Study of the Treatment of Organic Waste Gas Containing Benzene by a Low Temperature Plasma-Biological Degradation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
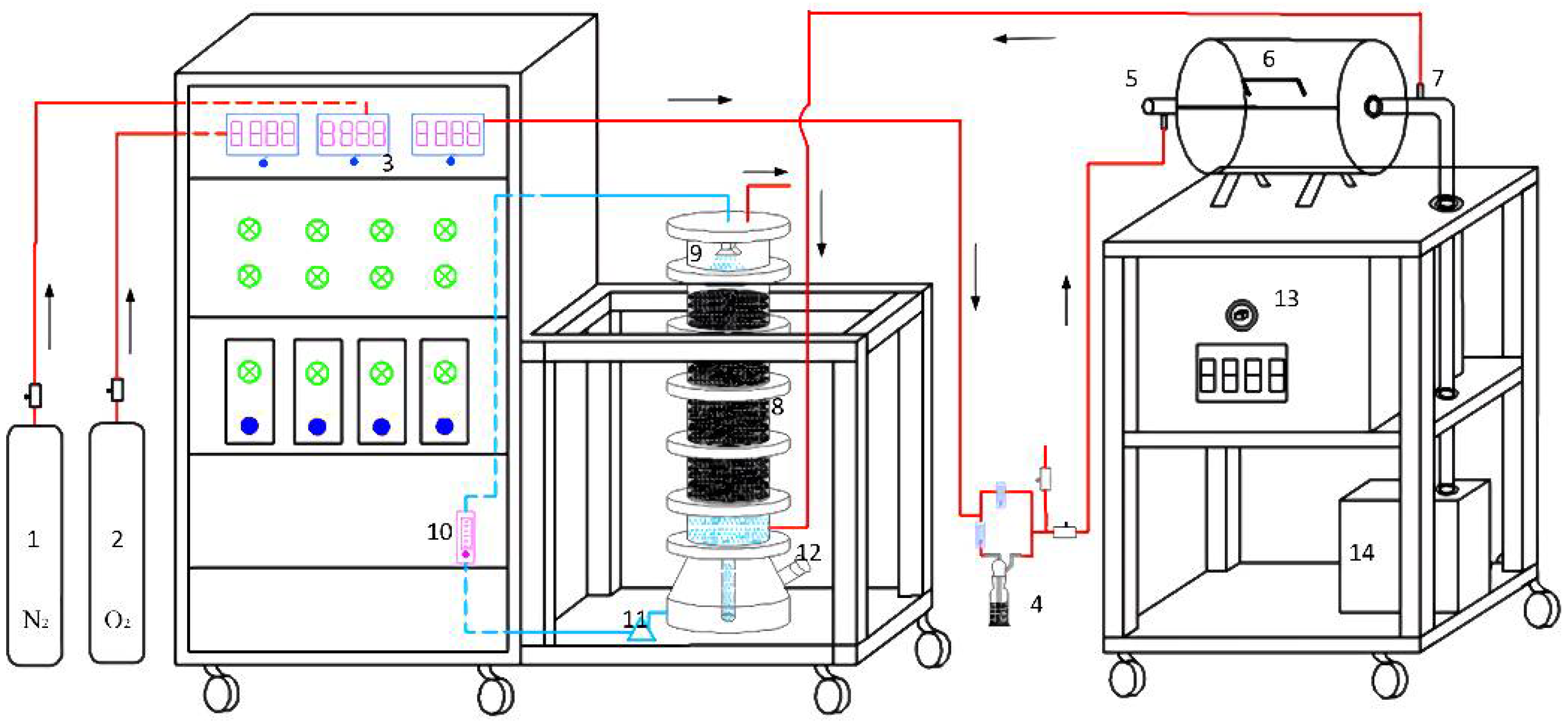
2.2. Apparatus
2.3. Experimental Methods
2.4. Analysis Method
3. Results and Discussion
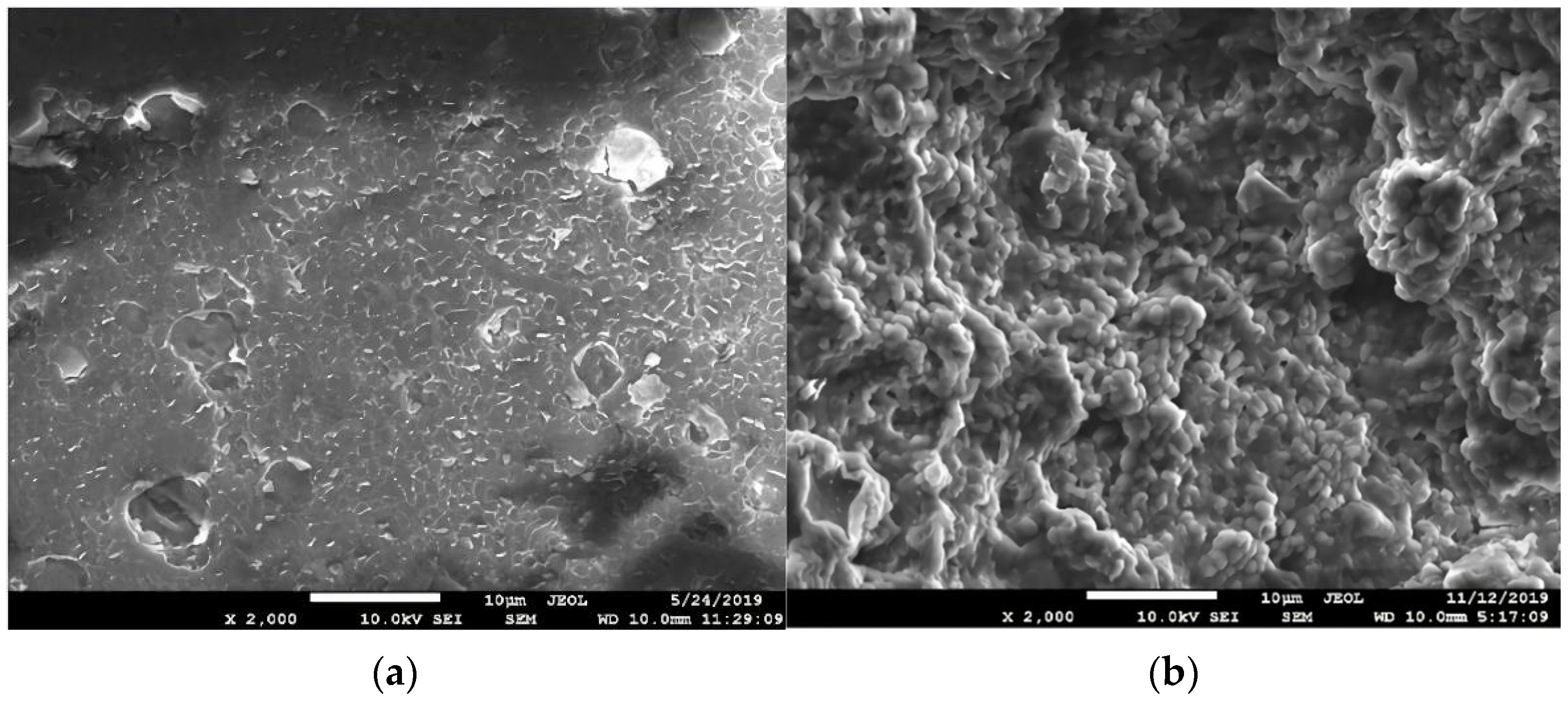
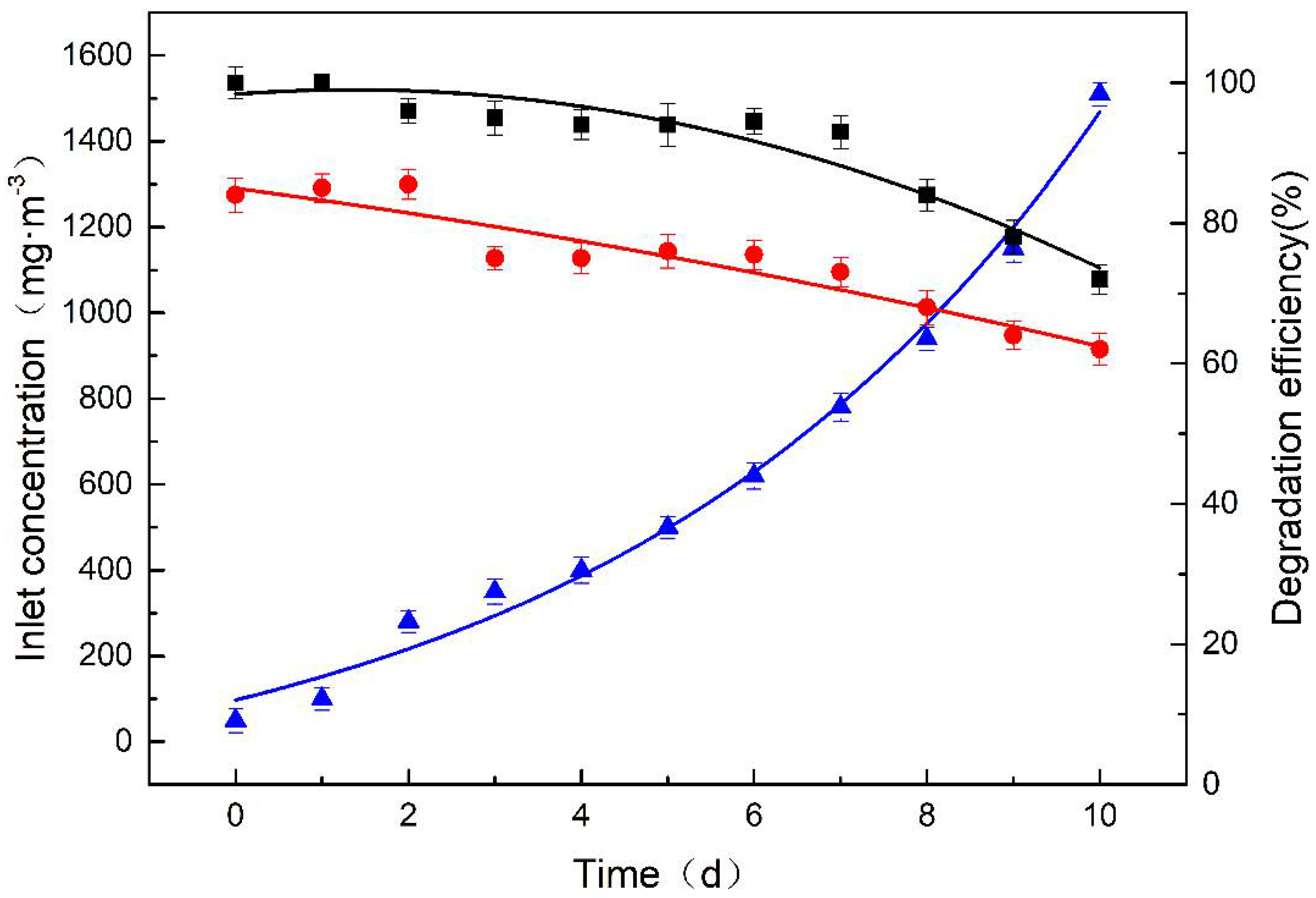
3.1. Reactor Start-Up Stage
3.2. Optimization of the Operating Parameters of the BTF
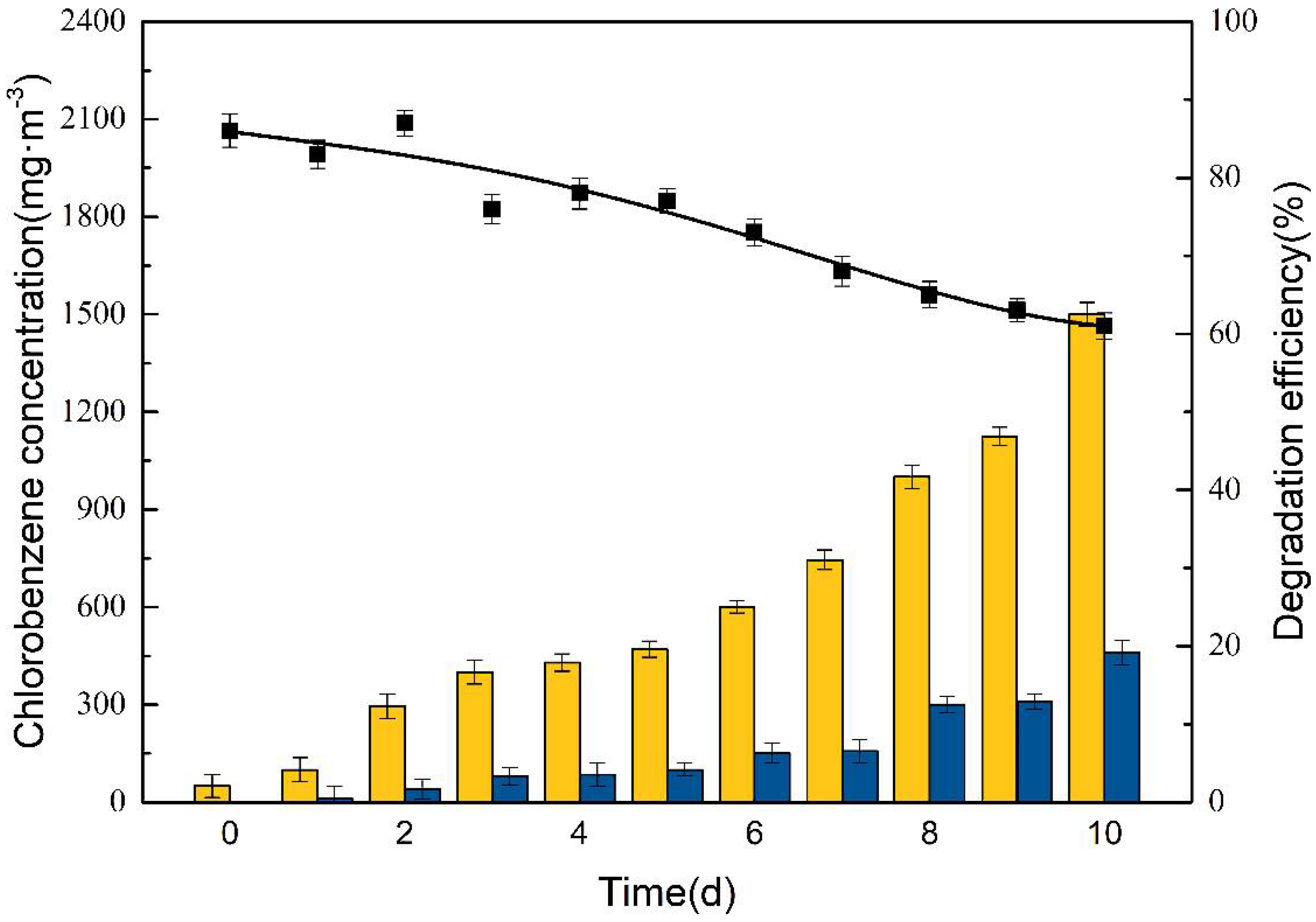
3.2.1. Influence of Inlet Concentration of Chlorobenzene on BTF
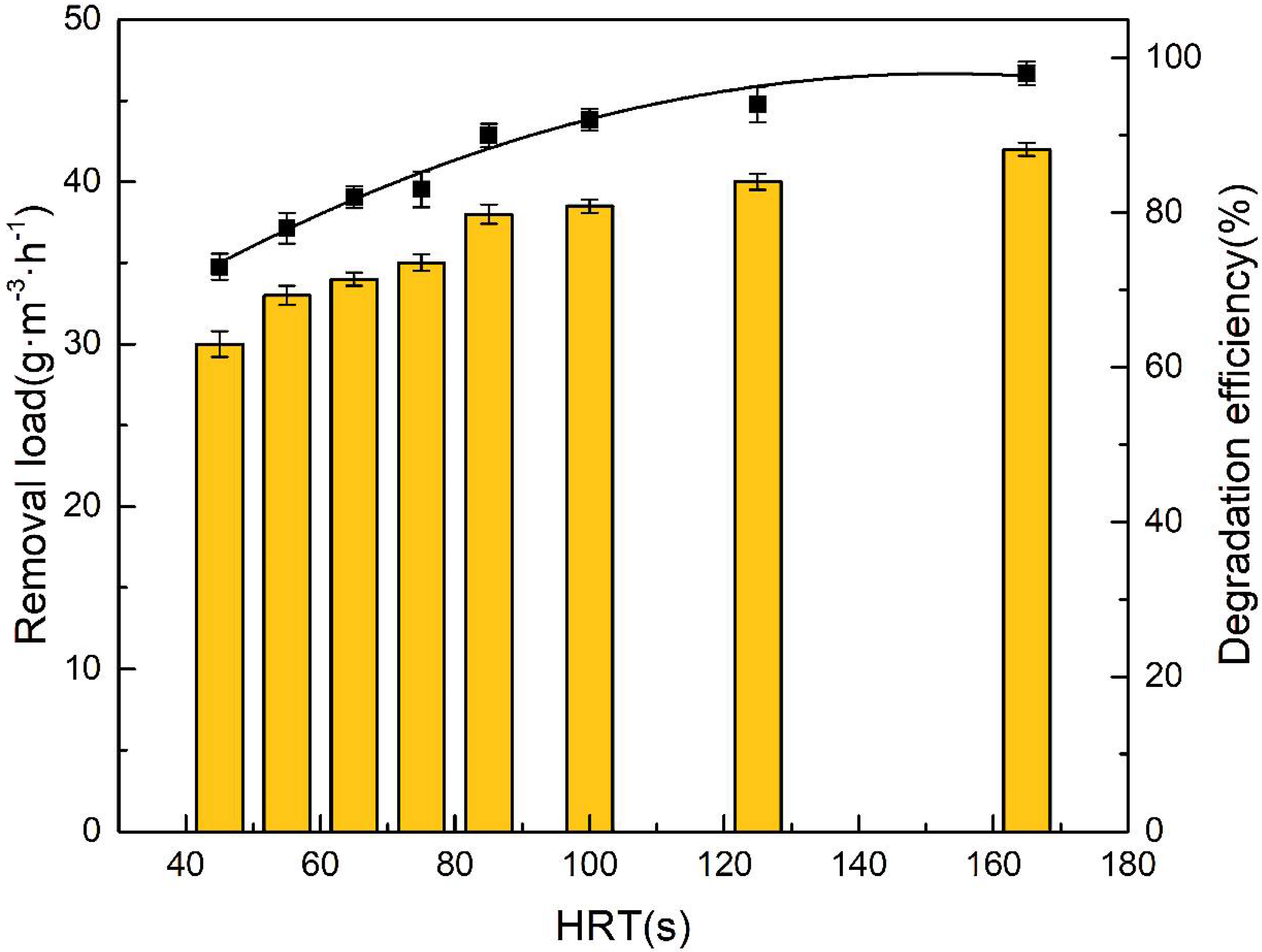
3.2.2. Influence of Hydraulic Retention Time on BTF
3.3. Optimization of the Operating Parameters of the DBD
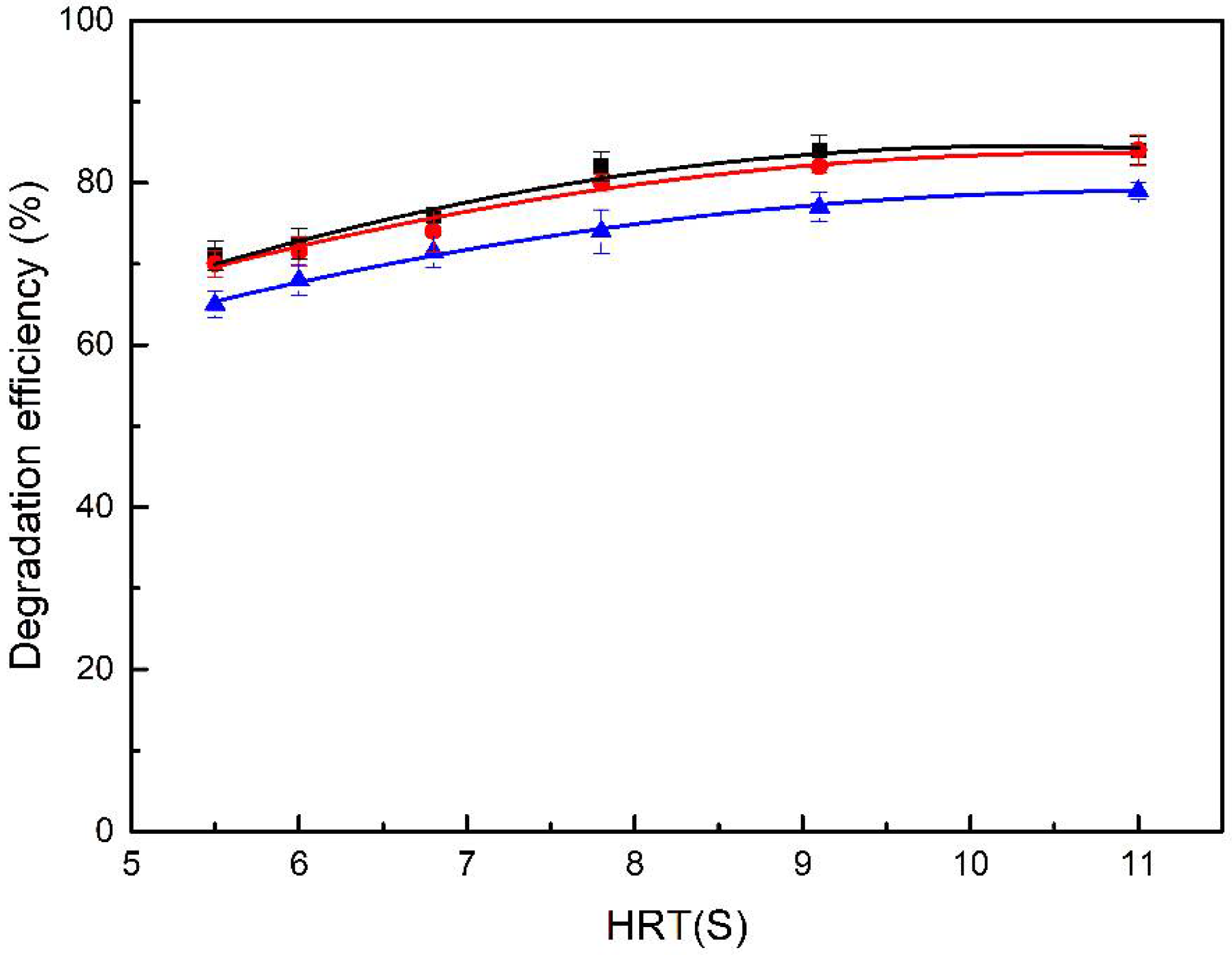
3.3.1. Influence of HRT on Degradation Efficiency of DBD
