Different Characteristics of PM2.5 Measured in Downtown and Suburban Areas of a Medium-Sized City in South Korea
Abstract
1. Introduction
2. Materials and Methods
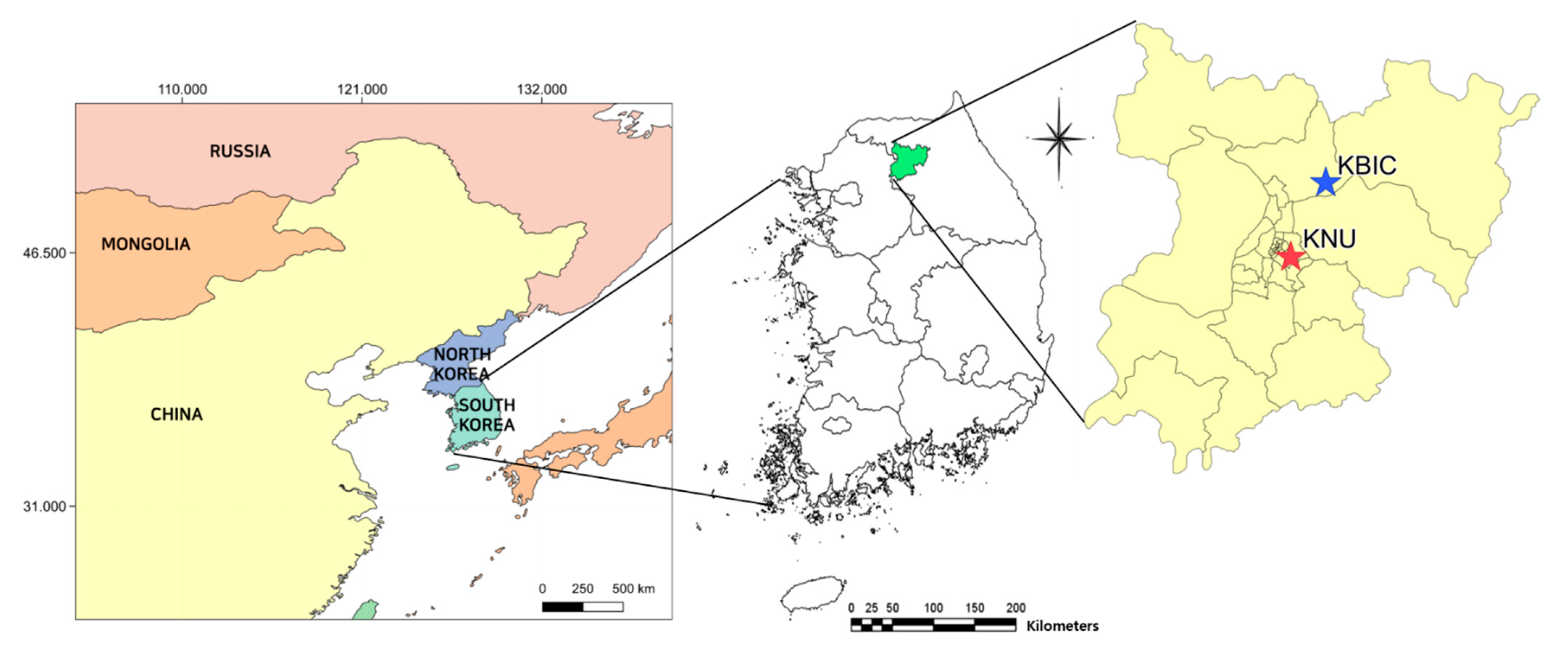
2.1. Sampling
2.2. Chemical Analysis
2.3. QA/QC and Statistical Analysis
3. Results
3.1. General Characteristics of PM2.5 at Two Locations
3.2. Relationship of PM2.5 Components between Two Locations
3.3. Higher PM2.5 Samples at the KBIC Site Than at the KNU Site
3.4. Correlations of PM2.5 Components with PM2.5 Mass and Relative Humidity
3.5. Secondary Organic Carbon
3.6. Size Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. J. Am. Med. Assoc. 2006, 295, 1127–1134. [Google Scholar] [CrossRef]
- Lu, F.; Xu, D.; Cheng, Y.; Dong, S.; Guo, C.; Jiang, X.; Zheng, X. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ. Res. 2015, 136, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.M.; Kaufman, J.; Hoek, G. Chronic Effects of Air Pollution on Cardiovascular Health. Cardiovasc. Eff. Inhaled Ultrafine Nanosized Part. 2011, 45–57. [Google Scholar] [CrossRef]
- Haywood, J.; Boucher, O. Estimates of the direct and indirect radiative forcing due to tropospheric aerosols: A review. Rev. Geophys. 2000, 38, 513–543. [Google Scholar] [CrossRef]
- Lohmann, U.; Feichter, J. Global indirect aerosol effects: A review. Atmos. Chem. Phys. 2005, 5, 715–737. [Google Scholar] [CrossRef]
- Kaufman, Y.J.; Tanré, D.; Boucher, O. A satellite view of aerosols in the climate system. Nature 2002, 419, 215–223. [Google Scholar] [CrossRef]
- Ye, B.; Ji, X.; Yang, H.; Yao, X.; Chan, C.K.; Cadle, S.H.; Chan, T.; Mulawa, P.A. Concentration and chemical composition of PM2.5 in Shanghai for a 1-year period. Atmos. Environ. 2003, 37, 499–510. [Google Scholar] [CrossRef]
- Liousse, C.; Penner, J.E.; Chuang, C.; Walton, J.J.; Eddleman, H.; Cachier, H. A global three-dimensional model study of carbonaceous aerosols. J. Geophys. Res. Atmos. 1996, 101, 19411–19432. [Google Scholar] [CrossRef]
- Chung, S.H.; Seinfeld, J.H. Global distribution and climate forcing of carbonaceous aerosols. J. Geophys. Res. Atmos. 2002, 107, AAC 14-1–AAC 14-33. [Google Scholar] [CrossRef]
