Modeling Air Pollution Health Risk for Environmental Management of an Internationally Important Site: The Salt Range (Kallar Kahar), Pakistan
Abstract
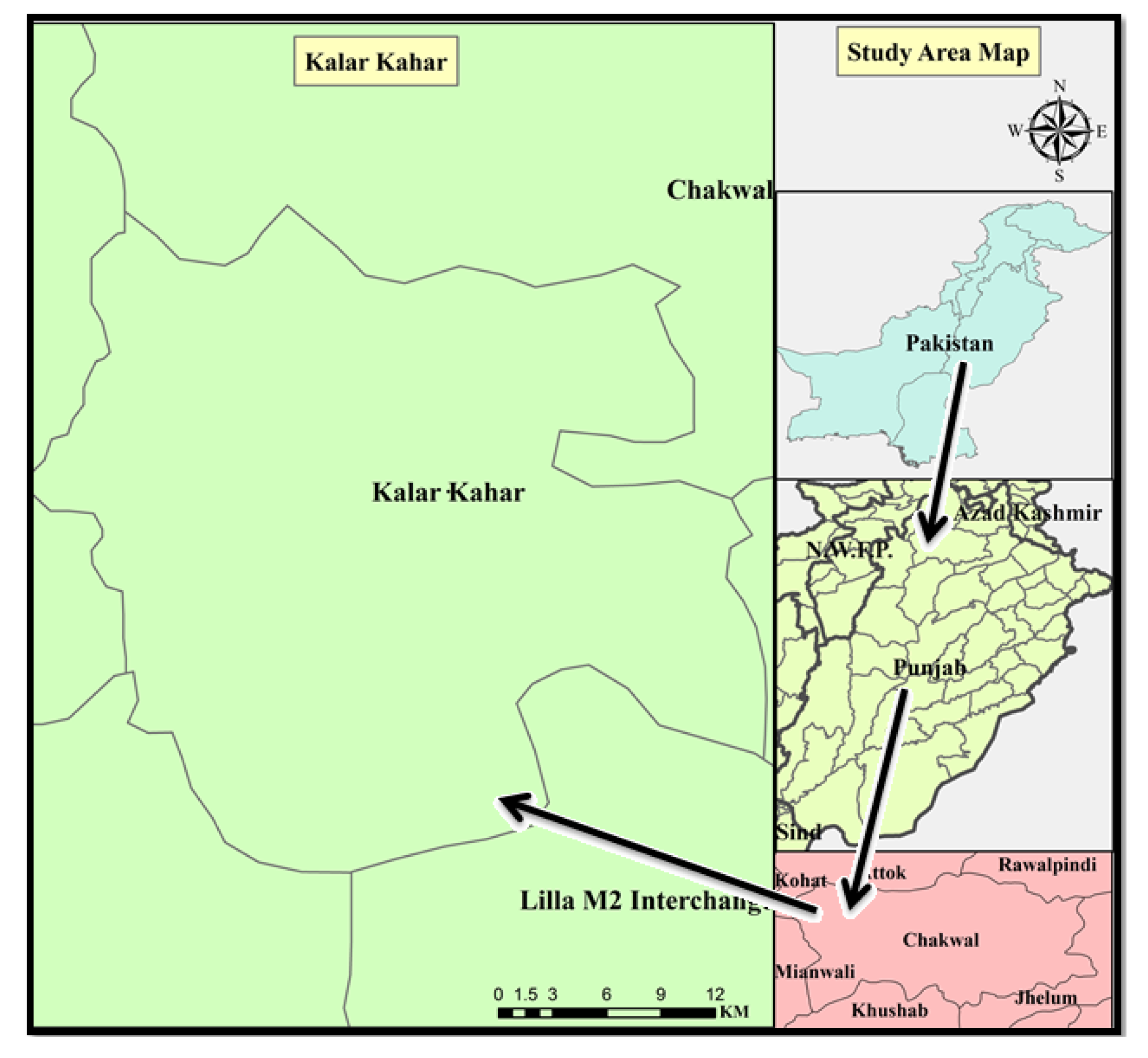
:1. Introduction
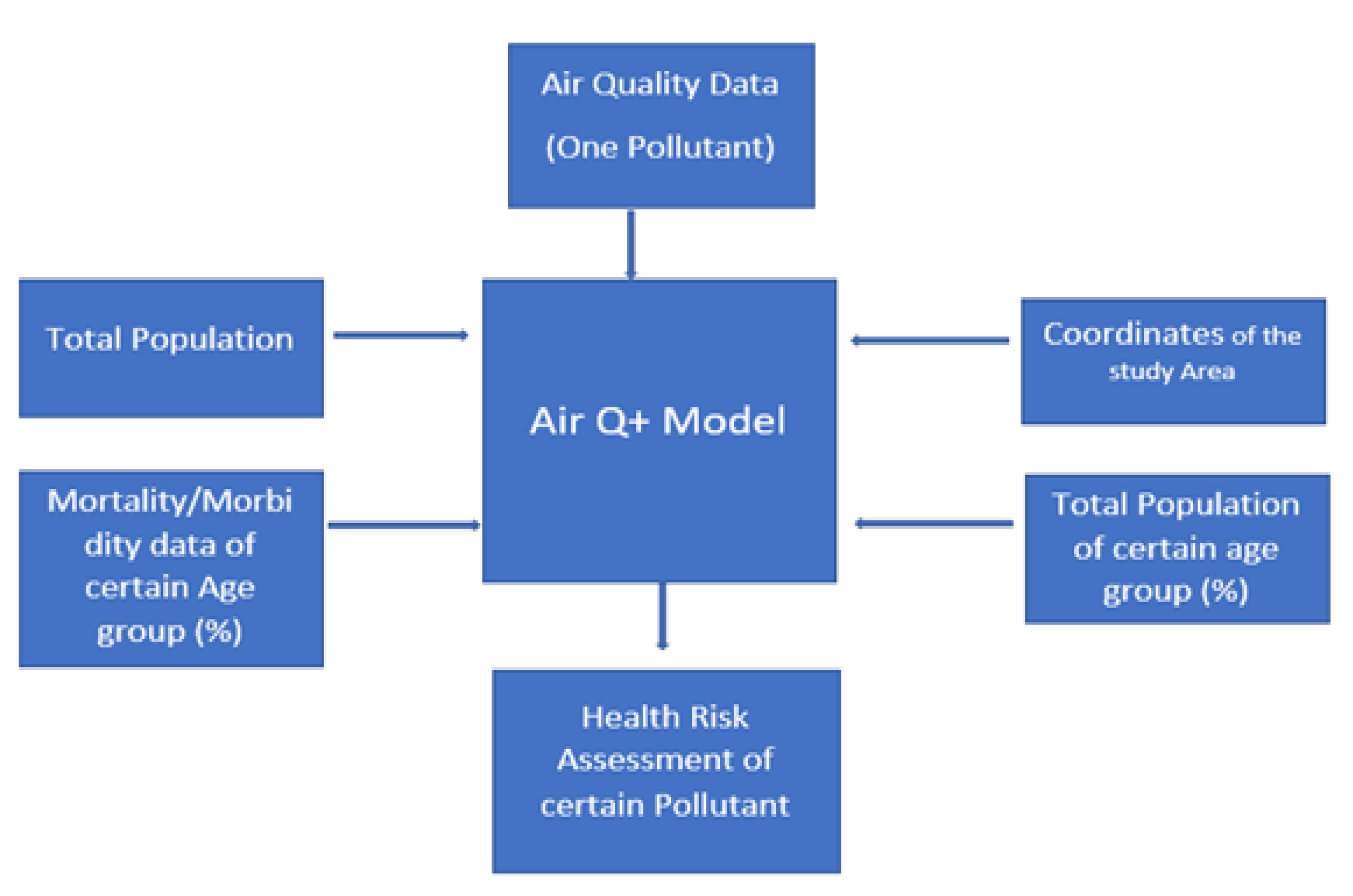
2. Materials and Methods
3. Results
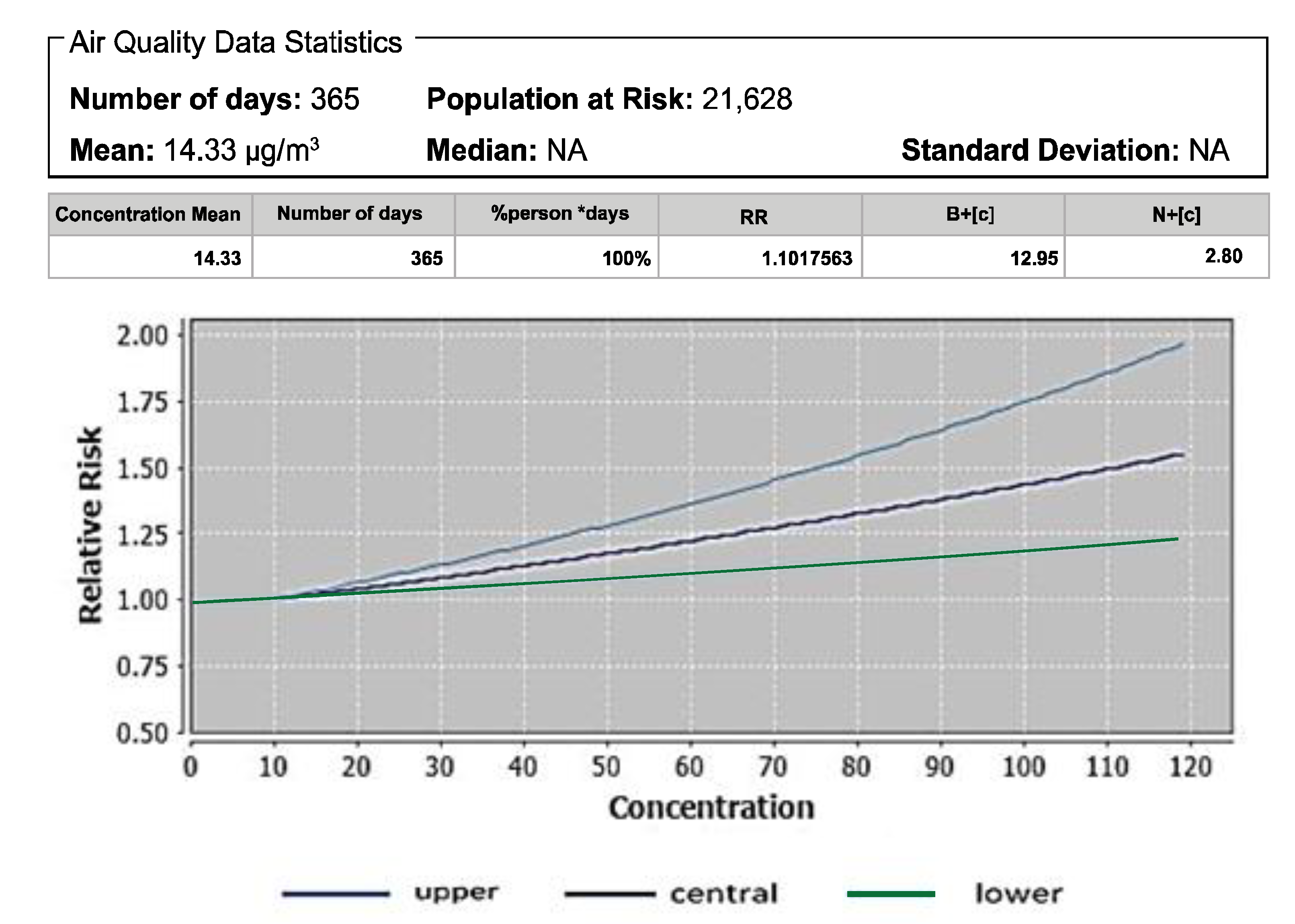
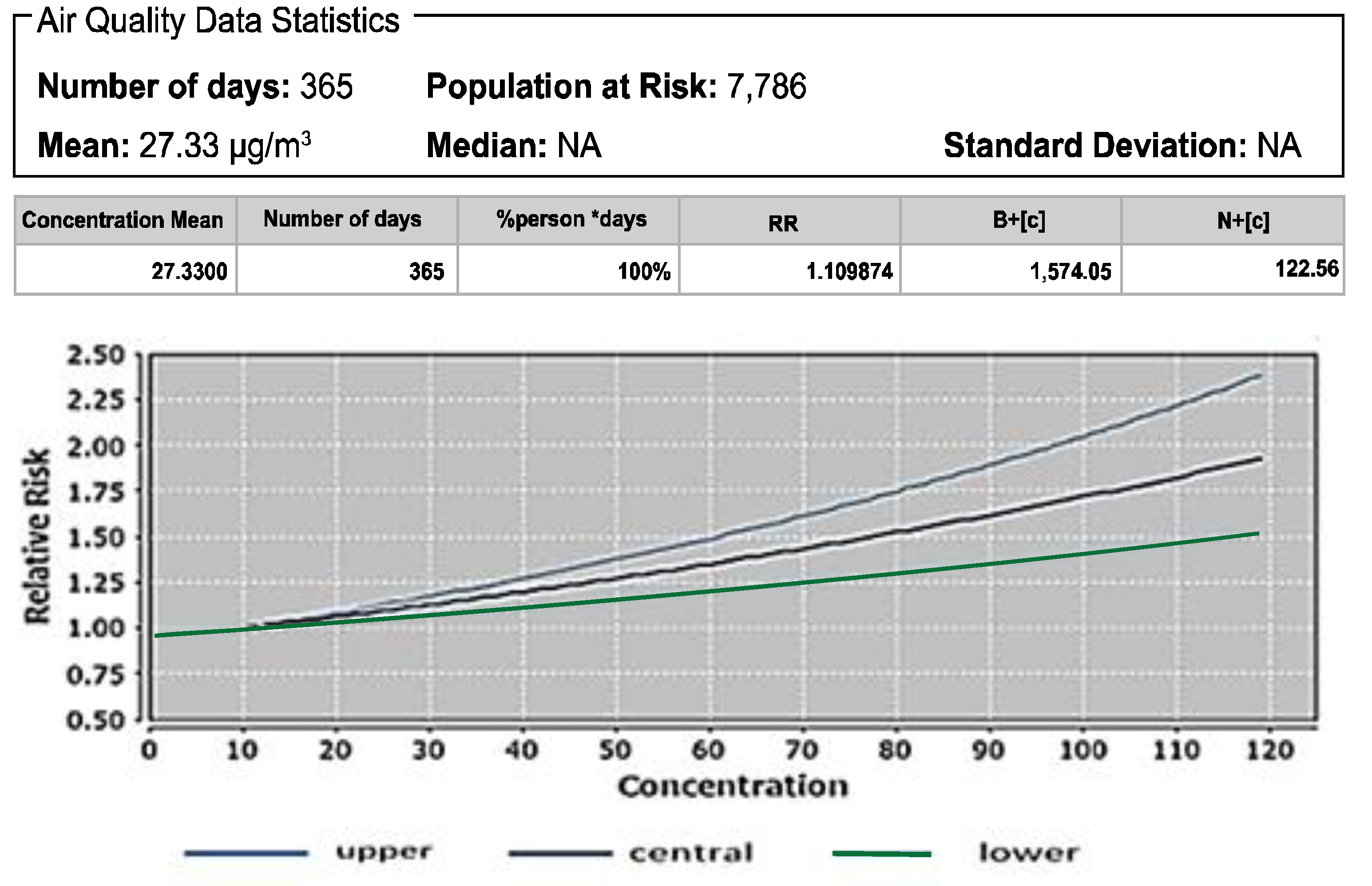
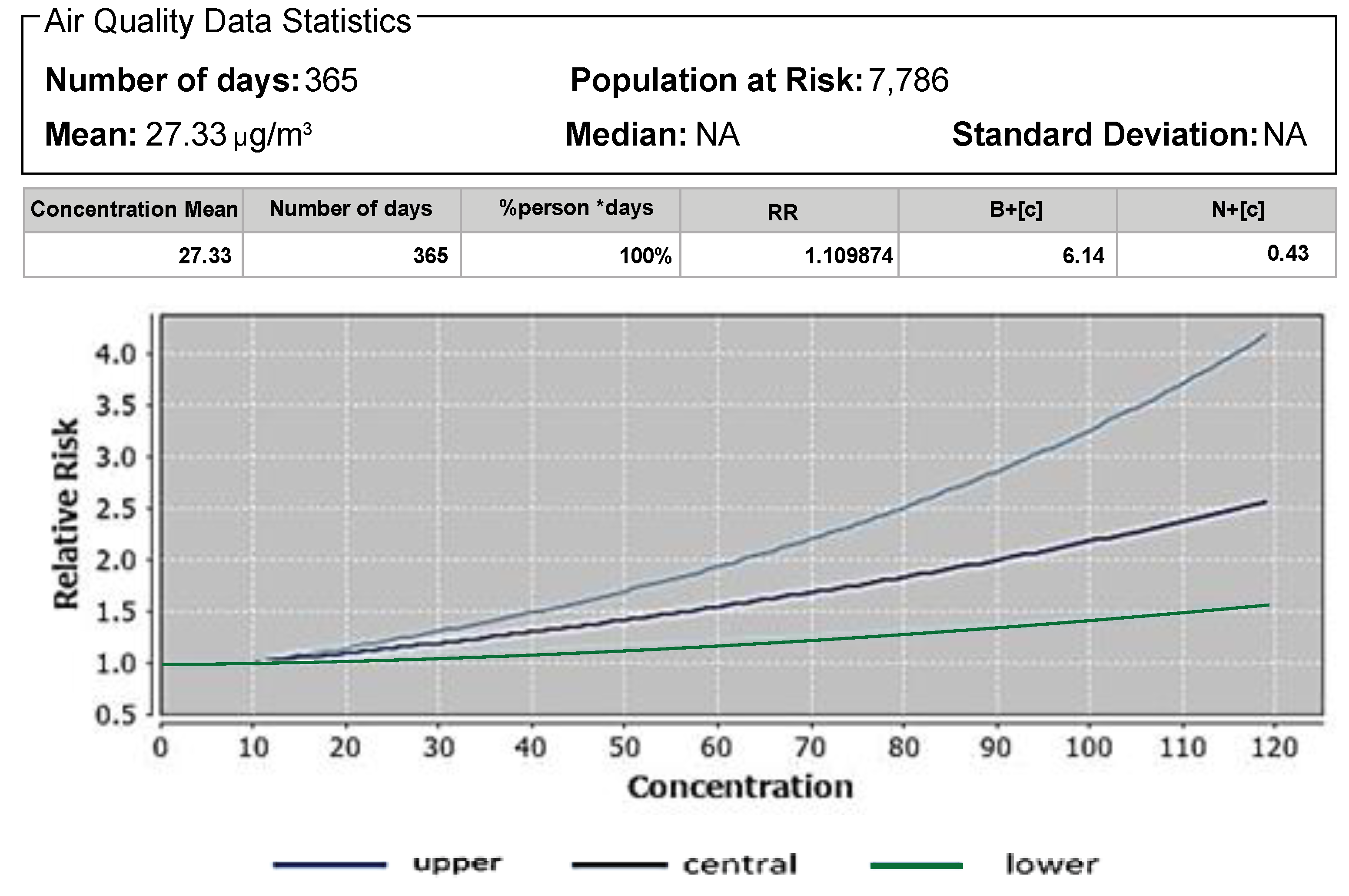
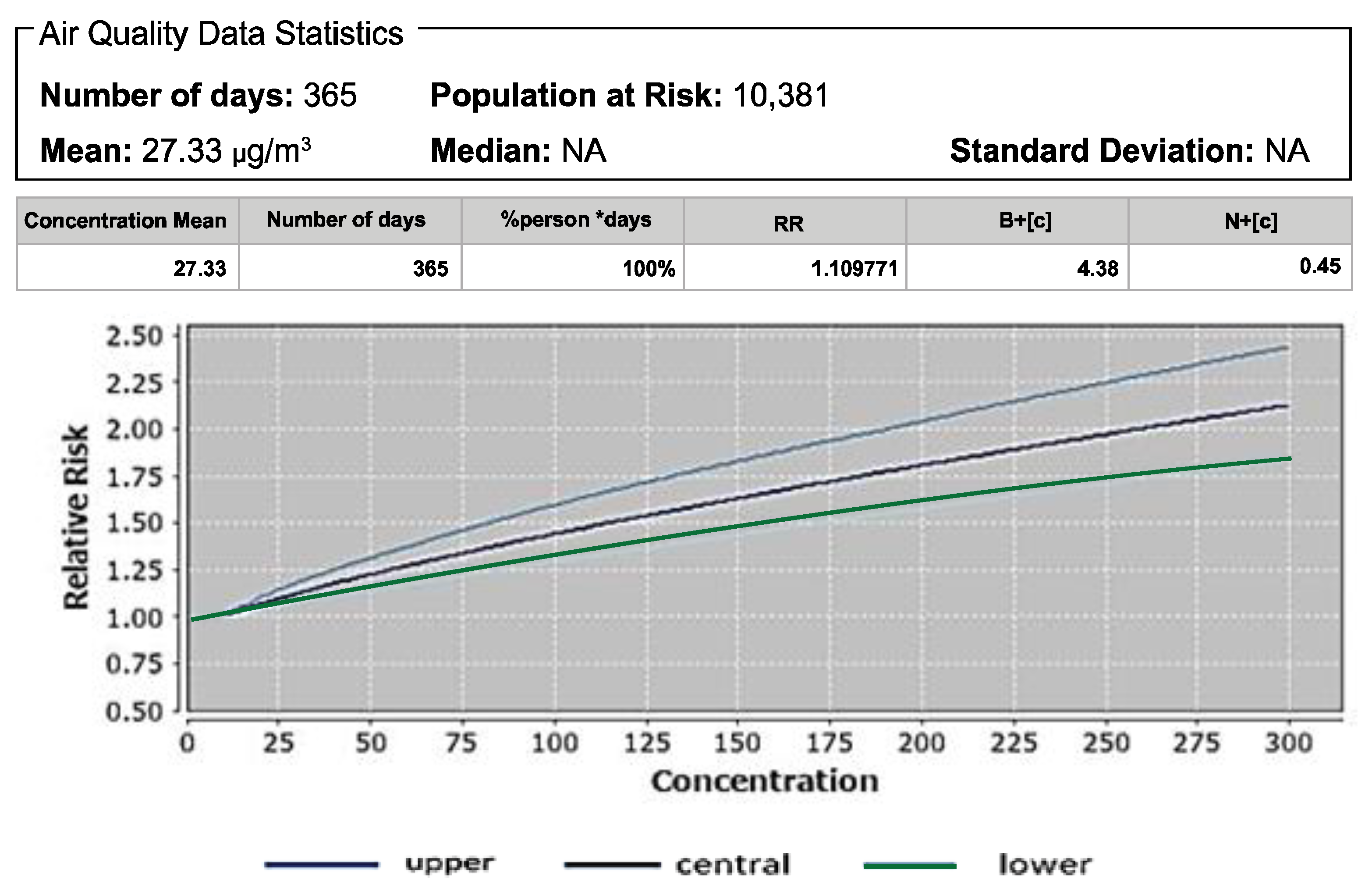
3.1. Atributale Proportion and Relative Risks of Pollutants
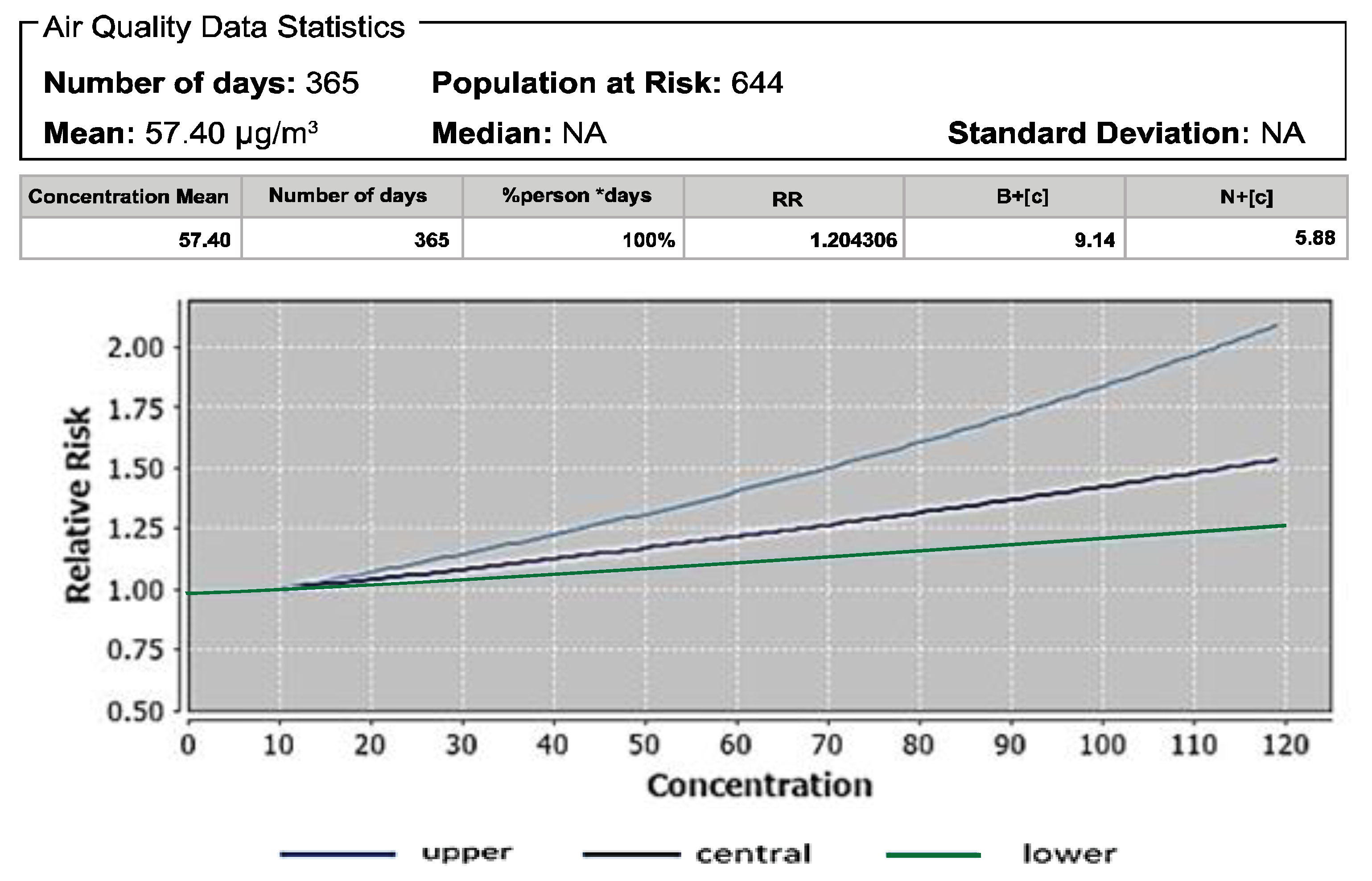
3.2. Effects of PM10 on Post-Neonatal Infant Mortality