3.3.2. Influence of Input Voltage on Degradation Efficiency of DBD
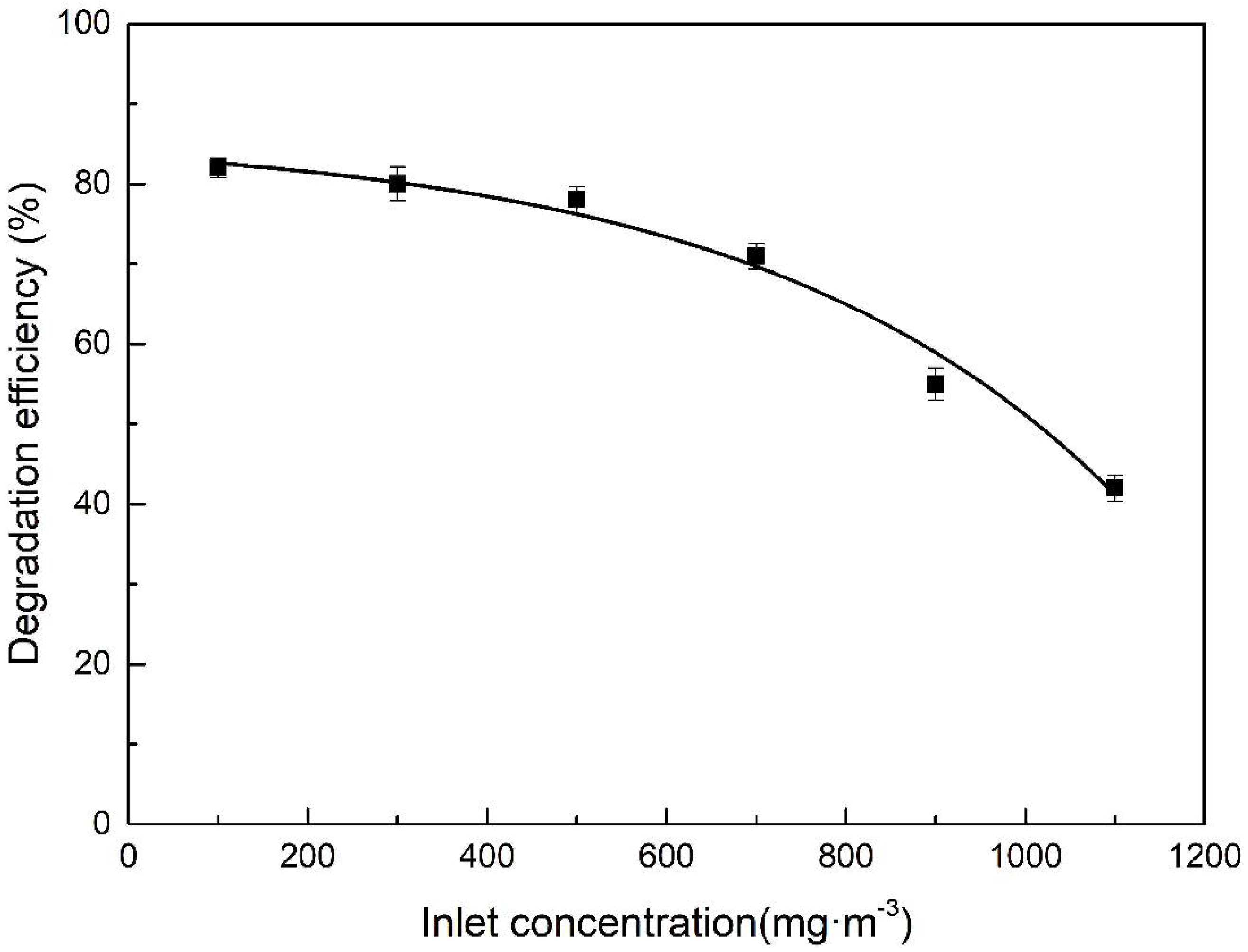
3.3.3. Influence of Inlet Concentration on Degradation Efficiency by DBD
3.4. Analysis of Chlorobenzene Degradation by DBD-Biological Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, J.J.; Wang, X.B.; Yang, Y.R.; Qin, Y.Y.; Shi, S.X.; Xu, P.H.; Chen, R.Z.; Zhou, X.M.; Tan, J.H.; Wang, X.M. Source apportionment of VOCs in a typical medium-sized city in North China Plain and implications on control policy—ScienceDirect. J. Environ. Sci. 2021, 107, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Du, H.B.; Guo, Y.Q.; Lin, Z.G.; Qiu, Y.M.; Xiao, X. Effects of the joint prevention and control of atmospheric pollution policy on air pollutants-A quantitative analysis of Chinese policy texts. J. Environ. Manag. 2021, 300, 113721. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.R.; Ma, T.; Gao, Z.J.; Gao, J.; Wang, Z.; Xue, L.K.; Liu, H.Q.; Liu, J.Y. Characteristics and sources of volatile organic compounds during high ozone episodes: A case study at a site in the eastern Guanzhong Plain, China. J. Chemosphere 2020, 265, 129072. [Google Scholar] [CrossRef] [PubMed]
- Mozaffar, A.; Zhang, Y.L. Atmospheric volatile organic compounds (VOCs) in China: A review. J. Curr. Pollut. Rep. 2020, 6, 250–263. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z.P. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. J. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Caceres, M.; Scott, F.; Díaz-Robles, L.; Aroca, G.; Vergara-Fernández, A. Biodegradation of benzo [alpha] pyrene, toluene, and formaldehyde from the gas phase by a consortium of Rhodococcus erythropolis and Fusarium solani. J. Appl. Microbiol. Biotechnol. 2017, 101, 6765–6777. [Google Scholar] [CrossRef]
- Zhu, R.Y.; Mao, Y.B.; Jiang, L.Y.; Chen, J.M. Performance of chlorobenzene removal in a nonthermal plasma catalysis reactor and evaluation of its byproducts. J. Chem. Eng. J. 2015, 279, 463–471. [Google Scholar] [CrossRef]
- Liu, S.H.; Fu, S.H.; Chen, C.Y.; Lin, C.W. Enhanced processing of exhaust gas and power generation by connecting mini-tubular microbial fuel cells in series with a biotrickling filter. J. Renew. Energy 2020, 156, 342–348. [Google Scholar] [CrossRef]
- Gu, S.; Guenther, A.; Faiola, C. Effects of anthropogenic and biogenic volatile organic compounds on Los Angeles air quality. J. Environ. Sci. Technol. 2021, 55, 12191–12201. [Google Scholar] [CrossRef]
