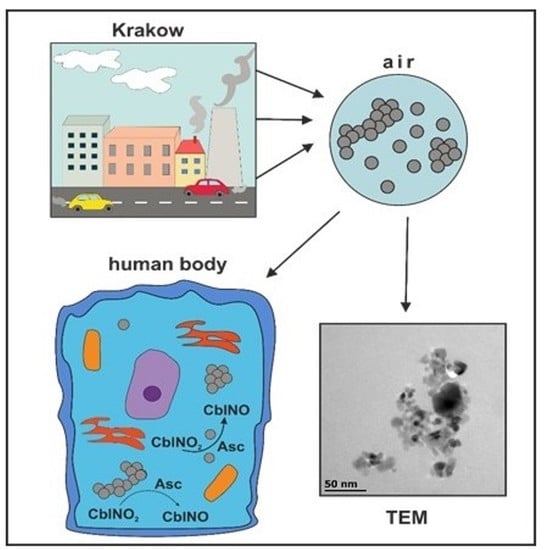
Influence of Krakow Winter and Summer Dusts on the Redox Cycling of Vitamin B12a in the Presence of Ascorbic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Methods
2.3. Experimental Procedures
2.4. UV-Vis Spectral Measurements
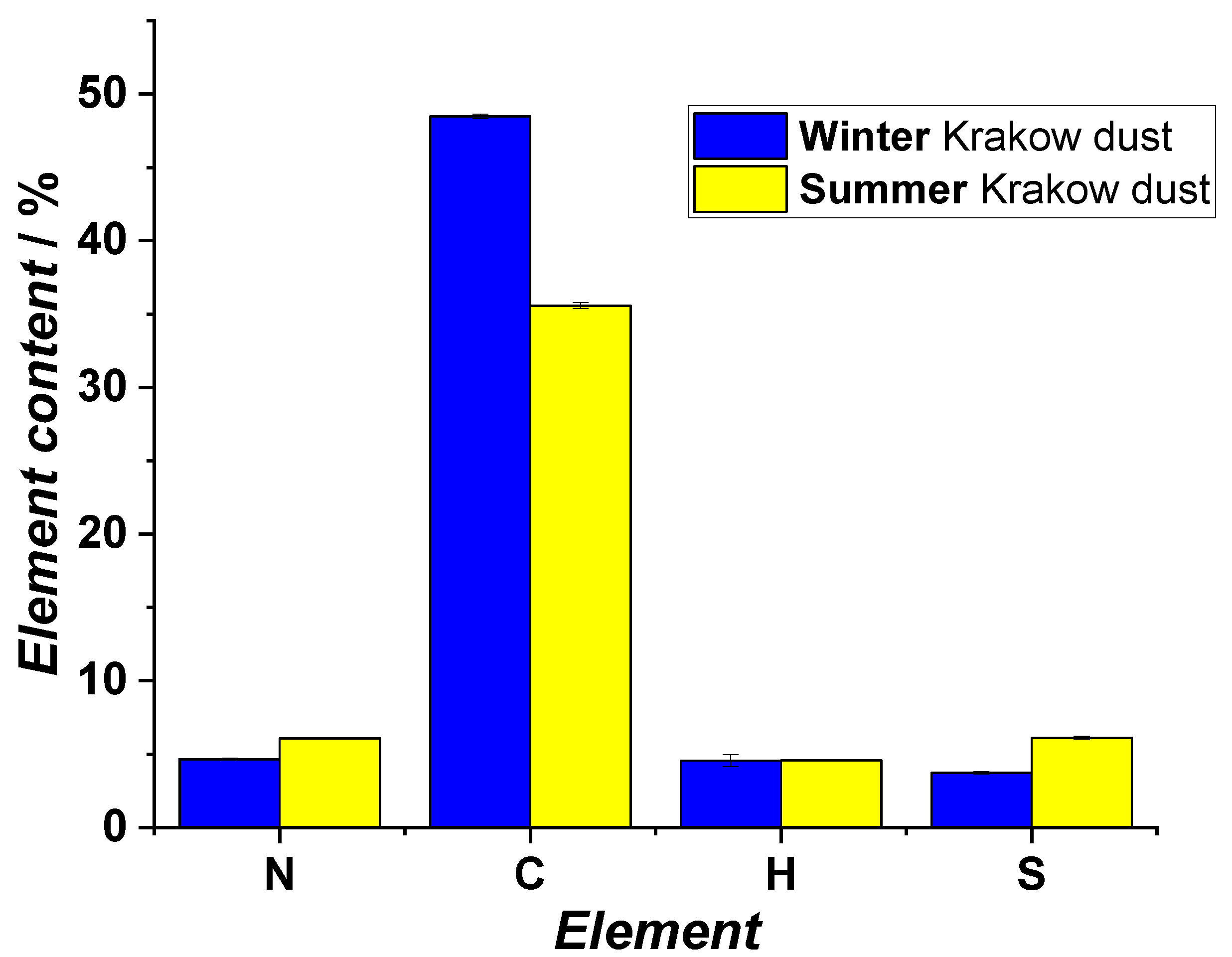
2.5. Elemental Analysis
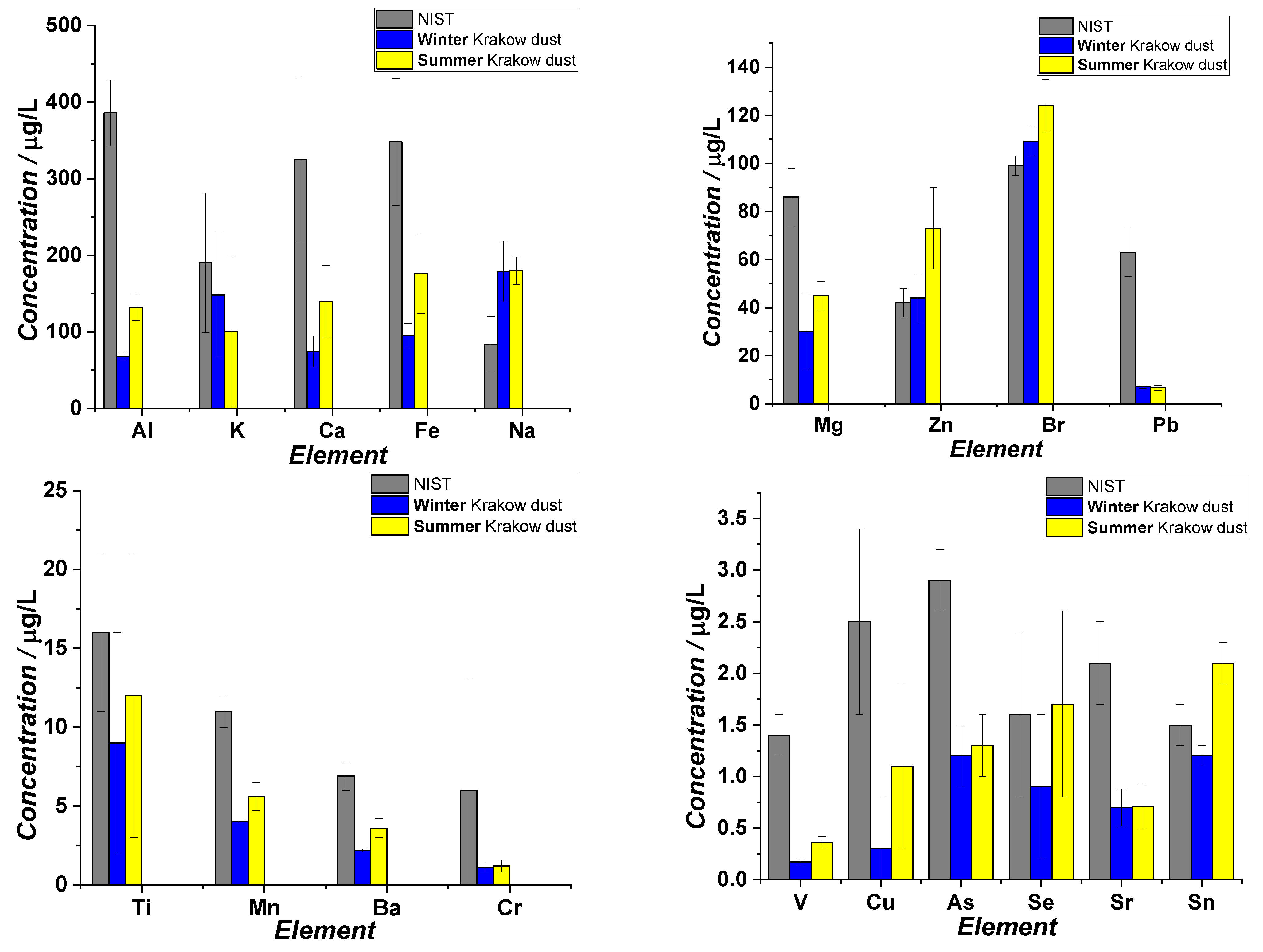
2.6. ICP MS
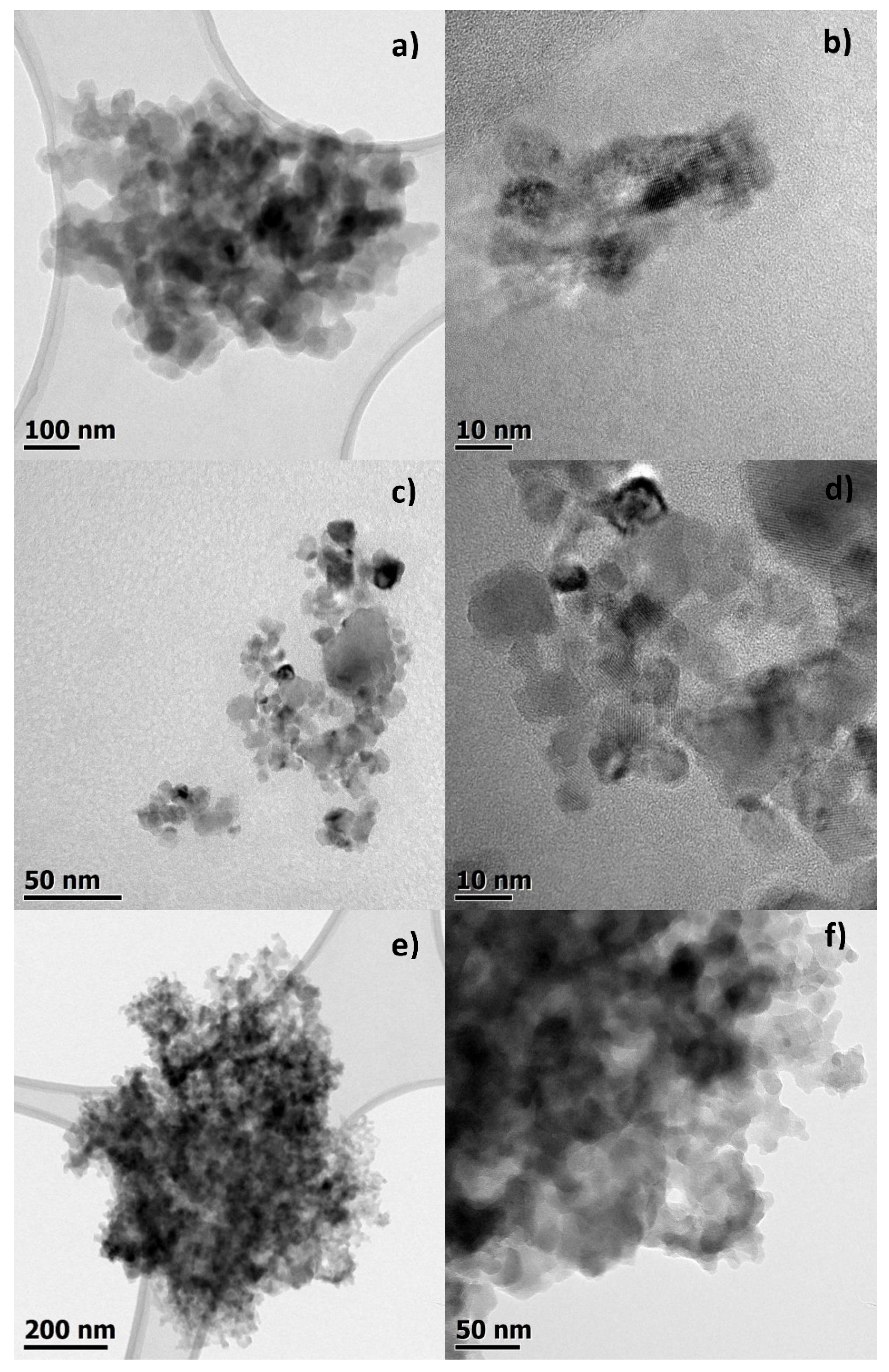
2.7. TEM Measurements
3. Results and Discussion