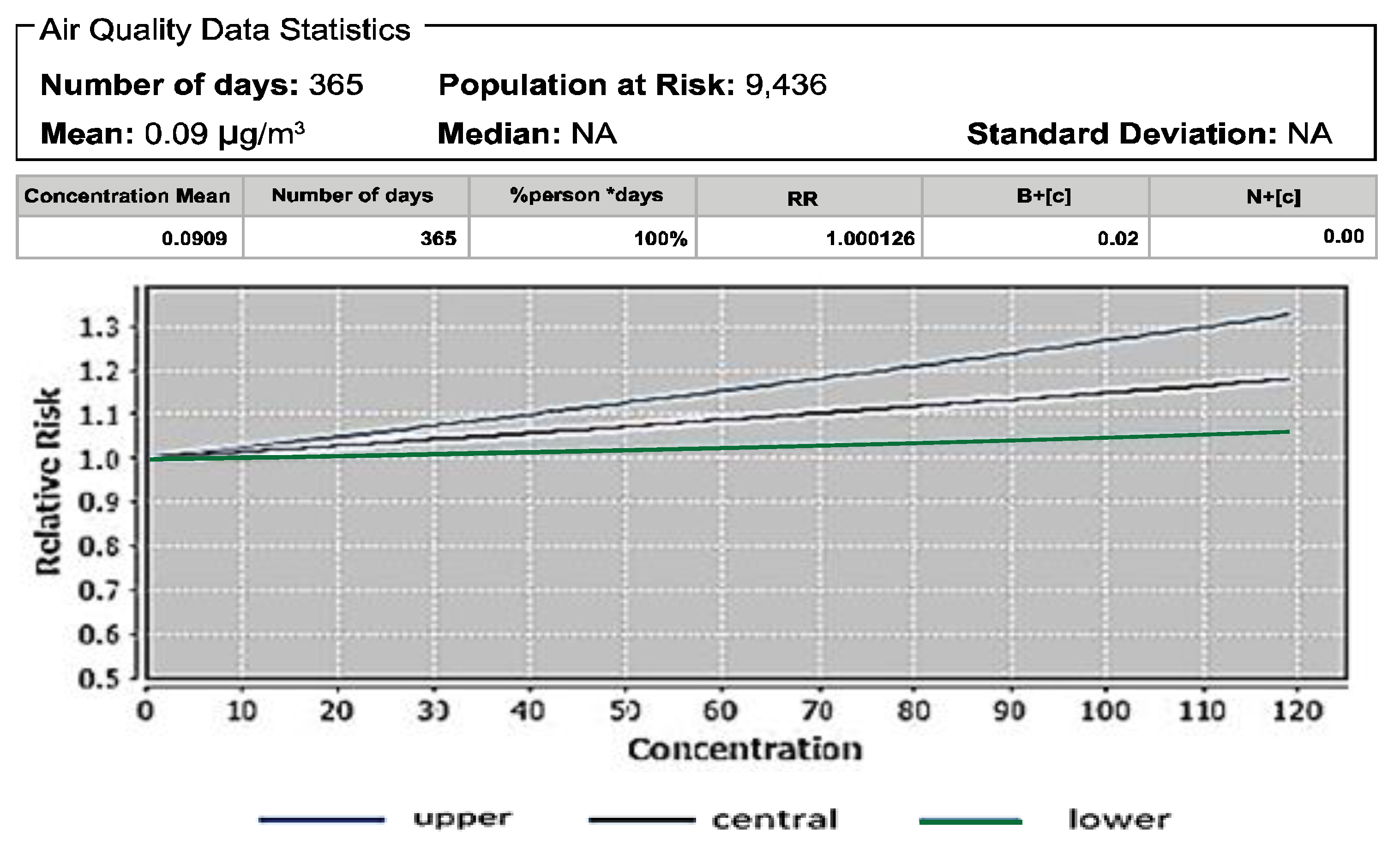
3.3. Mortality Resulting from O3 Exposure
3.4. Change in Relative Risk Value by 10% Reduction in Pollutant Concentrations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mudu, P.; Gapp, C.; Dunbar, M.; World Health Organization. AirQ+: 1.0 Example of Calculations (No. WHO/EURO: 2016-4103-43862-61760); World Health Organization, Regional Office for Europe: Geneva, Switzerland, 2016. [Google Scholar]
- Omidi Khaniabadi, Y.; Sicard, P.; Omidi Khaniabadi, A.; Mohammadinejad, S.; Keishams, F.; Takdastan, A.; Najafi, A.; De Marco, A.; Daryanoosh, M. Air quality modeling for health risk assessment of ambient PM10, PM2.5 and SO2 in Iran. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1298–1310. [Google Scholar] [CrossRef]
- Xayasouk, T.; Lee, H.; Lee, G. Air pollution prediction using long short-term memory (LSTM) and deep autoencoder (DAE) models. Sustainability 2020, 12, 2570. [Google Scholar] [CrossRef] [Green Version]
- Khamutian, R.; Najafi, F.; Soltanian, M.; Shokoohizadeh, M.J.; Poorhaghighat, S.; Dargahi, A.; Sharafi, K.; Afshari, A. The association between air pollution and weather conditions with increase in the number of admissions of asthmatic patients in emergency wards: A case study in Kermanshah. Med. J. Islamic Repub. Iran 2015, 29, 229. [Google Scholar]
- Traversi, D.; Schilirò, T.; Degan, R.; Pignata, C.; Alessandria, L.; Gilli, G. Involvement of nitro-compounds in the mutagenicity of urban PM2. 5 and PM10 in Turin. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2011, 726, 54–59. [Google Scholar] [CrossRef]
- Pope, C.A., III; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K.; et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Byford, S.; Torgerson, D.J.; Raftery, J. Cost of illness studies. BMJ 2000, 320, 1335. [Google Scholar] [CrossRef]
- World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization. Burden of Disease from Household Air Pollution for 2012; Summary of Results; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Atkinson, R.W.; Mills, I.C.; Walton, H.A.; Anderson, H.R. Fine particle components and health—a systematic review and meta-analysis of epidemiological time series studies of daily mortality and hospital admissions. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 208. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Phalkey, R.; Malik, A. A systematic review of air pollution as a risk factor for cardiovascular disease in South Asia: Limited evidence from India and Pakistan. Int. J. Hyg. Environ. Health 2014, 217, 133–144. [Google Scholar] [CrossRef]
- Irazola, V.E.; Gutierrez, L.; Bloomfield, G.; Carrillo-Larco, R.M.; Prabhakaran, D.; Gaziano, T.; Levitt, N.S.; Miranda, J.J.; Ortiz, A.B.; Steyn, K.; et al. Hypertension prevalence, awareness, treatment, and control in selected LMIC communities: Results from the NHLBI/UHG network of centers of excellence for chronic diseases. Glob. Heart 2016, 11, 47–59. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). Global Health Observatory. 2018. Available online: http://www.who.int/gho/ en/ (accessed on 18 October 2018).