- Donahue, N.M.; Posner, L.N.; Westervelt, D.M.; Li, Z.; Shrivastava, M.; Presto, A.A.; Sullivan, R.C.; Adams, P.J.; Pandis, S.N.; Robinson, A.L. Where Did This Particle Come From? Sources of Particle Number and Mass for Human Exposure Estimates; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Turpin, B.J.; Huntzicker, J.J.; Larson, S.M.; Cass, G.R. Los Angeles Summer Midday Particulate Carbon: Primary and Secondary Aerosol. Environ. Sci. Technol. 1991, 25, 1788–1793. [Google Scholar] [CrossRef]
- Chu, S.H. Stable estimate of primary OC/EC ratios in the EC tracer method. Atmos. Environ. 2005, 39, 1383–1392. [Google Scholar] [CrossRef]
- Timonen, H.; Aurela, M.; Carbone, S.; Saarnio, K.; Saarikoski, S.; Mäkelä, T.; Kulmala, M.; Kerminen, V.M.; Worsnop, D.R.; Hillamo, R. High time-resolution chemical characterization of the water-soluble fraction of ambient aerosols with PILS-TOC-IC and AMS. Atmos. Meas. Tech. 2010, 3, 1063–1074. [Google Scholar] [CrossRef]
- Bassett, M.E.; Seinfeld, J.H. Atmospheric equilibrium model of sulfate and nitrate aerosols—II. Particle size analysis. Atmos. Environ. 1967, 18, 1163–1170. [Google Scholar] [CrossRef]
- Mu, L.; Zheng, L.; Liang, M.; Tian, M.; Li, X.; Jing, D. Characterization and source analysis of water-soluble ions in atmospheric particles in Jinzhong, China. Aerosol Air Qual. Res. 2019, 19, 2396–2409. [Google Scholar] [CrossRef]
- Landreman, A.P.; Shafer, M.M.; Hemming, J.C.; Hannigan, M.P.; Schauer, J.J. A macrophage-based method for the assessment of the reactive oxygen species (ROS) activity of atmospheric particulate matter (PM) and application to routine (daily-24 h) aerosol monitoring studies. Aerosol Sci. Technol. 2008, 42, 946–957. [Google Scholar] [CrossRef]
- Sung Yong, G. A Study on the Health Impact and Management Policy of PM2.5 in Korea, I. Korea Environ. Inst. 2012. Available online: https://www.kei.re.kr/elibList.es?mid=a10101000000&elibName=researchreport&act=view&c_id=696516 (accessed on 28 April 2021).
