Assessing the Factors of Dengue Transmission in Urban Environments of Pakistan
Abstract
1. Introduction
2. Material and Methods
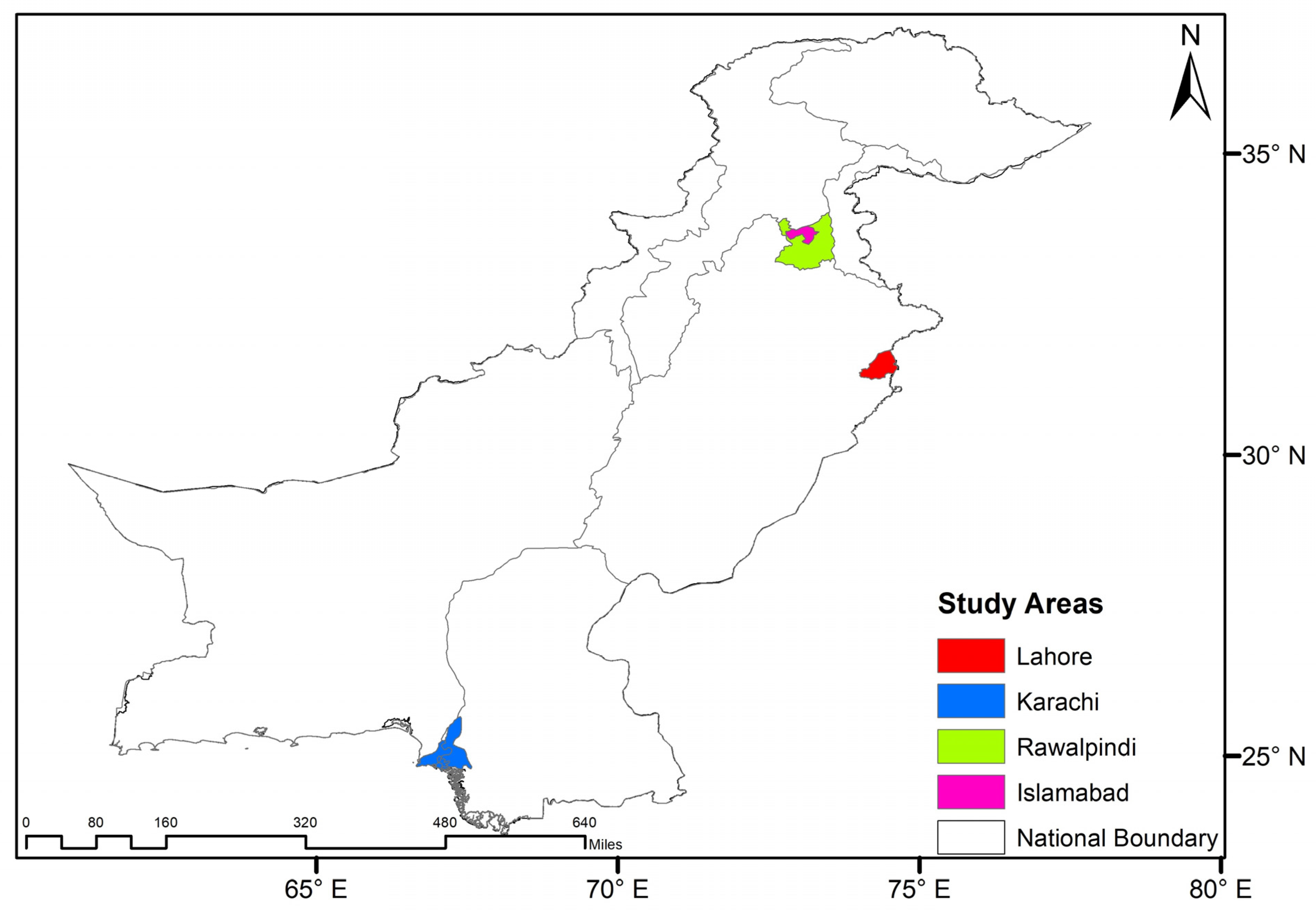
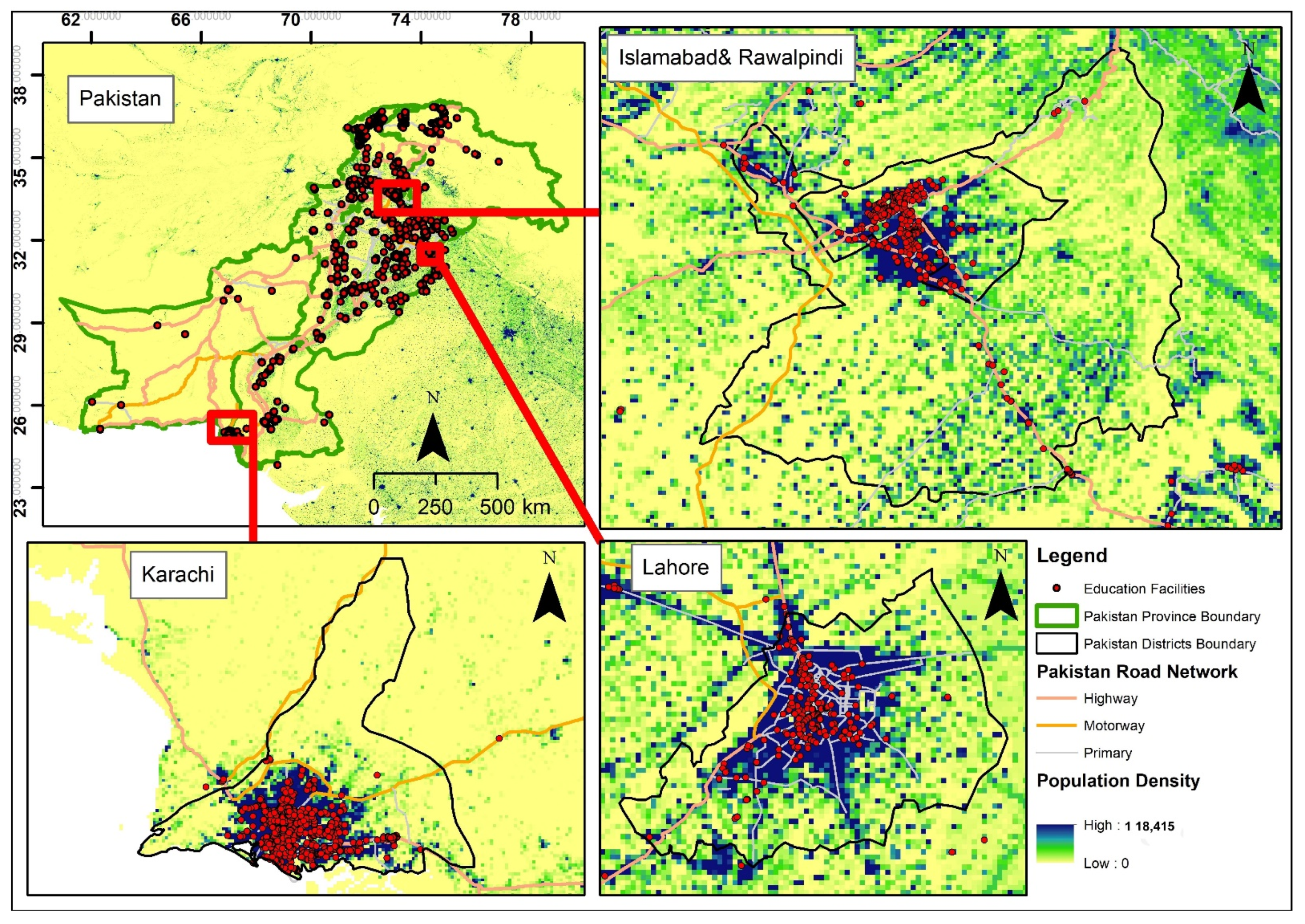
2.1. Study Region
2.2. Data
2.3. Data Analysis
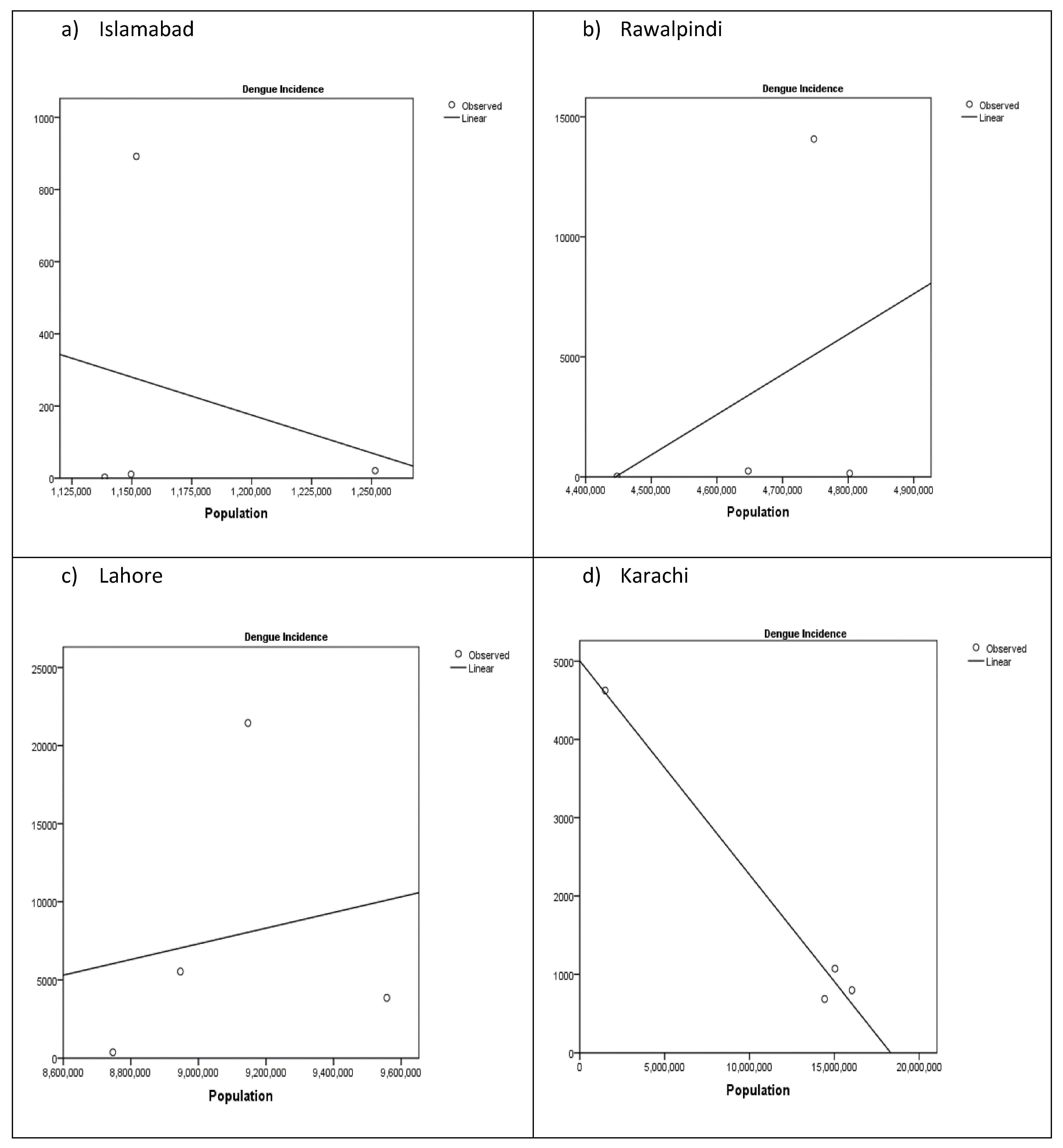
3. Results
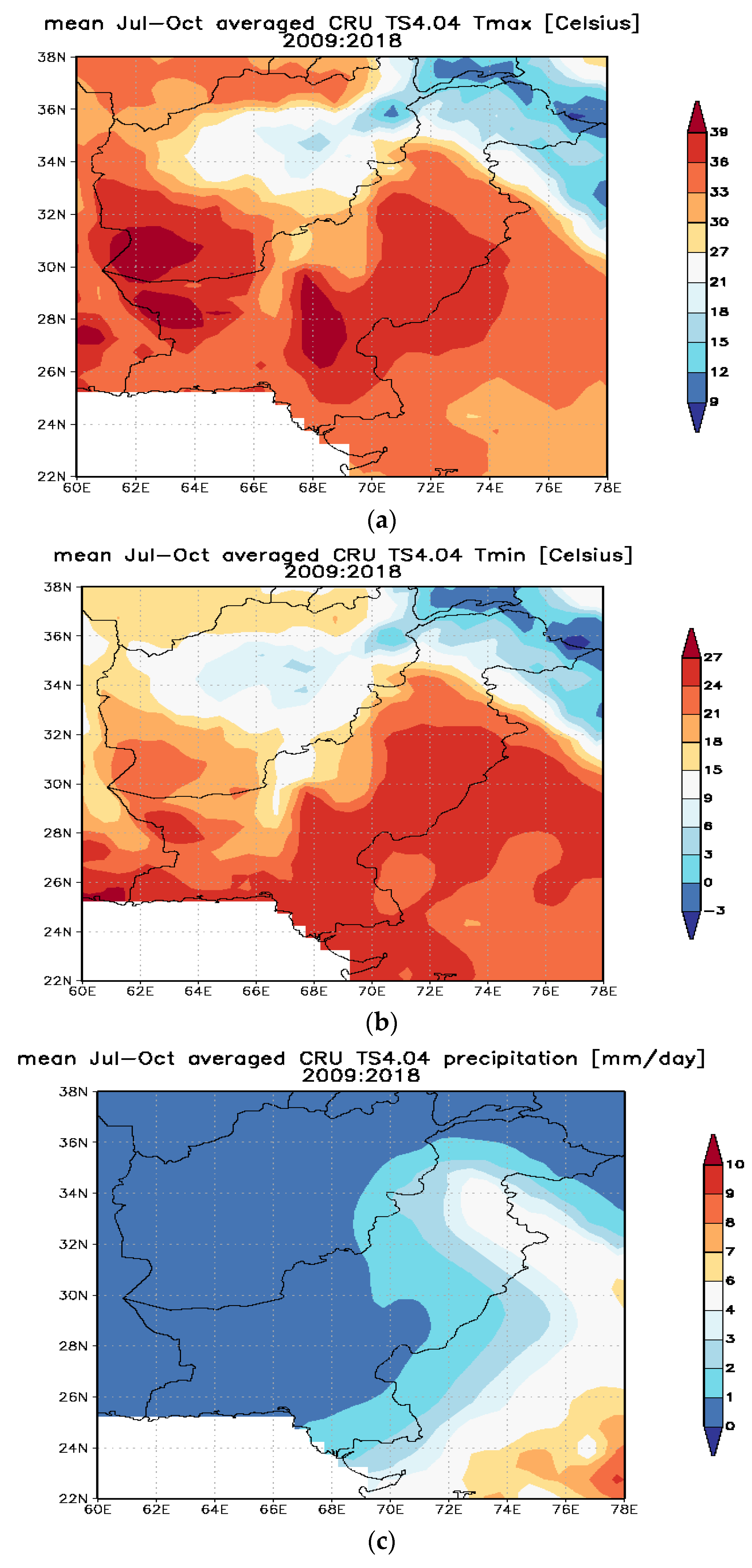
3.1. Minimum Temperature
3.2. Maximum Temperature
3.3. Rainfall
3.4. Human Population Density
3.5. Traveling toward Study Region
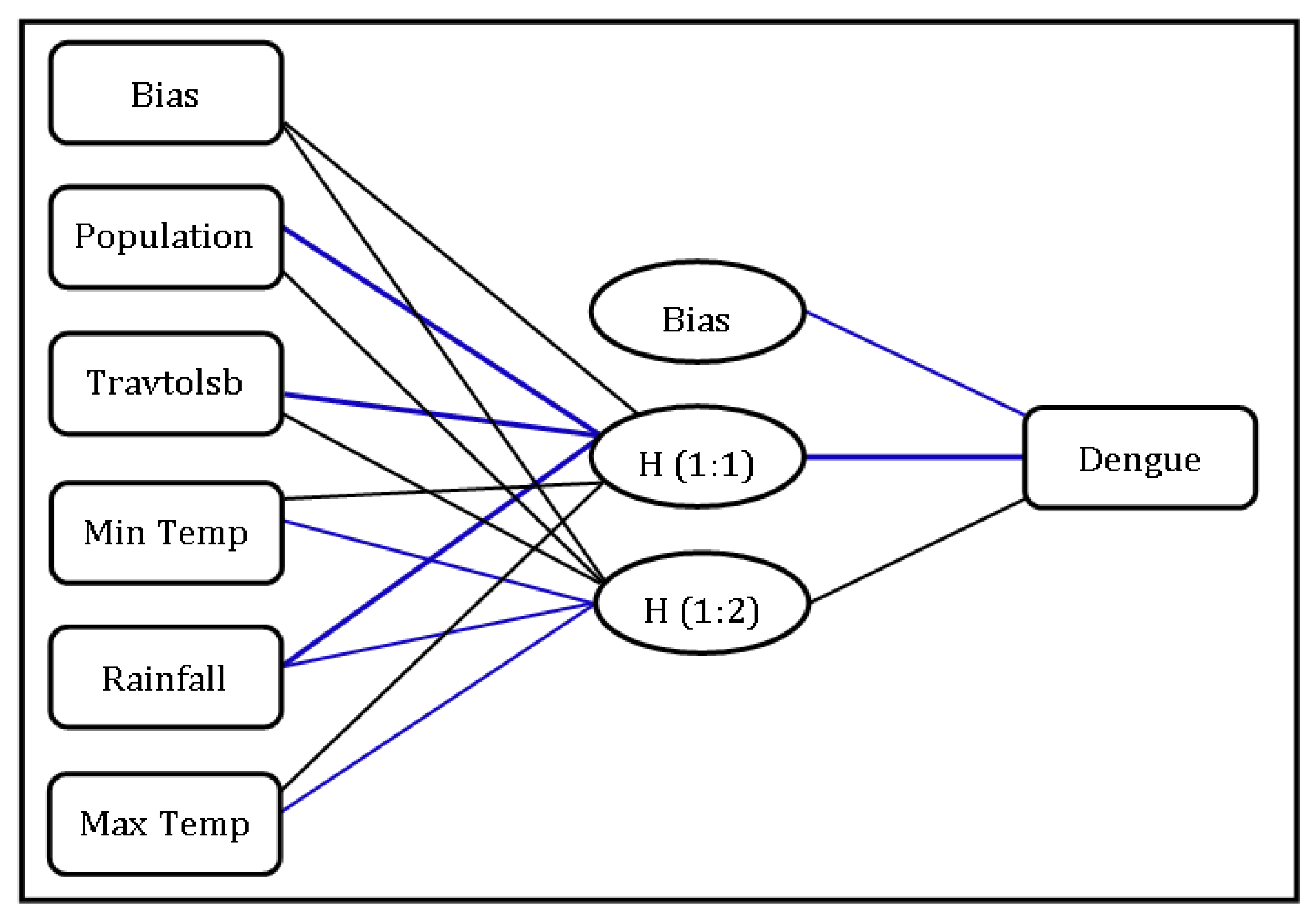
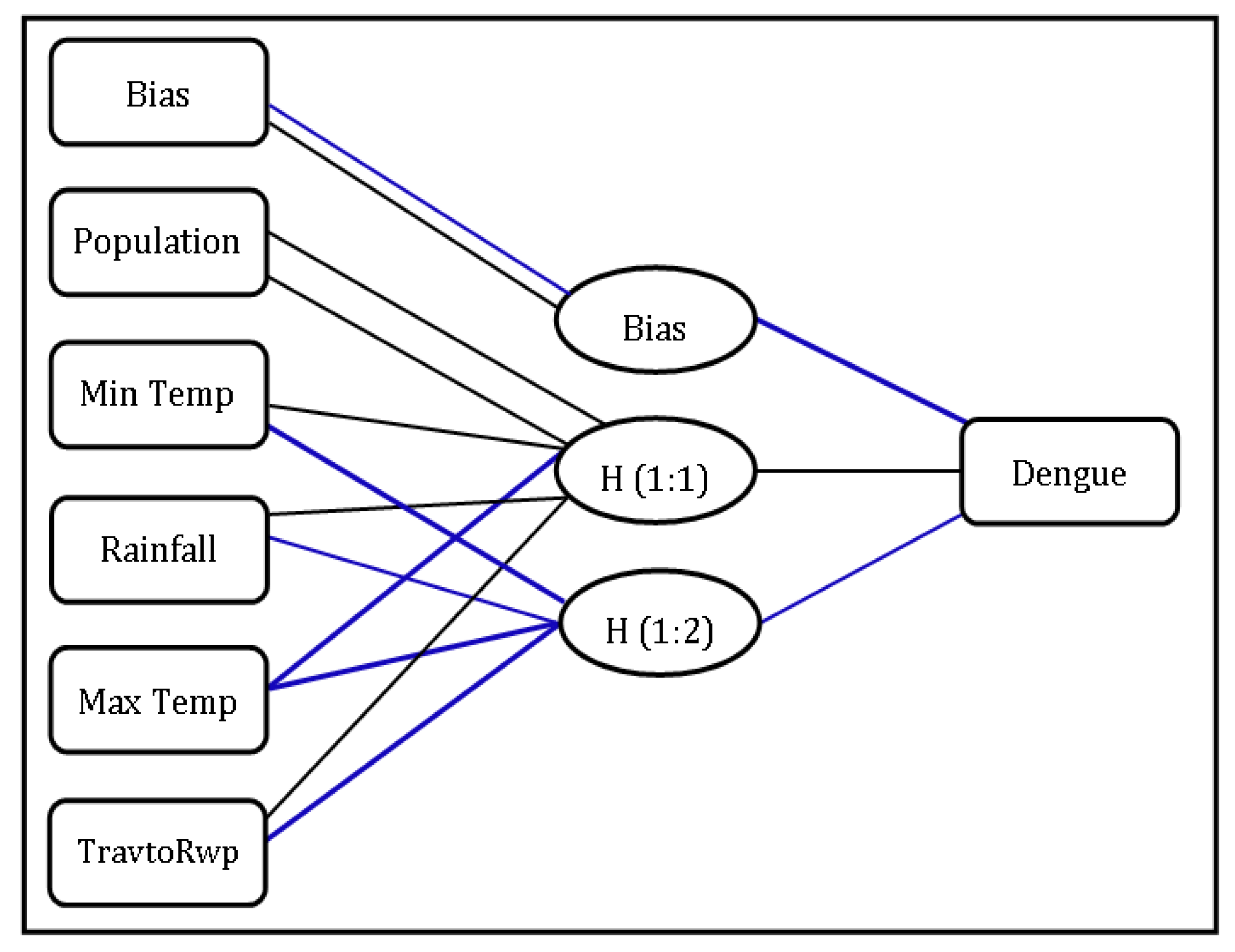
3.6. Random Effect Calculated by GLMM
3.7. Spatial Characteristics of Study Regions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, B.; Ghaffar, A. Dengue transmission based on urban environmental gradients in different cities of Pakistan. Int. J. Biometeor. 