- EHN. European Heart Network. European Cardiovascular Disease Statistics 2017; EHN: Brussels, Belgium, 2017. [Google Scholar]
- Chatkin, J.; Correa, L.; Santos, U. External environmental pollution as a risk factor for asthma. Clin. Rev. Allergy Immunol. 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Wang, Y.; Correia, A.W.; Ezzati, M.; Pope, C.A., III; Dockery, D.W. Chemical composition of fine particulate matter and life expectancy: In 95 US counties between 2002 and 2007. Epidemiology 2015, 26, 556. [Google Scholar] [CrossRef]
- Hayyat, M.U.; Nawaz, R.; Siddiq, Z.; Shakoor, M.B.; Mushtaq, M.; Ahmad, S.R.; Ali, S.; Hussain, A.; Irshad, M.A.; Alsahli, A.A.; et al. Investigation of Lithium Application and Effect of Organic Matter on Soil Health. Sustainability 2021, 13, 1705. [Google Scholar] [CrossRef]
- Jafar, T.H.; Qadri, Z.; Chaturvedi, N. Coronary artery disease epidemic in Pakistan: More electrocardiographic evidence of ischaemia in women than in men. Heart 2008, 94, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.A.; Nawaz, R.; Ahmad, S.; Arshad, M.; Rizwan, M.; Ahmad, N.; Nizami, M.; Ahmed, T. Evaluation of Anticipated Performance Index of Tree Species for Air Pollution Mitigation in Islamabad, Pakistan. J. Environ. Sci. Manag. 2020, 23, 50–59. [Google Scholar]
- Gobeli, D.; Schloesser, H.; Pottberg, T. Met one instruments BAM-1020 beta attenuation mass monitor US-EPA PM2.5 federal equivalent method field test results. In Proceedings of the Air & Waste Management Association (A&WMA) Conference, Kansas City, MO, USA, 24–27 June 2008. [Google Scholar]
- Rendón, A.M.; Salazar, J.F.; Palacio, C.A.; Wirth, V.J.J. Temperature inversion breakup with impacts on air quality in urban valleys influenced by topographic shading. Climatology 2015, 54, 302–321. [Google Scholar] [CrossRef]
- Mirzaei, A.; Tahriri, H.; Khorsandi, B. Comparison between AirQ+ and BenMAP-CE in estimating the health benefits of PM 2.5 reduction. Air Qual. Atmos. Health 2021, 14, 807–815. [Google Scholar] [CrossRef]
- Shaddick, G.; Thomas, M.L.; Green, A.; Brauer, M.; Donkelaar, A.; Burnett, R.; Chang, H.H.; Cohen, A.; Dingenen, R.V.; Dora, C.; et al. Data integration model for air quality: A hierarchical approach to the global estimation of exposures to ambient air pollution. J. R. Stat. Soc. Ser. C (Appl. Stat.) 2018, 67, 231–253. [Google Scholar] [CrossRef]
- Lee, H.-M.; Park, R.; Henze, D.K.; Lee, S.; Shim, C.; Shin, H.-J.; Moon, K.-J.; Woo, J.-H. PM2.5 source attribution for Seoul in May from 2009 to 2013 using GEOS-Chem and its adjoint model. Environ. Pollut. 2017, 221, 377–384. [Google Scholar] [CrossRef]
- Li, Y. Using BenMAP (Environmental Benefits Mapping and Analysis Program) to Evaluate Health Benefits of Air Pollution Control; East Tennessee State University: Johnson City, TN, USA, 2017. [Google Scholar]
- Baldauf, R.W.; Lane, D.D.; Marote, G.A. Ambient air quality monitoring network design for assessing human health impacts from exposures to airborne contaminants. Environ. Monit. Assess. 2001, 66, 63–76. [Google Scholar] [CrossRef]
- Karimi, A.; Shirmardi, M.; Hadei, M.; Birgani, Y.T.; Neisi, A.; Takdastan, A.; Goudarzi, G. Concentrations and health effects of short-and long-term exposure to PM2. 5, NO2, and O3 in ambient air of Ahvaz city, Iran (2014–2017). Ecotoxicol. Environ. Saf. 2019, 180, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, M.; Hadei, M.; Nazari, S.S.H.; Conti, G.O.; Alipour, M.R.; Ferrante, M.; Shahsavani, A. Mortality assessment attributed to long-term exposure to fine particles in ambient air of the megacity of Tehran, Iran. Environ. Sci. Pollut. Res. 2018, 25, 14254–14262. [Google Scholar] [CrossRef] [PubMed]
- Adeyanju, E.; Okeke, C.A. Exposure effect to cement dust pollution: A mini review. SN Appl. Sci. 2019, 1, 1572. [Google Scholar] [CrossRef] [Green Version]
- Gharavandi, S.; Nasori, M.; Ghobadi, M.; Besharatipur, M.; Jabari, M.; Omidianidost, A. Exposure to Respirable Dust and Crystalline Silica in a Cement Plant. Arch. Occup. Health 2019, 3, 366–370. [Google Scholar] [CrossRef]
- Majeed, F.A.; Azeem, A.R.; Farhan, N. Lung cancer in Pakistan, where do we stand? JPMA J. Pak. Med. Assoc. 2019, 69, 405–408. [Google Scholar] [PubMed]
- Sultana, T.; Afzal, A.; Sultana, S.; Al-Ghanim, K.; Shahid, T.; Jabeen, Z.; Turab, N.; Ahmed, Z.; Mahboob, S. Epidemiological estimates of Respiratory diseases in the hospital population, Faisalabad, Pakistan. Braz. Arch. Biol. Technol. 2017, 60. [Google Scholar] [CrossRef]
- Ansari, M.; Ehrampoush, M.H. Meteorological correlates and AirQ+ health risk assessment of ambient fine particulate matter in Tehran, Iran. Environ. Res. 2019, 170, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Badyda, A.J.; Grellier, J.; Dąbrowiecki, P. Ambient PM2.5 exposure and mortality due to lung cancer and cardiopulmonary diseases in Polish cities. In Respiratory Treatment and Prevention; Springer: Berlin/Heidelberg, Germany, 2016; pp. 9–17. [Google Scholar]