- Godri, K.J.; Harrison, R.M.; Evans, T.; Baker, T.; Dunster, C.; Mudway, I.S.; Kelly, F.J. Increased oxidative burden associated with traffic component of ambient particulate matter at roadside and Urban background schools sites in London. PLoS ONE 2011, 6, e21961. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Traub, A.; Huang, A.; Hilker, N.; Wang, J.M.; Herod, D.; Dabek-Zlotorzynska, E.; Celo, V.; Evans, G.J. Long-term analysis of PM2.5 from 2004 to 2017 in Toronto: Composition, sources, and oxidative potential. Environ. Pollut. 2020, 263, 114652. [Google Scholar] [CrossRef]
- Kelly, F.J. Oxidative stress: Its role in air pollution and adverse health effects. Occup. Environ. Med. 2003, 60, 612–616. [Google Scholar] [CrossRef]
- Jung, J.-H.; Han, Y.-J. Study on Characteristics of PM 2.5 and Its Ionic Constituents in Chuncheon, Korea. J. Korean Soc. Atmos. Environ. 2008, 24, 682–692. [Google Scholar] [CrossRef]
- Han, Y.J.; Kim, S.R.; Jung, J.H. Long-term measurements of atmospheric PM 2.5 and its chemical composition in rural Korea. J. Atmos. Chem. 2011, 68, 281–298. [Google Scholar] [CrossRef]
- Cho, S.-H.; Kim, P.-R.; Han, Y.-J.; Kim, H.-W.; Yi, S.-M. Characteristics of Ionic and Carbonaceous Compounds in PM 2.5 and High Concentration Events in Chuncheon, Korea. J. Korean Soc. Atmos. Environ. 2016, 32, 435–447. [Google Scholar] [CrossRef]
- Byun, J.Y.; Kim, H.; Han, Y.J.; Lee, S.D.; Park, S.W. High pm2.5 concentrations in a small residential city with low anthropogenic emissions in south korea. Atmosphere 2020, 11, 1159. [Google Scholar] [CrossRef]
- Choi, S.Y.; Park, S.W.; Byun, J.Y.; Han, Y.J. Characteristics of locally occurring high pm2.5 concentration episodes in a small city in south korea. Atmosphere 2021, 12, 86. [Google Scholar] [CrossRef]
- USEPA. Compendium of Methods for the Determination of Inorganic Compounds in Compendium of Methods for the determination of Inorganic Compounds in Ambient Air, Compendium Method IO-4.2: Determination of Reactive Acidic and Basic Gases and Strong Acidity of Atmos; Center for Environmental Research Information Office of Research and Development U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999; Volume 126, pp. 20–56. [Google Scholar]
- Butt, C.M.; Mabury, S.A.; Kwan, M.; Wang, X.; Muir, D.C.G. Spatial trends of perfluoroalkyl compounds in ringed seals (Phoca hispida) from the Canadian arctic. Environ. Toxicol. Chem. 2008, 27, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.B.; Hopke, P.K.; Yi, S.M. Source apportionment of PM2.5 in seoul, korea. Atmos. Chem. Phys. 2009, 9, 4957–4971. [Google Scholar] [CrossRef]
- Ofosu, F.G.; Hopke, P.K.; Aboh, I.J.K.; Bamford, S.A. Characterization of fine particulate sources at Ashaiman in Greater Accra, Ghana. Atmos. Pollut. Res. 2012, 3, 301–310. [Google Scholar] [CrossRef]
- Kim, E.; Hopke, P.K.; Qin, Y. Estimation of organic carbon blank values and error structures of the speciation trends network data for source apportionment. J. Air Waste Manag. Assoc. 2005, 55, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Wayne, R.O. Environmental Statistics and Data Analysis, 1st ed.; CRC Press: Boca Rtaon, FL, USA, 1995. [Google Scholar] [CrossRef]
- Gray, H.A.; Cass, G.R.; Huntzicker, J.J.; Heyerdahl, E.K.; Rau, J.A. Characteristics of Atmospheric Organic and Elemental Carbon Particle Concentrations in Los Angeles. Environ. Sci. Technol. 1986, 20, 580–589. [Google Scholar] [CrossRef]