2015, 59, 267–283. [Google Scholar] [CrossRef]
- Khalid, B.; Ghaffar, A. Environmental risk factors and hotspot analysis of dengue distribution in Pakistan. Int. J. Biometeor. 2015, 59, 1721–1746. [Google Scholar] [CrossRef]
- Caley, T.; Malaizé, B.; Zaragosi, S.; Rossignol, L.; Bourget, J.; Martinez, P.; Giraudeau, J.; Charlier, K.; Zimmermann, N. New Arabian Sea records help deciphers orbital timing of Indo-Asian monsoon. Earth Planet. Sci. Lett. 2011, 308, 433–444. [Google Scholar] [CrossRef]
- Morin, C.; Comrie, A.C.; Ernst, K. Climate and dengue transmission: Evidence and implications. Environ. Health Perspect. 2013, 121, 1264–1272. [Google Scholar] [CrossRef]
- Kureshy, K. Geography of Pakistan; National Book Service Lahore: Lahore, Pakistan, 1988. [Google Scholar]
- Luckge, A.; Doose-Rolinski, H.; Khan, A.; Schulz, H.; von Rad, U. Monsoonal variability in the northeastern Arabian Sea during the past 5000-years: Geochemical evidence from laminated sediments. Palaeogeog. Palaeoclim. Palaeoecol. 2001, 167, 273–286. [Google Scholar] [CrossRef]
- Ali, S.; Khalid, B.; Kiani, R.S.; Babar, R.; Nasir, S.; Rehman, N.; Adnan, M.; Goheer, M.A. Spatio-temporal variability of summer monsoon onset over Pakistan. Asia-Pac. J. Atmos. Sci. 2020, 56, 147–172. [Google Scholar] [CrossRef]