3.1. Studies on Characterization of Krakow Dust Samples
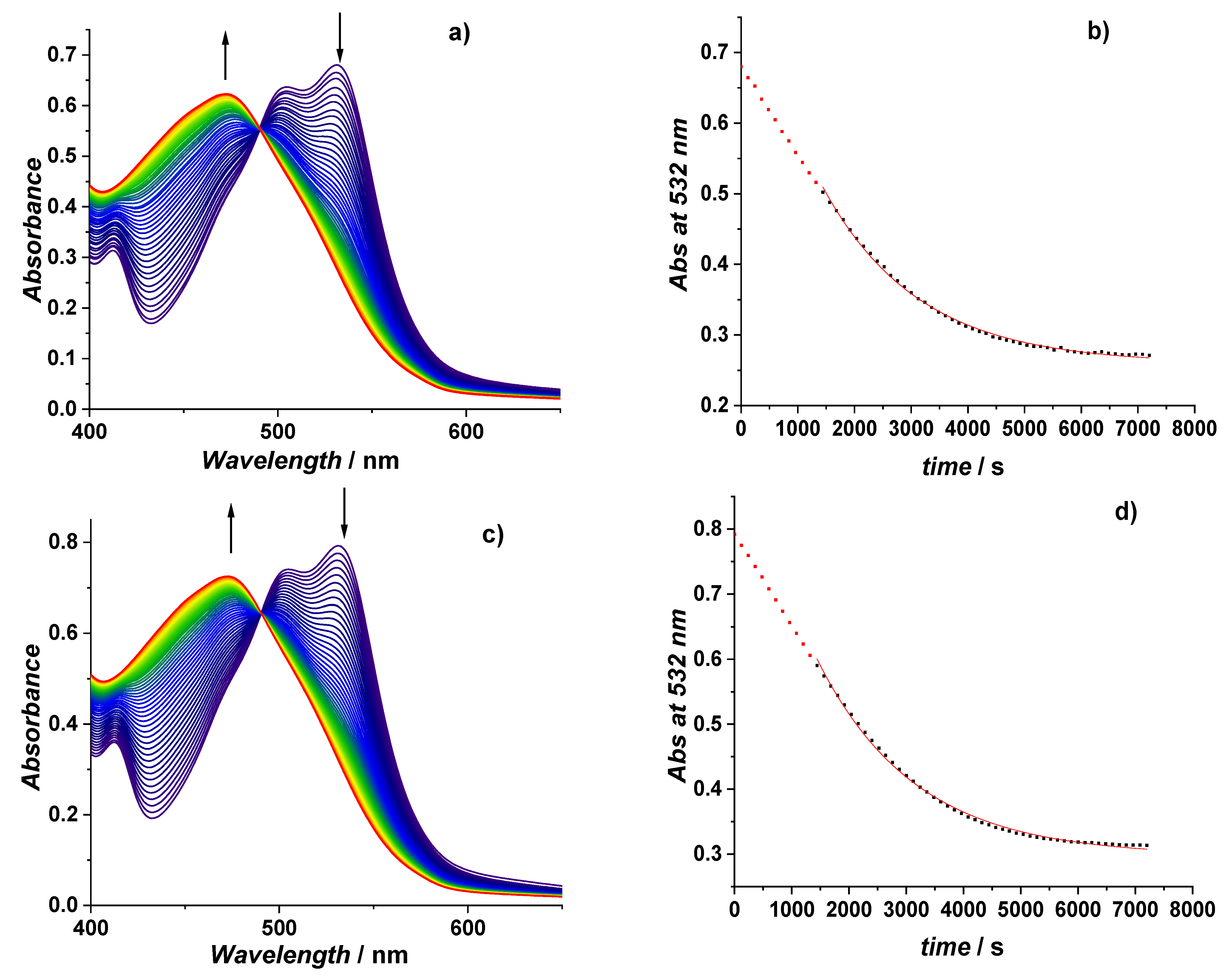
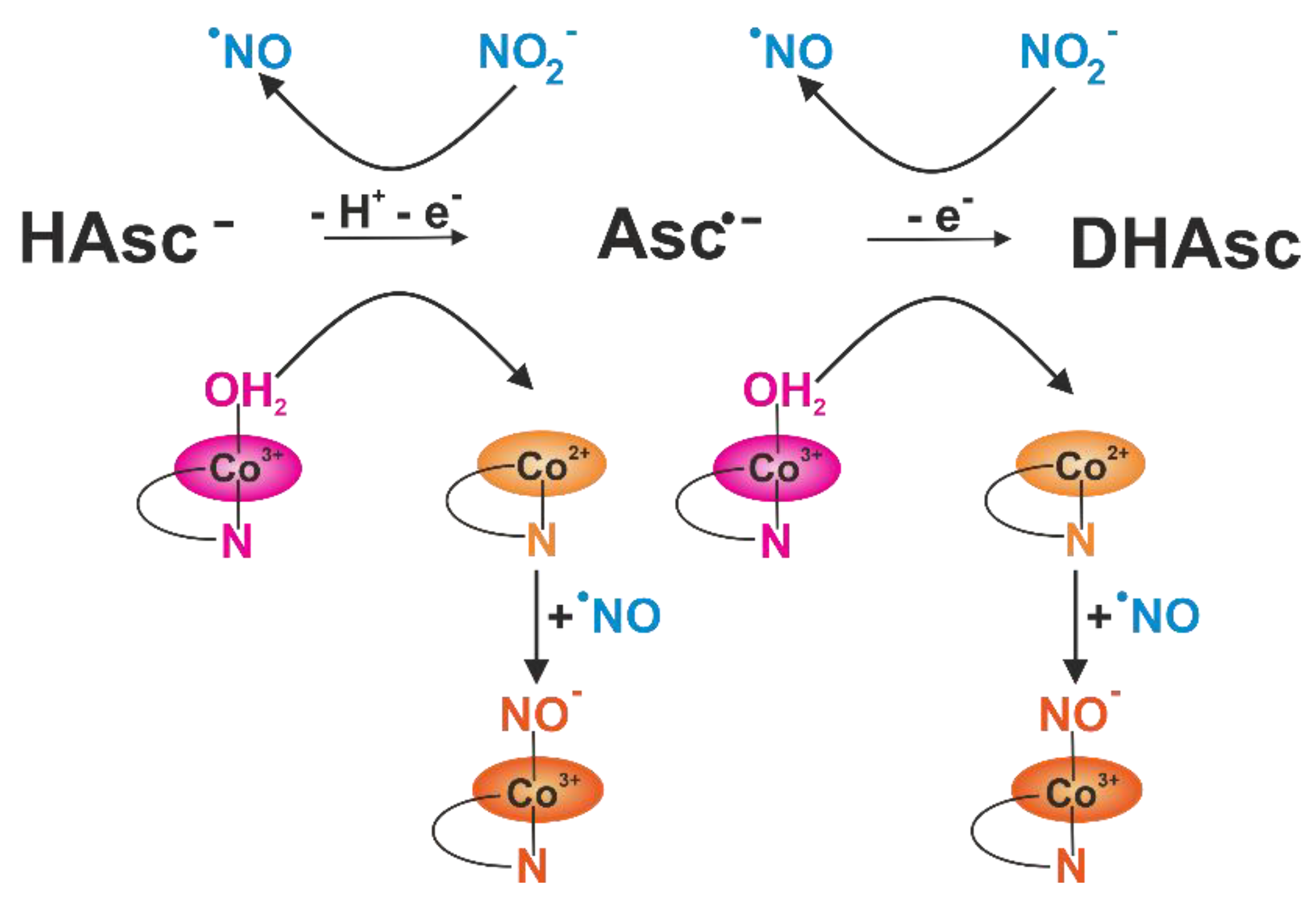
3.2. Influence of Krakow Dust on Nitrocobalamin and Ascorbic Acid Interaction at pH 4.3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortiz, A.G.; De Brito Beirao Guerreiro, C.; Horalek, J. Air Quality in Europe-2019; European Environmental Agency: Copenhagen, Denmark, 2019. [Google Scholar]
- Ortiz, A.G.; De Brito Beirao Guerreiro, C. Air Quality in Europe-2020; European Environmental Agency: Copenhagen, Denmark, 2020. [Google Scholar]
- Poland—Air Pollution Country Fact Sheet. Available online: https://www.eea.europa.eu/themes/air/country-fact-sheets/2020-country-fact-sheets/poland (accessed on 2 June 2021).