- Mayol-Bracero, O.L.; Gabriel, R.; Andreae, M.O.; Kirchstetter, T.W.; Novakov, T.; Ogren, J.; Sheridan, P.; Streets, D.G. Carbonaceous aerosols over the Indian Ocean during the Indian Ocean Experiment (INDOEX): Chemical characterization, optical properties, and probable sources. J. Geophys. Res. Atmos. 2002, 107. [Google Scholar] [CrossRef]
- Ram, K.; Sarin, M.M. Day-night variability of EC, OC, WSOC and inorganic ions in urban environment of Indo-Gangetic Plain: Implications to secondary aerosol formation. Atmos. Environ. 2011, 45, 460–468. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, J.; Zhang, G.; Zotter, P.; Huang, R.J.; Tang, J.H.; Wacker, L.; Prévoît, A.S.H.; Szidat, S. Radiocarbon-based source apportionment of carbonaceous aerosols at a regional background site on Hainan Island, South China. Environ. Sci. Technol. 2014, 48, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C. Measurement methods to determine compliance with ambient air quality standards for suspended particles. J. Air Waste Manag. Assoc. 1995, 45, 320–382. [Google Scholar] [CrossRef]
- Andreae, M.O. Soot carbon and excess fine potassium: Long-range transport of combustion-derived aerosols. Science 1983, 220, 10–13. Available online: http://www.sciencemag.org/content/220/4602/1148.short (accessed on 31 March 2021). [CrossRef] [PubMed]
- Rattigan, O.V.; Dirk, F.H.; Bae, M.S.; Schwab, J.J.; Demerjian, K.L. Multi-year hourly PM2.5 carbon measurements in New York: Diurnal, day of week and seasonal patterns. Atmos. Environ. 2010, 44, 2043–2053. [Google Scholar] [CrossRef]
- Park, J.M.; Han, Y.J.; Cho, S.H.; Kim, H.W. Characteristics of carbonaceous PM 2.5 in a small residential city in Korea. Atmosphere 2018, 9, 490. [Google Scholar] [CrossRef]
- Urban, R.C.; Lima-Souza, M.; Caetano-Silva, L.; Queiroz, M.E.C.; Nogueira, R.F.P.; Allen, A.G.; Cardoso, A.A.; Held, G.; Campos, M.L.A.M. Use of levoglucosan, potassium, and water-soluble organic carbon to characterize the origins of biomass-burning aerosols. Atmos. Environ. 2012, 61, 562–569. [Google Scholar] [CrossRef]
- Devi, N.L.; Kumar, A.; Yadav, I.C. PM10 and PM2.5 in Indo-Gangetic Plain (IGP) of India: Chemical characterization, source analysis, and transport pathways. Urban. Clim. 2020, 33, 100663. [Google Scholar] [CrossRef]
- Liu, H.; Tian, H.; Zhang, K.; Liu, S.; Cheng, K.; Yin, S.; Liu, Y.; Liu, X.; Wu, Y.; Liu, W.; et al. Seasonal variation, formation mechanisms and potential sources of PM2.5 in two typical cities in the Central Plains Urban Agglomeration, China. Sci. Total Environ. 2019, 657, 657–670. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Liu, Q.; Qi, X.; Qu, J.; Zhang, S.; Wang, X.; Jia, K.; Zhu, M. Study on chemical components and sources of PM2.5 during heavy air pollution periods at a suburban site in Beijing of China. Atmos. Pollut. Res. 2021, 12, 188–199. [Google Scholar] [CrossRef]
- Park, J.; Park, E.H.; Schauer, J.J.; Yi, S.M.; Heo, J. Reactive oxygen species (ROS) activity of ambient fine particles (PM2.5) measured in Seoul, Korea. Environ. Int. 2018, 117, 276–283. [Google Scholar] [CrossRef]