- Pope, C.A.; Ezzati, M.; Cannon, J.B.; Allen, R.T.; Jerrett, M.; Burnett, R.T. Mortality risk and PM 2.5 air pollution in the USA: An analysis of a national prospective cohort. Air Qual. Atmos. Health 2018, 11, 245–252. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, H.; Zheng, R.; Li, S.; Pereira, G.; Liu, Q.; Chen, W.; Huxley, R. The burden of lung cancer mortality attributable to fine particles in China. Sci. Total Environ. 2017, 579, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Faridi, S.; Shamsipour, M.; Krzyzanowski, M.; Künzli, N.; Amini, H.; Azimi, F.; Malkawi, M.; Momeniha, F.; Gholampour, A.; Hassanvand, M.S. Long-term trends and health impact of PM2.5 and O3 in Tehran, Iran, 2006–2015. Environ. Int. 2018, 114, 37–49. [Google Scholar] [CrossRef]
- Dastoorpoor, M.; Riahi, A.; Yazdaninejhad, H.; Borsi, S.H.; Khanjani, N.; Khodadadi, N.; Mohammadi, M.J.; Aghababaeian, H. Exposure to particulate matter and carbon monoxide and cause-specific Cardiovascular-Respiratory disease mortality in Ahvaz. Toxin Rev. 2020, 1–11. [Google Scholar] [CrossRef]
- Barzeghar, V.; Sarbakhsh, P.; Hassanvand, M.S.; Faridi, S.; Gholampour, A. Long-term trend of ambient air PM10, PM2. 5, and O3 and their health effects in Tabriz city, Iran, during 2006–2017. Sustain. Cities Soc. 2020, 54, 101988. [Google Scholar] [CrossRef]
- Mehmood, T.; Tianle, Z.; Ahmad, I.; Li, X. Integration of AirQ+ and particulate matter mass concentration to calculate health and ecological constraints in Islamabad, Pakistan. In Proceedings of the 2019 16th International Bhurban Conference on Applied Sciences and Technology (IBCAST), Islamabad, Pakistan, 8–12 January 2019. [Google Scholar]
- Habeebullah, T.M. Modeling particulate matter (PM10) in Makkah, Saudi Arabia—A viewpoint of health impact. J. Clean Energy Technol. 2014, 2, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Amoatey, P.; Takdastan, A.; Sicard, P.; Hopke, P.K.; Baawain, M.; Omidvarborna, H.; Allahyari, S.; Esmaeilzadeh, A.; De Marco, A.; Khanaibadi, Y.O.; et al. Short and long-term impacts of ambient ozone on health in Ahvaz, Iran. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1336–1351. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, Y.; Liu, H.; Si, Y.; Wu, Y.; Wang, X.; Wang, M.; Wu, J.; Chen, L.; Wei, C.; et al. The impact of ambient ozone pollution on pneumonia: A nationwide time-series analysis. Environ. Int. 2020, 136, 105498. [Google Scholar] [CrossRef] [PubMed]

| Pollutant | Model | Range | Method | Detection Limit |
|---|---|---|---|---|
| CO | AM-5300 | 0–50 ppm | non-dispersive infrared ray method (ISO4224) | 0.1 ppm |
| NO/NO2/NOx | AM-5200 | 0–1 ppm | Chemiluminescence (ISO7996) method | 0.5 ppb |
| Sulfur Dioxide | AM-5100 | 0–0.5 ppm | U.V. fluorescence method (ISO10498) | 1 ppb |
| Ozone | AM-5400 | 0–1 ppm | UV photometry method | 0.5 ppb |
| PM2.5/PM10 | DPM-6000 | 0–5 mg m3 | Beta attenuation method | <1 µg/m3 |
| Pollutant | Mortality | Baseline Incidence (Per 100,000 Population) | Attributable Proportion (%) | Relative Risk (RR) |
|---|---|---|---|---|
| PM2.5 | Mortality due to LC in adults age 25+ | 44.25 [32] | 9.89 | 1.10 |
| Mortality due to LC in adults age 30+ | 44.25 [32] | 13.88 | 1.16 | |
| All-cause mortality in adults age 30+ | 15,900 | 9.9 | 1.10 | |
| PM10 | Post neonatal infant mortality | 5386 | 16.96 | 1.20 |
| NO2 | All-cause mortality | 750 | 1.73 | 1.01 |
| O3 | Respiratory disease mortality | 181 [33] | 0.01 | 1.00 |
| Current Situation | Reductions by 10% | ||||
|---|---|---|---|---|---|
| Pollutant | Mortality | Mean Concentrations (µg/m3) | Relative Risk (RR) | Mean Concentrations (µg/m3) | Relative Risk (RR) |
| PM2.5 | Mortality due to LC in adults age 25+ Mortality due to LC in adults age 30+ All-cause mortality in adults age 30+ | 27.33 27.33 27.33 | 1.10 1.16 1.10 | 24.60 24.60 24.60 | 1.09 1.13 1.09 |
| PM10 | Post neonatal infant mortality | 57.39 | 1.20 | 47.10 | 1.15 |
| NO2 | All-cause mortality | 14.33 | 1.01 | 12.90 | 1.01 |
| O3 | Respiratory disease mortality | 0.0909 | 1.0000 | 0.0818 | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasir, A.H.; Nawaz, R.; Haider, R.; Irshad, M.A. Modeling Air Pollution Health Risk for Environmental Management of an Internationally Important Site: The Salt Range (Kallar Kahar), Pakistan. Atmosphere 2022, 13, 100. https://doi.org/10.3390/atmos13010100
Nasir AH, Nawaz R, Haider R, Irshad MA. Modeling Air Pollution Health Risk for Environmental Management of an Internationally Important Site: The Salt Range (Kallar Kahar), Pakistan. Atmosphere. 2022; 13(1):100. https://doi.org/10.3390/atmos13010100
Chicago/Turabian StyleNasir, Abdul Hafeez, Rab Nawaz, Rizwan Haider, and Muhammad Atif Irshad. 2022. "Modeling Air Pollution Health Risk for Environmental Management of an Internationally Important Site: The Salt Range (Kallar Kahar), Pakistan" Atmosphere 13, no. 1: 100. https://doi.org/10.3390/atmos13010100
APA StyleNasir, A. H., Nawaz, R., Haider, R., & Irshad, M. A. (2022). Modeling Air Pollution Health Risk for Environmental Management of an Internationally Important Site: The Salt Range (Kallar Kahar), Pakistan. Atmosphere, 13(1), 100. https://doi.org/10.3390/atmos13010100