- Wilczyńska-Michalik, W.; Michalik, M. Air Pollution in Krakow—A glance into the future from a historical perspective. Acta Geobalc. 2017, 3, 78–92. [Google Scholar] [CrossRef]
- Traczyk, P.; Gruszecka-Kosowska, A. The Condition of Air Pollution in Kraków, Poland, in 2005–2020, with Health Risk Assessment. Int. J. Environ. Res. Public Health 2020, 17, 6063. [Google Scholar] [CrossRef] [PubMed]
- Bokwa, A. Environmental impacts of long-term air pollution changes in Krakow, Poland. Pol. J. Environ. Stud. 2008, 17, 673–686. [Google Scholar]
- Bokwa, A. The climate of the city and air pollution. Aura 2016, 9, 8–13. (In Polish) [Google Scholar]
- Adamiec, E.; Jarosz-Krzemińska, E.; Wieszała, R. Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environ. Monit. Assess. 2016, 188, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Melly, S.; Spengler, J. Intraurban and longitudinal variability of classical pollutants in Kraków, Poland, 2000–2010. Int. J. Environ. Res. Public Health 2015, 12, 4967–4991. [Google Scholar] [CrossRef]
- Jędruszkiewicz, J.; Czernecki, B.; Marosz, M. The variability of PM(10) and PM(2.5) concentrations in selected Polish agglomerations: The role of meteorological conditions, 2006–2016. Int. J. Environ. Health Res. 2017, 27, 441–462. [Google Scholar] [CrossRef]
- Samek, L.; Furman, L.; Mikrut, M.; Regiel-Futyra, A.; Macyk, W.; Stochel, G.; van Eldik, R. Chemical composition of submicron and fine particulate matter collected in Krakow, Poland. Consequences for the APARIC project. Chemosphere 2017, 187, 430–439. [Google Scholar] [CrossRef]
- Mikrut, M.; Macyk, W.; van Eldik, R.; Stochel, G. Physicochemical analysis of water extracts of particulate matter from polluted air in the area of Kraków, Poland. Atmosphere 2021, 12, 565. [Google Scholar] [CrossRef]
- Mikrut, M.; Regiel-Futyra, A.; Samek, L.; Macyk, W.; Stochel, G.; van Eldik, R. Generation of hydroxyl radicals and singlet oxygen by particulate matter and its inorganic components. Environ. Pollut. 2018, 238, 638–646. [Google Scholar] [CrossRef]
- Đurović, M.; Oszajca, M.; Stochel, G.; van Eldik, R. The Influence of Redox-Active Transition Metal Containing Micro- and Nanoparticles on the Properties of Representative Bioinorganic Reaction Systems. Eur. J. Inorg. Chem. 2018, 2018, 1229–1235. [Google Scholar] [CrossRef]