- Goswami, B. Increasing trend of extreme rain events over India in a Warming Environment. Science 2006, 314, 1442–1445. [Google Scholar] [CrossRef]
- von Rad, U.; Schaaf, M.; Michels, K.; Schulzc, H.; Bergerd, W.H.; Sirocko, F. A 5000-yr record of climate change in varved sediments from the oxygen minimum zone off Pakistan, Northeastern Arabian Sea. Quat. Res. 1999, 51, 39–53. [Google Scholar] [CrossRef]
- Singapore Red Cross Society. Pakistan Floods the Deluge of Disaster-Facts & Figures as of 15 September 2010; Singapore Red Cross Society: Singapore, 2010. [Google Scholar]
- Dyurgerov, M.; Meier, M. Glaciers and the Changing Earth System: A 2004 Snapshot; Report of Institute of Arctic and Alpine Research: Boulder, CO, USA, 2005. [Google Scholar]
- Ahern, M.; Kovats, R.; Wilkinson, P.; Few, R.; Matthies, F. Global health impacts of floods: Epidemiologic evidence. Epidemiol. Rev. 2005, 27, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Thavara, U.; Chitnumsup, P.; Tawatsin, A.; Chansang, C.; Campbell-Lendrum, D. Climatic and social risk factors for Aedes infestation in rural Thailand. Trop. Med. Int. Health 2003, 8, 650–659. [Google Scholar] [CrossRef] [PubMed]
- WHO. Flooding and Communicable Diseases Fact Sheet: Risk Assessment and Preventive Measures; The World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Qaiser, G. Climate of Pakistan; National Drought Monitoring Center, Pakistan Meteorological Department: Islamabad, Pakistan, 2011.