- Kim, Y.; Yi, S.M.; Heo, J. Fifteen-year trends in carbon species and PM2.5 in Seoul, South Korea (2003–2017). Chemosphere 2020, 261, 127750. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical characteristics of biomass ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Magdziarz, A.; Gajek, M.; Nowińska, K.; Nowak, W. Alkali metals association in biomass and their impact on ash melting behaviour. Fuel 2020, 261, 116421. [Google Scholar] [CrossRef]
- Bray, C.D.; Battye, W.; Aneja, V.P.; Tong, D.Q.; Lee, P.; Tang, Y. Ammonia emissions from biomass burning in the continental United States. Atmos. Environ. 2018, 187, 50–61. [Google Scholar] [CrossRef]
- Xiao, H.W.; Wu, J.F.; Luo, L.; Liu, C.; Xie, Y.J.; Xiao, H.Y. Enhanced biomass burning as a source of aerosol ammonium over cities in central China in autumn. Environ. Pollut. 2020, 266, 115278. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pósfai, M.; Hobbs, P.V.; Buseck, P.R. Individual aerosol particles from biomass burning in southern Africa: 2. Compositions and aging of inorganic particles. J. Geophys. Res. Atmos. 2003, 108, 1–12. [Google Scholar] [CrossRef]
- Huang, R.J.; Wang, Y.; Cao, J.; Lin, C.; Duan, J.; Chen, Q.; Li, Y.; Gu, Y.; Yan, J.; Xu, W.; et al. Primary emissions versus secondary formation of fine particulate matter in the most polluted city (Shijiazhuang) in North China. Atmos. Chem. Phys. 2019, 19, 2283–2298. [Google Scholar] [CrossRef]
- Xu, W.; Han, T.; Du, W.; Wang, Q.; Chen, C.; Zhao, J.; Zhang, Y.; Li, J.; Fu, P.; Wang, Z.; et al. Effects of aqueous-phase and photochemical processing on secondary organic aerosol formation and evolution in Beijing, China. Environ. Sci. Technol. 2017, 51, 762–770. [Google Scholar] [CrossRef]
- Galon-Negru, A.G.; Olariu, R.I.; Arsene, C. Chemical characteristics of size-resolved atmospheric aerosols in Iasi, north-eastern Romania: Nitrogen-containing inorganic compounds control aerosol chemistry in the area. Atmos. Chem. Phys. 2018, 18, 5879–5904. [Google Scholar] [CrossRef]
- Sharma, M.; Kishore, S.; Tripathi, S.N.; Behera, S.N. Role of atmospheric ammonia in the formation of inorganic secondary particulate matter: A study at Kanpur, India. J. Atmos. Chem. 2007, 58, 1–17. [Google Scholar] [CrossRef]
- Pathak, R.K.; Wang, T.; Wu, W.S. Nighttime enhancement of PM2.5 nitrate in ammonia-poor atmospheric conditions in Beijing and Shanghai: Plausible contributions of heterogeneous hydrolysis of N2O5 and HNO3 partitioning. Atmos. Environ. 2011, 45, 1183–1191. [Google Scholar] [CrossRef]
- Wu, C.; Yu, J.Z. Determination of primary combustion source organic carbon-to-elemental carbon (OC/EC) ratio using ambient OC and EC measurements: Secondary OC-EC correlation minimization method. Atmos. Chem. Phys. 2016, 16, 5453–5465. [Google Scholar] [CrossRef]
- Lim, H.J.; Turpin, B.J. Origins of primary and secondary organic aerosol in Atlanta: Results of time-resolved measurements during the Atlanta Supersite Experiment. Environ. Sci. Technol. 2002, 36, 4489–4496. [Google Scholar] [CrossRef]
- John, H.; Seinfeld, S.N.P. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons, Inc.: New York, NY, USA, 2016. [Google Scholar]
- Xing, L.; Wu, J.; Elser, M.; Tong, S.; Liu, S.; Li, X.; Liu, L.; Cao, J.; Zhou, J.; El-Haddad, I.; et al. Wintertime secondary organic aerosol formation in Beijing-Tianjin-Hebei (BTH): Contributions of HONO sources and heterogeneous reactions. Atmos. Chem. Phys. 2019, 19, 2343–2359. [Google Scholar] [CrossRef]
