figure | Analysis Method | Selected References |
|---|---|---|---|---|
 3-methyl-3-butenetriol + tautomer (C5-alkene triol) 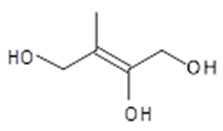 3-methyl-2-butene-1,2,4-triol (C5-alkene triol) | C5H10O3 118 | – | GC/MS | [6,18,20] |
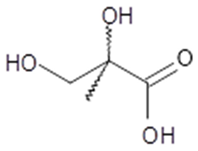 2,3-dihydroxy-2-methyl- propanoic acid (2-MG) | C4H8O4 120 | 3 | GC/MS | [8,17,21,22] |
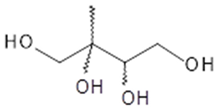 2-methylbutane-1,2,3,4-tetrol (2-methyltetrol) | C5HO4 136 | 1 2 | GC/MS | [1,6,10,17,19] |
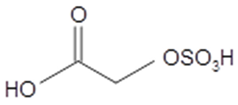 sulfoxyethanoic acid (glycolic acid OS) | C2H4O6S 156 | – | LC/MS | [39,40,47,48] |
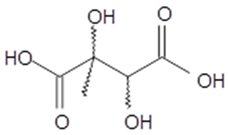 2,3-dihydroxy-2-methyl- butanedioic acid (2-methyltartaric acid) | C5H8O6 164 | – | GC/MS | [1,68] |
 2-sulfoxypropionic acid (lactic acid OS) | C3H6O6S 170 | – | LC/MS | [39,40,47] |
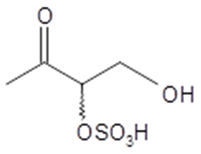 3-oxo-2-sulfoxybutane-1-ol | C4H8O6S 184 | – | LC/MS | [40] |
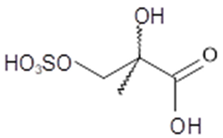 2-hydroxy-2-methyl- 3-sulfoxypropanoic acid (2-MG OS) | C4H8O7S 200 | 3 | LC/MS | [8,38,39,40] |
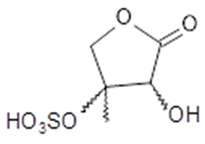 + isomers 3-hydroxy-4-methyl-4-sulf- oxy-2(3H)-dihydrofuranone | C5H8O7S 212 | 1 | LC/MS | [41,42,46,47] |
 + isomers 2,3-dihydroxy-4-methyl- 4-sulfoxytetrahydrofurane | C5H10O7S 214 | 1 | LC/MS | [41,46,47] |
 3-methyl-2-sulfoxy- butane-1,3,4-triol + isomers (2-methyltetrol OS) | C5H12O7S 216 | 1 | LC/MS | [12,38,39] |
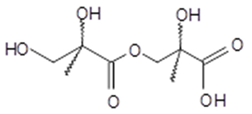 several isomers (2-MG dimer) | C8H14O7 222 | 3 | GC/MS | [21] |
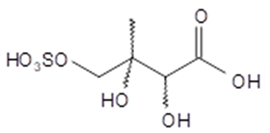 2,3-dihydroxy-3-methyl-4- sulfoxybutanoic acid (2-methylthreonic acid OS) | C5H10O8S 230 | – | LC/MS | [49] |
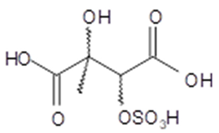 2-hydroxy-2-methyl- 3-sulfoxybutanedioic acid (2-methyltartaric acid OS) | C5H9O9S 244 | – | LC/MS | [49] |
 3-methyl-4-nitrooxy- 3-sulfoxybutane-1,2-diol – several isomers (2-methyltetrol NOS) | C5H11O9NS 261 | – | LC/MS | [38,39] |
 (2-MG dimer OS) | C8H14O10S 302 | – | LC/MS | [8,38] |
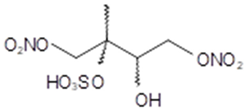 1,4-dinitrooxy-3-methyl- 3-sulfoxybutane-2-ol - several isomers (2-methyltetrol NOS) | C5H10O11N2S 306 | – | LC/MS | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claeys, M.; Maenhaut, W. Secondary Organic Aerosol Formation from Isoprene: Selected Research, Historic Account and State of the Art. Atmosphere 2021, 12, 728. https://doi.org/10.3390/atmos12060728
Claeys M, Maenhaut W. Secondary Organic Aerosol Formation from Isoprene: Selected Research, Historic Account and State of the Art. Atmosphere. 2021; 12(6):728. https://doi.org/10.3390/atmos12060728
Chicago/Turabian StyleClaeys, Magda, and Willy Maenhaut. 2021. "Secondary Organic Aerosol Formation from Isoprene: Selected Research, Historic Account and State of the Art" Atmosphere 12, no. 6: 728. https://doi.org/10.3390/atmos12060728
APA StyleClaeys, M., & Maenhaut, W. (2021). Secondary Organic Aerosol Formation from Isoprene: Selected Research, Historic Account and State of the Art. Atmosphere, 12(6), 728. https://doi.org/10.3390/atmos12060728