- Zhang, Y.; Bi, P.; Hiller, J.E. Climate change and the transmission of vector-borne diseases: A review. Asia Pac. J. Public Health 2008, 20, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Chowell, G.; Duen, D.J.C.; Miller, A.; Velazco, A.J.M.; Hyman, P.W.; Fenimore, C. Estimation of the reproduction number of dengue fever from spatial epidemic data. Math. Biosci. 2007, 208, 571–589. [Google Scholar] [CrossRef]
- Yu, H.; Yang, S.; Yen, H.; Christakos, G. A spatio-temporal climate-based model of early dengue fever warning in southern Taiwan. Stoch. Environ. Res. Risk Assess. 2011, 25, 485–494. [Google Scholar] [CrossRef]
- Pinto, E.; Coelho, M.; Oliver, L.; Massad, E. The influence of climate variables on dengue in Singapore. Int. J. Environ. Health Res. 2011, 21, 415–426. [Google Scholar] [CrossRef]
- Favier, C.; Degallier, N.; Dubois, M. Dengue epidemic modeling: Stakes and pitfalls. Asia Pac. 2005, 22, 1191–1194. [Google Scholar]
- Wu, P.; Lay, J.; Guo, H.; Lung, S.; Su, H. Higher temperature and urbanization affect the spatial patterns of dengue fever transmission in subtropical Taiwan. Sci. Total Environ. 2009, 407, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.; Weld, L.; Smith, W.A. Seasonality, annual trends, and characteristics of dengue among ill returned travelers, 1997–2006. Emerg. Infect. Dis. 2008, 14, 1081–1088. [Google Scholar] [CrossRef]
- Berrington, W.R.; Hitti, J.; Casper, C. A case report of dengue virus infection and acalculous cholecystitis in a pregnant returning traveler. Trav. Med. Infect. 2007, 5, 251–253. [Google Scholar] [CrossRef]
- Karch, S.; Dellile, M.-F.; Guillet, P.; Mouchet, J. African Malaria vectors in European aircraft. Lancet 2001, 357, 235. [Google Scholar] [CrossRef]
- Kritsiriwuthinan, K.; Ngrenngarmlert, W. Asymptomatic Malaria infections among foreign migrant workers in Thailand. Asian Pac. J. Trop. Med. 2011, 1, 560–563. [Google Scholar] [CrossRef]