- Claeys, M.; Graham, B.; Vas, G.; Wang, W.; Vermeylen, R.; Pashynska, V.; Cafmeyer, J.; Guyon, P.; Andreae, M.O.; Artaxo, P.; et al. Formation of Secondary Organic Aerosols Through Photooxidation of Isoprene. Science 2004, 303, 1173–1176. [Google Scholar] [CrossRef]
- Edney, E.O.; Kleindienst, T.E.; Jaoui, M.; Lewandowski, M.; Offenberg, J.H.; Wang, W.; Claeys, M. Formation of 2-methyl tetrols and 2-methylglyceric acid in secondary organic aerosol from laboratory irradiated isoprene/NOX/SO 2/air mixtures and their detection in ambient PM2.5 samples collected in the eastern United States. Atmos. Environ. 2005, 39, 5281–5289. [Google Scholar] [CrossRef]
- Kroll, J.H.; Ng, N.L.; Murphy, S.M.; Flagan, R.C.; Seinfeld, J.H. Secondary organic aerosol formation from isoprene photooxidation. Environ. Sci. Technol. 2006, 40, 1869–1877. [Google Scholar] [CrossRef]
- Budisulistiorini, S.H.; Li, X.; Bairai, S.T.; Renfro, J.; Liu, Y.; Liu, Y.J.; McKinney, K.A.; Martin, S.T.; McNeill, V.F.; Pye, H.O.T.; et al. Examining the effects of anthropogenic emissions on isoprene-derived secondary organic aerosol formation during the 2013 Southern Oxidant and Aerosol Study (SOAS) at the Look Rock, Tennessee ground site. Atmos. Chem. Phys. 2015, 15, 8871–8888. [Google Scholar] [CrossRef]
- Robinson, A.L.; Donahue, N.M.; Shrivastava, M.K.; Weitkamp, E.A.; Sage, A.M.; Grieshop, A.P.; Lane, T.E.; Pierce, J.R.; Pandis, S.N. Rethinking organic aerosols: Semivolatile emissions and photochemical aging. Science 2007, 315, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- Shrivastava, M.; Cappa, C.D.; Fan, J.; Goldstein, A.H.; Guenther, A.B.; Jimenez, J.L.; Kuang, C.; Laskin, A.; Martin, S.T.; Ng, N.L.; et al. Recent advances in understanding secondary organic aerosol: Implications for global climate forcing. Rev. Geophys. 2017, 55, 509–559. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wang, Q.; Qin, Q.; Ye, J.; Han, Y.; Li, L.; Zhen, W.; Zhi, Q.; Zhang, Y.; et al. Increased secondary aerosol contribution and possible processing on polluted winter days in China. Environ. Int. 2019, 127, 78–84. [Google Scholar] [CrossRef]
- Kim, P.R. Atmospheric Mercury: Characteristics of speciation Hg concentration and Dry deposition Flux. Ph.D. Thesis, Kangwon National University, Chuncheon-si, Korea, 2017. [Google Scholar]
- Cabada, J.C.; Rees, S.; Takahama, S.; Khlystov, A.; Pandis, S.N.; Davidson, C.I.; Robinson, A.L. Mass size distributions and size resolved chemical composition of fine particulate matter at the Pittsburgh supersite. Atmos. Environ. 2004, 38, 3127–3141. [Google Scholar] [CrossRef]
- Hu, M.; Peng, J.; Sun, K.; Yue, D.; Guo, S.; Wiedensohler, A.; Wu, Z. Estimation of size-resolved ambient particle density based on the measurement of aerosol number, mass, and chemical size distributions in the winter in Beijing. Environ. Sci. Technol. 2012, 46, 9941–9947. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.Y. Source Estimation Based on Chemical Characteristics and Size Distribution of Fine Particle in Chuncheon, Korea. Master’s Thesis, Kangwon National University, Kangwon-do, Korea, 2018. [Google Scholar]
- Moreno, T.; Reche, C.; Rivas, I.; Cruz Minguillón, M.; Martins, V.; Vargas, C.; Buonanno, G.; Parga, J.; Pandolfi, M.; Brines, M.; et al. Urban air quality comparison for bus, tram, subway and pedestrian commutes in Barcelona. Environ. Res. 2015, 142, 495–510. [Google Scholar] [CrossRef]