- Wang, D.S.; Zhu, X.M.; Yang, X.F.; Jiao, R.Y.; Zhao, S.; Song, R.W.; Lv, M.H.; Yang, M. VOCs and odors control and development in pharmaceutical fermentation industry. J. Environ. Sci. 2019, 40, 1990–1998. [Google Scholar]
- Deng, W.; Tang, Q.X.; Huang, S.S.; Zhang, L.; Jia, Z.Y.; Guo, L.M. Low temperature catalytic combustion of chlorobenzene over cobalt based mixed oxides derived from layered double hydroxides. J. Appl. Catal. B Environ. 2020, 278, 119336. [Google Scholar] [CrossRef]
- Jin, X.P.; Wang, G.C.; Lian, L.P.; Gao, F.; Zhang, R.X.; Zhao, W.X.; Hou, J.Y.; Chen, S.P.; Zhang, R. Chlorobenzene removal using DBD coupled with CuO/gamma-Al2O3 catalyst. J. Appl. Sci. 2021, 11, 6433. [Google Scholar] [CrossRef]
- Li, Y.Z.; Fan, Z.Y.; Shi, J.W.; Liu, Z.Y.; Zhou, J.W.; Shangguan, W.F. Removal of volatile organic compounds (VOCs) at room temperature using dielectric barrier discharge and plasma-catalysis. J. Plasma Chem. Plasma Processing 2014, 34, 801–810. [Google Scholar] [CrossRef]