- Oszajca, M.; Wądołek, A.; Hooper, J.; Brindell, M.; van Eldik, R.; Stochel, G. Urban Particulate Matter-Induced Decomposition of S-Nitrosoglutathione Relevant to Aberrant Nitric Oxide Biological Signaling. ChemSusChem 2019, 12, 661–671. [Google Scholar] [CrossRef]
- Polaczek, J.; Stochel, G.; van Eldik, R. Can Particulate Matter and Nano Metal Oxide Particles Affect the Redox Cycling of Nitrosylcobalamin in Weakly Acidic Aqueous Solution? Eur. J. Inorg. Chem. 2021, 2021, 2325–2333. [Google Scholar] [CrossRef]
- Basudhar, D.; Ridnour, L.A.; Cheng, R.; Kesarwala, A.H.; Heinecke, J.; Wink, D.A. Biological signaling by small inorganic molecules. Coord. Chem. Rev. 2016, 306, 708–723. [Google Scholar] [CrossRef] [Green Version]
- Impert, O.; Katafias, A.; Wrzeszcz, G.; Muzioł, T.; Hrynkiewicz, K.; Olejnik, N.; Chrzanowska, M.; van Eldik, R. Synthesis and detailed characterization of cis-dichloridobispicolinatoruthenate(III) as solid and in solution. J. Coord. Chem. 2016, 69, 2107–2120. [Google Scholar] [CrossRef]
- Polaczek, J.; Orzel, L.; Stochel, G.; van Eldik, R. Can nitrocobalamin be reduced by ascorbic acid to nitroxylcobalamin? Some surprising mechanistic findings. J. Biol. Inorg. Chem. 2018, 23, 377–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.A.W.; Gantt, B.; McDow, S. The reduction of sulfate and switch from summertime to wintertime PM2.5 concentration maxima in the United States. Atmos. Environ. 2017, 175, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hand, J.L.; Schichtel, B.A.; Pitchford, M.; Malm, W.C.; Frank, N.H. Seasonal composition of remote and urban fine particulate matter in the United States. J. Geophys. Res. 2012, 117, D05209. [Google Scholar] [CrossRef]
- Ghio, A.J.; Stoneheurner, J.; McGee, J.K.; Kinsey, J.S. Sulfate content correlates with iron concentrations in ambient air pollution particles. Inhal Toxicol. 1999, 11, 293–307. [Google Scholar] [PubMed]
- Wise, S.; Watters, R. Standard Reference Material 1648a, Certificate of Analysis; National Institute of Standards & Technology: Gaithersburg, MD, USA, 2008. [Google Scholar]