- Kumar, P.; Rivas, I.; Singh, A.P.; Ganesh, V.J.; Ananya, M.; Frey, H.C. Dynamics of coarse and fine particle exposure in transport microenvironments. Npj Clim. Atmos. Sci. 2018, 1. [Google Scholar] [CrossRef]
- Almeida, S.M.; Pio, C.A.; Freitas, M.C.; Reis, M.A.; Trancoso, M.A. Source apportionment of fine and coarse particulate matter in a sub-urban area at the Western European Coast. Atmos. Environ. 2005, 39, 3127–3138. [Google Scholar] [CrossRef]
- Wilson, W.E.; Suh, H.H. Fine particles and coarse particles: Concentration relationships relevant to epidemiologic studies. J. Air Waste Manag. Assoc. 1997, 47, 1238–1249. [Google Scholar] [CrossRef] [PubMed]






| Site | Month | PM2.5 | OC | EC | WSOC | WIOC | NH4+ | NO3− | SO42− | OC/EC | K+ | Mg2+ | Ca2+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KNU | SEP. | 28.1 ± 12.0 | 8.6 ± 2.9 | 1.0 ± 0.40 | 2.5 ± 1.3 | 6.1 ± 3.4 | 1.87 ± 0.61 | 0.73 ± 0.12 | 1.69 ± 1.48 | 9.6 ± 4.9 | 81.5 ± 32.3 | 12.1 ± 11.9 | 28.6 ± 33.0 |
| OCT. | 21.5 ± 12.3 | 8.0 ± 4.8 | 1.3 ± 0.77 | 4.7 ± 3.1 | 3.3 ± 2.4 | 1.37 ± 0.38 | 1.57 ± 0.80 | 1.19 ± 0.82 | 5.9 ± 0.9 | 214.5 ± 60.3 | 23.8 ± 5.4 | 73.0 ± 17.2 | |
| NOV. | 26.8 ± 12.1 | 7.2 ± 3.1 | 1.1 ± 0.61 | 3.5 ± 2.2 | 3.7 ± 1.0 | 2.01 ± 1.18 | 2.91 ± 0.00 | 1.03 ± 0.00 | 6.9 ± 1.0 | 180.6 ± 84.2 | 2.6 ± 4.4 | 26.0 ± 46.1 | |
| DEC. | 33.7 ± 11.2 | 8.3 ± 2.1 | 1.1 ± 0.37 | 3.7 ± 0.8 | 4.5 ± 1.3 | 3.10 ± 1.18 | 4.23 ± 2.95 | 2.07 ± 1.32 | 8.1 ± 0.7 | 192.1 ± 77.9 | 10.2 ± 8.6 | 64.8 ± 64.3 | |
| JAN. | 31.3 ± 13.3 | 9.8 ± 3.1 | 1.3 ± 0.26 | 4.6 ± 1.3 | 5.2 ± 2.1 | 3.80 ± 2.06 | 6.19 ± 4.20 | 2.89 ± 1.74 | 7.5 ± 2.2 | 301.1 ± 154.9 | 21.5 ± 11.7 | 150.0 ± 70.1 | |
| AVG. | 28.3 ± 12.2 | 8.6 ± 3.0 | 1.2±0.45 | 3.8 ± 1.8 | 4.8 ± 2.3 | 2.50 ± 1.54 | 3.19 ± 3.48 | 1.97 ± 1.52 | 7.9 ± 2.8 | 191.7 ± 121.3 | 13.8 ± 11.7 | 70.6 ± 69.4 | |
| KBIC | SEP. | 23.5 ± 10.7 | 5.3 ± 0.89 | 0.9 ± 0.3 | 2.5 ± 0.9 | 2.8 ± 1.0 | 5.16 ± 2.31 | 1.15 ± 0.61 | 2.84 ± 2.22 | 6.7 ± 2.2 | 88.8 ± 62.9 | 13.2 ± 11.5 | 61.8 ± 103.7 |
| OCT. | 19.7 ± 10.0 | 5.9 ± 2.3 | 0.9 ± 0.4 | 2.4 ± 1.5 | 3.6 ± 1.2 | 2.24 ± 0.60 | 2.11 ± 0.71 | 1.18 ± 0.82 | 6.8 ± 0.9 | 200.2 ± 68.6 | 18.6 ± 3.6 | 46.0 ± 14.2 | |
| NOV. | 20.3 ± 11.1 | 6.4 ± 2.3 | 0.9 ± 0.3 | 3.2 ± 1.8 | 3.2 ± 0.8 | 4.79 ± 3.52 | No data | No data | 7.4 ± 1.0 | 182.0 ± 107.1 | 0.4 ± 1.1 | 1.9 ± 4.9 | |
| DEC. | 49.4 ± 26.0 | 11.4 ± 4.2 | 1.6 ± 0.3 | 5.7 ± 2.4 | 5.7 ± 1.9 | 8.23 ± 5.18 | No data | No data | 7.1 ± 1.8 | 383.8 ± 223.0 | 2.8 ± 3.7 | 31.5 ± 36.1 | |
| JAN. | 33.4 ± 19.1 | 8.2 ± 3.2 | 1.3 ± 0.7 | 3.9 ± 2.1 | 4.3 ± 1.3 | 4.67 ± 3.12 | No data | No data | 7.2 ± 1.8 | 246.4 ± 137.2 | 6.2 ± 13.0 | 93.7 ± 195.2 | |
| AVG. | 28.8 ± 18.8 | 7.5 ± 3.4 | 1.1 ± 0.48 | 3.6 ± 2.1 | 3.9 ± 1.5 | 4.94 ± 3.60 | 1.52 ± 0.79 | 2.20 ± 1.95 | 7.1 ± 1.6 | 210.1± 155.7 | 7.8 ± 10.3 | 49.2 ± 106.3 | |
| F.B. 1 (μg m−3) | 0.4 | 0.51 | 0.00 | 1.42 | – | 0.0 | 0.14 | 0.19 | – | 0.02 | 0.0 | 0.0 | |
| MDL 2 (μg m−3) | 1.6 | 1.11 | 0.00 | 1.06 | – | 0.0 | 0.44 | 0.39 | – | 0.04 | 0.0 | 0.0 | |
| < MDL* (%) | 0.0 | 0.0 | 0.0 | 0.0 | –. | 0.0 | 7.1 | 10.7 | – | 0.00 | 0.0 | 0.0 | |
| RPD 3(%) | – | 0.4 | 3.8 | 1.4 | –. | 2.3 | 1.6 | 0.5 | – | 0.9 | 0.4 | 1.2 | |