- Alaya-Bouafif, N.B.; Chahed, M.K.; Bez, H.; Bellali, H.; Ayari, L.; Achour, N. Completeness of Malaria notification in Tunisia assessed by capture recapture method. Asian Pac. J. Trop. Dis. 2011, 1, 187–191. [Google Scholar] [CrossRef]
- Thaver, A.; Sobanim, A.; Qazib, F.; Khan, M.; Zafar, A.; Beg, M.A. Assessing the need for training: General practitioners’ knowledge, attitude and practice concerning dengue and Malaria in Karachi, Pakistan. Int. Health 2011, 3, 126–130. [Google Scholar] [CrossRef]
- Huguet, J.; Punpuing, S. International Migration in Thailand; International Organization for Migration: Bangkok, Thailand, 2005. [Google Scholar]
- Lowe, R.; Bailey, T.; Stephenson, D.; Graham, R.; Coelho, C.; Carvelho, M.; Barcello, C. Spatio-temporal modelling of climate-sensitive disease risk: Towards an early warning system for dengue in Brazil. Comput. Geosci. 2011, 3, 371–381. [Google Scholar] [CrossRef]
- Browne, W.; Draper, D. A comparison of Bayesian and likelihood-based methods for fitting multilevel models. Bayesian Anal. 2006, 1, 473–514. [Google Scholar] [CrossRef]
- Zhao, Y.; Staudenmayer, J.; Coull, B.; Wand, M. General design Bayesian generalized linear mixed models. Stat. Sci. 2006, 21, 35–51. [Google Scholar] [CrossRef]
- Alabi, M.A.; Issa, S.; Afolayan, R.B. An application of artificial intelligent neural network and discriminant analyses on credit scoring. Math Theory Model 2013, 3, 20–28. [Google Scholar]
- Rajalakshmi, P. Mode choice modelling based on work trips–artificial neural network model. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 360–369. [Google Scholar]
- Kausar, R.; Mirza, S.; Saboor, A.; Saleem, A.; Khalid, B. Role of ecotourism in promoting and sustaining conservation of nature: A case study of Murree forest recreational resort. Pak. J. Agric. Sci. 2013, 50, 463–468. [Google Scholar]