- Polaczek, J.; Orzel, L.; Stochel, G.; van Eldik, R. Mechanistic information on the nitrite-controlled reduction of aquacob(III)alamin by ascorbate at physiological pH. J. Biol. Inorg. Chem. 2015, 20, 1069–1078. [Google Scholar] [CrossRef] [Green Version]
- Walker, D.T.; Dassanayake, R.S.; Garcia, K.A.; Mukherjee, R.; Brasch, N.E. Mechanistic studies on the reaction of nitrocobalamin with glutathione: Kinetic evidence for formation of an aquacobalamin intermediate. Eur. J. Inorg. Chem. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, H.M.; Knapton, L. Factors affecting the rate of ligand substitution reactions of aquacobalamin (vitamin B12a). J. Chem. Soc. Dalt. Trans. 1997, 20, 3827–3834. [Google Scholar] [CrossRef]
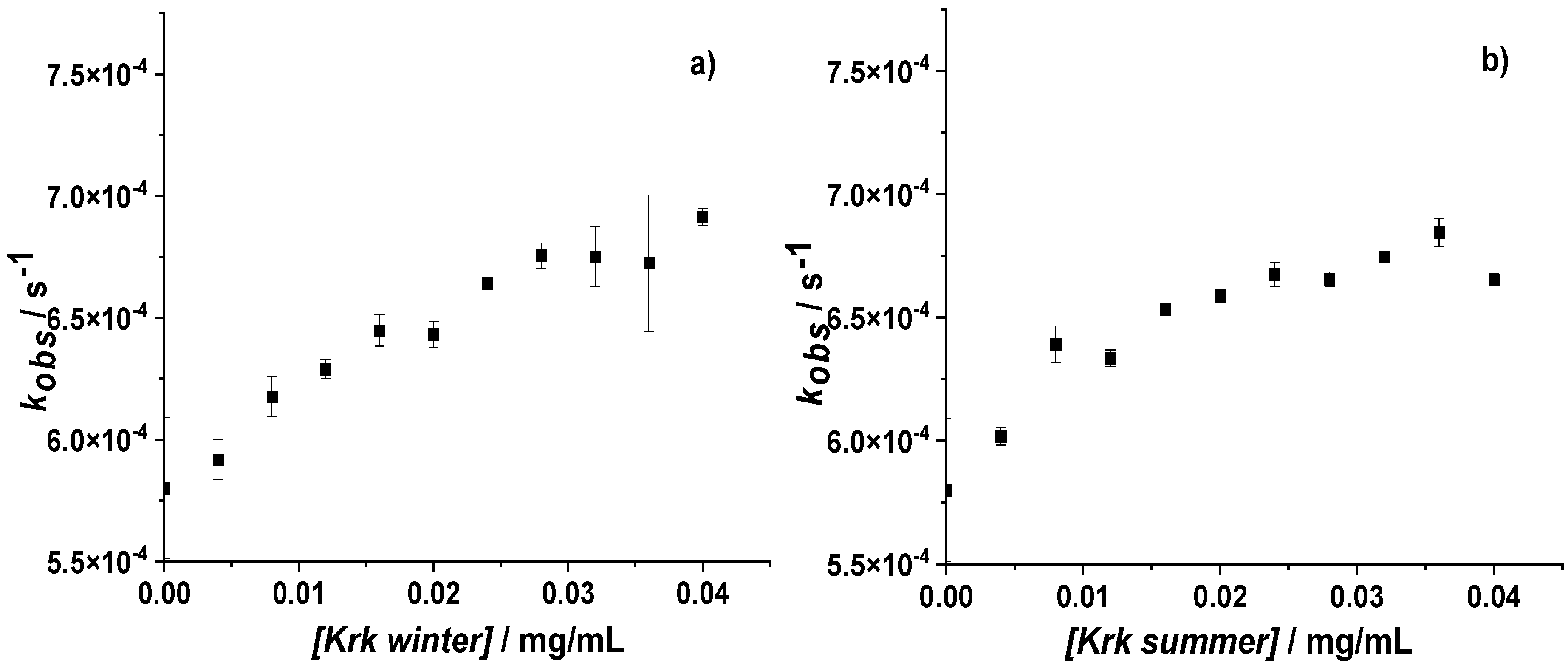
| Parameters | Setting Value |
|---|---|
| Nebulizer gas flow L/min | 1.13 |
| Plasma gas flow L/min | 15 |
| Auxiliary gas flow L/min | 1.2 |
| ICP RF power W | 1600 |
| Analog stage voltage | −1825 |
| Pulse stage voltage | 1000 |
| Discriminator threshold | 12 |
| Deflector voltage | −14 |
| Cell entrance voltage | −2 |
| Cell exit voltage | −2 |
| Cell rod offset | −13 |
| Sample | Average Concentration μg/L | +/− |
|---|---|---|
| Krakow Winter | 0.3 | 0.3 |
| Krakow Summer | 1.2 | 0.9 |
| NIST | 2.7 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polaczek, J.; Jodłowska, A.; Stochel, G.; van Eldik, R. Influence of Krakow Winter and Summer Dusts on the Redox Cycling of Vitamin B12a in the Presence of Ascorbic Acid. Atmosphere 2021, 12, 1050. https://doi.org/10.3390/atmos12081050
Polaczek J, Jodłowska A, Stochel G, van Eldik R. Influence of Krakow Winter and Summer Dusts on the Redox Cycling of Vitamin B12a in the Presence of Ascorbic Acid. Atmosphere. 2021; 12(8):1050. https://doi.org/10.3390/atmos12081050
Chicago/Turabian StylePolaczek, Justyna, Angelika Jodłowska, Grażyna Stochel, and Rudi van Eldik. 2021. "Influence of Krakow Winter and Summer Dusts on the Redox Cycling of Vitamin B12a in the Presence of Ascorbic Acid" Atmosphere 12, no. 8: 1050. https://doi.org/10.3390/atmos12081050
APA StylePolaczek, J., Jodłowska, A., Stochel, G., & van Eldik, R. (2021). Influence of Krakow Winter and Summer Dusts on the Redox Cycling of Vitamin B12a in the Presence of Ascorbic Acid. Atmosphere, 12(8), 1050. https://doi.org/10.3390/atmos12081050