| Site | Chuncheon KNU | Chuncheon KBIC | India (New Delhi) | China (Zhengzhou) | China (Beijing) | Korea (Seoul) | Korea (Seoul) |
|---|---|---|---|---|---|---|---|
| Period | 26 August 2017~29 January 2018 | June 2017 | 2017 | June 2016~June 2017 | 2017 | September 2013–May 2015 | |
| Reference | This study | [42] | [43] | [44] | [45] | [46] | |
| PM2.5 | 28.3 | 28.8 | 91.5 | 70.5 | 134.7 | 31.3 | 52.7 |
| OC | 8.63 | 7.46 | 20.3 | 10.5 | 24.2 | 6.01 | 7.1 |
| EC | 1.16 | 1.10 | 4.92 | 4.4 | 6.0 | 0.80 | 2.2 |
| WSOC | 3.81 | 3.56 | - | - | - | - | 1.9 |
| NH4+ | 2.50 | 4.94 | 3.78 | 8.8 | 7.8 | 4.1 | |
| NO3− | 3.19 | 1.52 | 0.09 | 11.7 | 21.4 | 8.0 | |
| SO42− | 1.97 | 2.20 | 0.81 | 7.9 | 20.2 | 7.4 | |
| K+ | 0.19 | 0.21 | 3.17 | 0.9 | 1.9 | - | |
| Mg2+ | 0.01 | 0.01 | 1.09 | - | 0.3 | - | |
| Ca2+ | 0.07 | 0.05 | 0.69 | - | 2.32 | - | |
| KNU | OC | EC | WSOC | NH4+ | K+ | Mg2+ | Ca2+ | NO3− | SO42− |
|---|---|---|---|---|---|---|---|---|---|
| PM2.5 | 0.620 ** | 0.582 ** | 0.560 ** | 0.699 ** | 0.616 ** | 0.194 | 0.347 * | 0.6350 ** | 0.837 ** |
| OC | 1 | ||||||||
| EC | 0.692 ** | 1 | |||||||
| WSOC | 0.660 ** | 0.773 ** | 1 | ||||||
| NH4+ | 0.441 * | 0.336 * | 0.441 * | 1 | |||||
| K+ | 0.483 ** | 0.447 ** | 0.611 ** | 0.784 ** | 1 | ||||
| Mg2+ | 0.193 | 0.273 | 0.183 | 0.160 | 0.378 * | 1 | |||
| Ca2+ | 0.347 * | 0.339 | 0.409 * | 0.534 ** | 0.719 ** | 0.606 ** | 1 | ||
| NO3− | 0.635 ** | 0.372 | 0.468 * | 0.940 ** | 0.904 ** | 0.321 | 0.751 ** | 1 | |
| SO42− | 0.837 ** | 0.425 * | 0.496 * | 0.851 ** | 0.746 ** | 0.307 | 0.590 ** | 0.775 ** | 1 |
| KBIC | OC | EC | WSOC | NH4+ | K+ | Mg2+ | Ca2+ | NO3− | SO42− |
| PM2.5 | 0.930 ** | 0.832 ** | 0.906 ** | 0.813 ** | 0.890 ** | 0.194 | 0.416 * | 0.388 | 0.815 ** |
| OC | 1 | ||||||||
| EC | 0.890 ** | 1 | |||||||
| WSOC | 0.958 ** | 0.870 ** | 1 | ||||||
| NH4+ | 0.759 ** | 0.655 ** | 0.720 ** | 1 | |||||
| K+ | 0.889 ** | 0.740 ** | 0.843 ** | 0.719 ** | 1 | ||||
| Mg2+ | 0.012 | 0.155 | 0.029 | 0.111 | 0.185 | 1 | |||
| Ca2+ | 0.298 | 0.411 * | 0.306 | 0.393 * | 0.405 * | 0.688 ** | 1 | ||
| NO3− | 0.652 * | 0.599 * | 0.307 | –0.204 | 0.483 | 0.106 | 0.007 | 1 | |
| SO42− | 0.306 | 0.526 | 0.471 | 0.620 * | 0.181 | 0.325 | 0.593 * | 0.094 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-W.; Choi, S.-Y.; Byun, J.-Y.; Kim, H.; Kim, W.-J.; Kim, P.-R.; Han, Y.-J. Different Characteristics of PM2.5 Measured in Downtown and Suburban Areas of a Medium-Sized City in South Korea. Atmosphere 2021, 12, 832. https://doi.org/10.3390/atmos12070832
Park S-W, Choi S-Y, Byun J-Y, Kim H, Kim W-J, Kim P-R, Han Y-J. Different Characteristics of PM2.5 Measured in Downtown and Suburban Areas of a Medium-Sized City in South Korea. Atmosphere. 2021; 12(7):832. https://doi.org/10.3390/atmos12070832
Chicago/Turabian StylePark, Sung-Won, Su-Yeon Choi, Jin-Yeo Byun, Hekap Kim, Woo-Jin Kim, Pyung-Rae Kim, and Young-Ji Han. 2021. "Different Characteristics of PM2.5 Measured in Downtown and Suburban Areas of a Medium-Sized City in South Korea" Atmosphere 12, no. 7: 832. https://doi.org/10.3390/atmos12070832
APA StylePark, S.-W., Choi, S.-Y., Byun, J.-Y., Kim, H., Kim, W.-J., Kim, P.-R., & Han, Y.-J. (2021). Different Characteristics of PM2.5 Measured in Downtown and Suburban Areas of a Medium-Sized City in South Korea. Atmosphere, 12(7), 832. https://doi.org/10.3390/atmos12070832