- Khan, J.; Khan, A. Incidence of dengue in 2013: Dengue outbreak in district Swat, Khyber Pakhtunkhwa, Pakistan. Int. J. Fauna Biol. Stud. 2015, 2, 1–7. [Google Scholar]



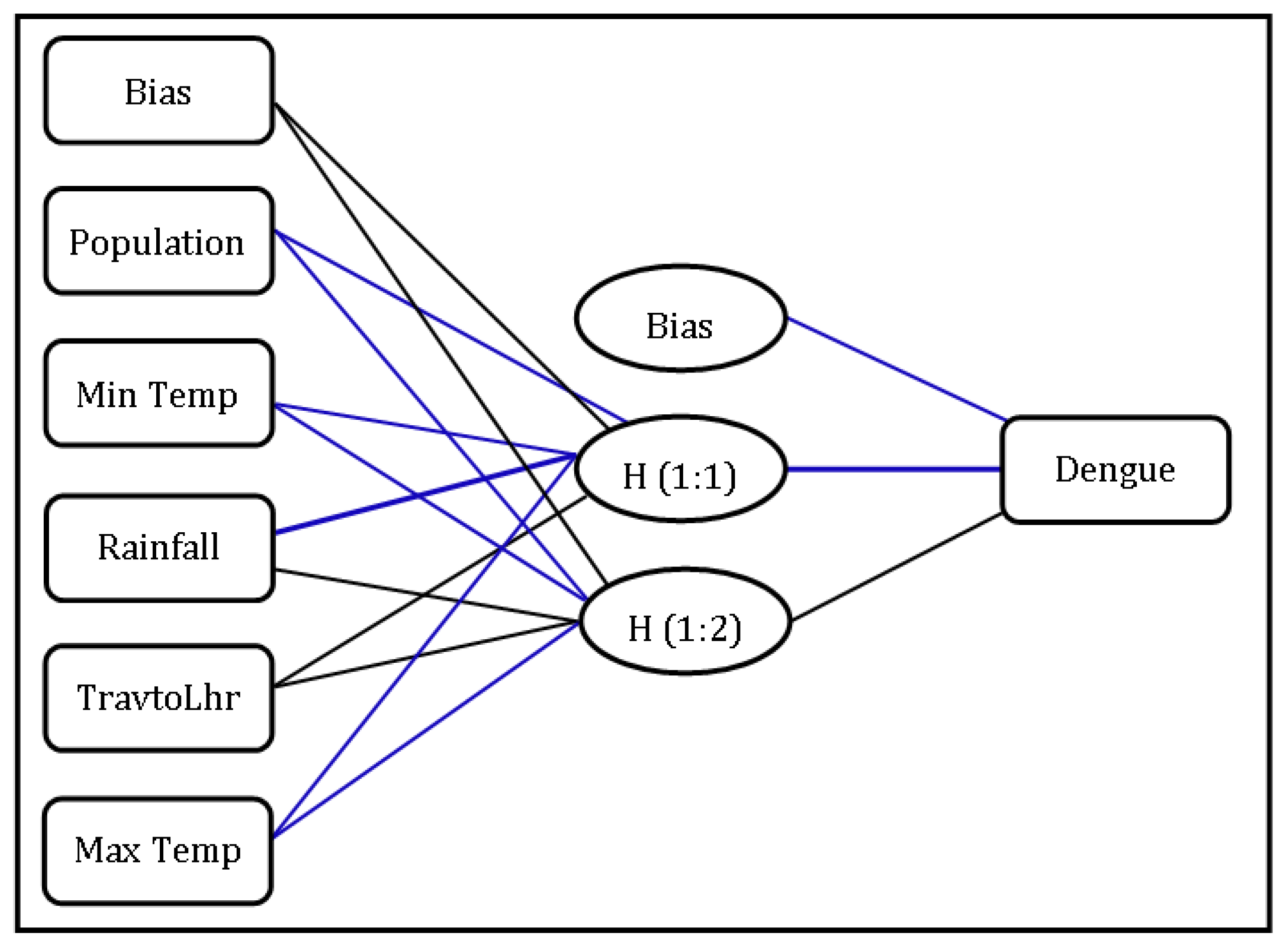
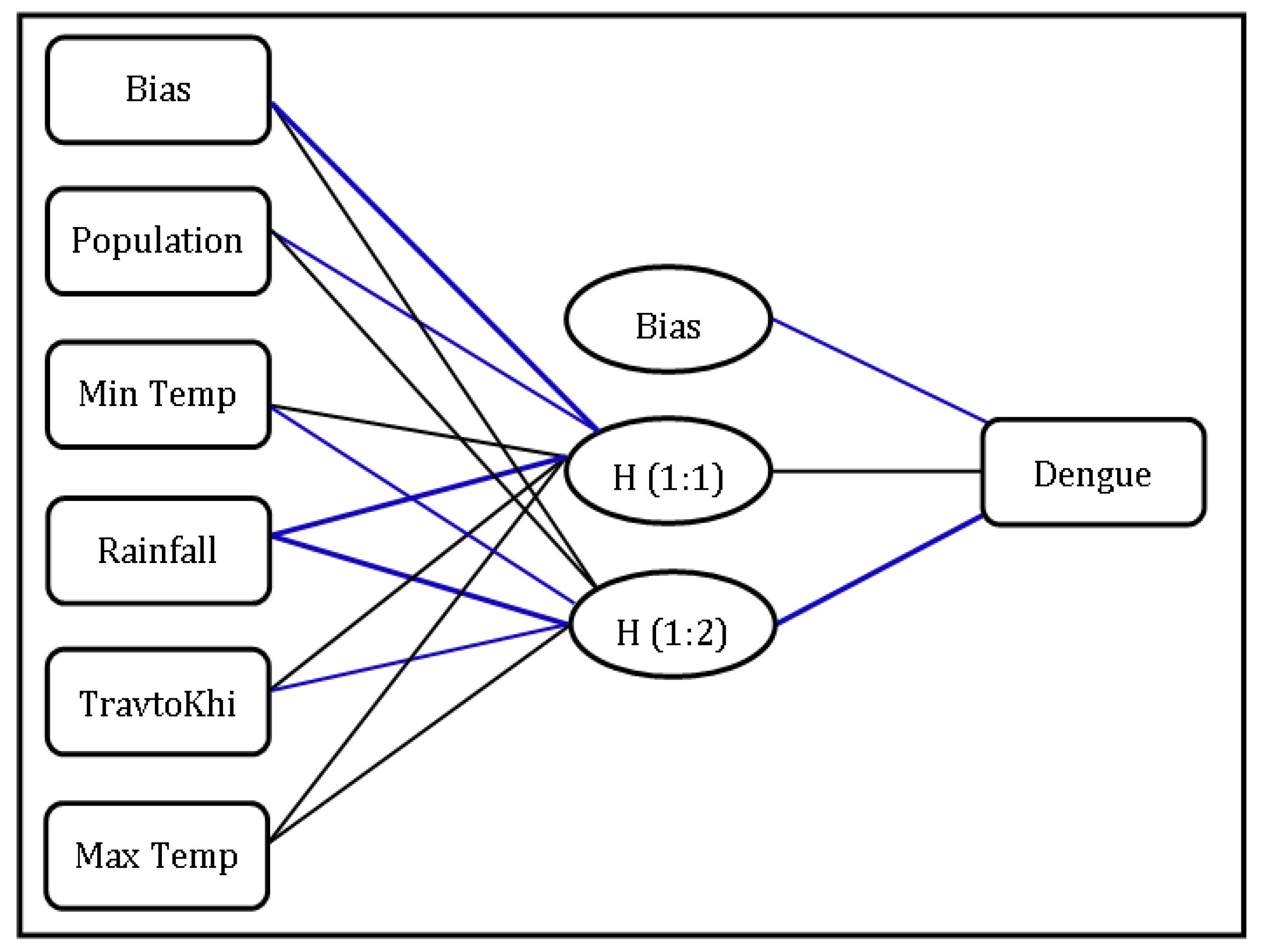
| Factors | Importance | Normalized Importance |
|---|---|---|
| Population | 0.210 | 67.8% |
| TravtoIsb | 0.240 | 74.8% |
| MinTemp | 0.152 | 49.2% |
| Rainfall | 0.309 | 100.0% |
| MaxTemp | 0.097 | 31.5% |
| Factors | Importance | Normalized Importance |
|---|---|---|
| Population | 0.137 | 35.1% |
| MinTemp | 0.366 | 93.8% |
| Rainfall | 0.391 | 100.0% |
| Maxtemp | 0.021 | 6.7% |
| TravtoRwp | 0.080 | 20.4% |
| Factors | Importance | Normalized Importance |
|---|---|---|
| Population | 0.144 | 20.2% |
| MinTemp | 0.103 | 24.7% |
| Rainfall | 0.582 | 100.0% |
| TravtoLhr | 0.069 | 11.8% |
| MaxTemp | 0.109 | 14.1% |
| Factors | Importance | Normalized Importance |
|---|---|---|
| Population | 0.140 | 42.2% |
| MinTemp | 0.332 | 100.0% |
| Rainfall | 0.161 | 48.5% |
| TravtoKhi | 0.210 | 63.2% |
| MaxTemp | 0.156 | 46.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, B.; Bueh, C.; Ghaffar, A. Assessing the Factors of Dengue Transmission in Urban Environments of Pakistan. Atmosphere 2021, 12, 773. https://doi.org/10.3390/atmos12060773
Khalid B, Bueh C, Ghaffar A. Assessing the Factors of Dengue Transmission in Urban Environments of Pakistan. Atmosphere. 2021; 12(6):773. https://doi.org/10.3390/atmos12060773
Chicago/Turabian StyleKhalid, Bushra, Cholaw Bueh, and Abdul Ghaffar. 2021. "Assessing the Factors of Dengue Transmission in Urban Environments of Pakistan" Atmosphere 12, no. 6: 773. https://doi.org/10.3390/atmos12060773
APA StyleKhalid, B., Bueh, C., & Ghaffar, A. (2021). Assessing the Factors of Dengue Transmission in Urban Environments of Pakistan. Atmosphere, 12(6), 773. https://doi.org/10.3390/atmos12060773