- Mo, Z.; Lu, S.; Shao, M. Volatile organic compound (VOC) emissions and health risk assessment in paint and coatings industry in the Yangtze River delta, China. J. Environ. Pollut. 2020, 269, 115740. [Google Scholar] [CrossRef]
- Zheng, H.; Kong, S.F.; Yan, Y.Y.; Chen, N.; Yao, L.Q.; Liu, X.; Wu, F.Q.; Cheng, Y.; Niu, Z.Z.; Zheng, S.R.; et al. Compositions, sources and health risks of ambient volatile organic compounds (VOCs) at a petrochemical industrial park along the Yangtze River. J. Sci. Total Environ. 2020, 703, 135505. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.H.; Lin, X. Research progress in treatment of VOCs by dielectric barrier plasma cooperating catalyst. J. Environ. Eng. 2019, 37, 128–131. [Google Scholar] [CrossRef]
- Martinez-Soria, V.; Gabaldon, C.; Penya-Roja, J.M.; Palauj, J.; Alvarez-Hornos, F.J.; Sempere, F.; Soriano, C. Performance of a pilot-scale biotrickling filter in controlling the volatile organic compound emissions in a furniture manufacturing facility. J. Air Waste Manag. Assoc. 2009, 59, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Yue, Q.; Hao, W.; Yin, Z.; Fang, Y.Y.; Yin, C.R. Effect of static magnetic field on trichloroethylene removal in a biotrickling filter. J. Bioresour. Technol. 2017, 239, 7–16. [Google Scholar]
- Qi, Y.Q.; Shen, L.; Zhang, J.L.; Yao, J.; Lu, R.; Miyakoshi, T. Species and release characteristics of VOCs in furniture coating process. J. Environ. Pollut. 2019, 245, 810–819. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Li, S.; Cheng, Z.W.; Chen, J.M.; Nie, G.F. Treatment of 1, 2-dichloroethane and n-hexane in a combined system of non-thermal plasma catalysis reactor coupled with a biotrickling filter. J. Chem. Technol. Biotechnol. 2018, 93, 127–137. [Google Scholar] [CrossRef]
- Zhou, L.; Ma, C.; Horlyck, J.; Liu, R.J.; Yun, J. Development of pharmaceutical VOCs elimination by catalytic processes in China. J. Catal. 2020, 10, 668. [Google Scholar] [CrossRef]
- Dai, Z.; Xiong, L.; Jiao, Q. Application of biotechnology in organic waste gas treatment. J. AER-Adv. Eng. Res. 2016, 104, 738–741. [Google Scholar]
- Liu, Y.H.C.; Lian, L.P.; Zhao, W.X.; Zhang, R.X.; Hou, H.Q. DBD coupled with MnO x /γ-Al2O3 catalysts for the degradation of chlorobenzene. J. Plasma Sci. Technol. 2020, 22, 034016. [Google Scholar] [CrossRef]
- Wei, W.; Li, Y.; Wang, Y.T.; Cheng, S.Y.; Wang, L.T. Characteristics of VOCs during haze and non-haze days in Beijing, China: Concentration, chemical degradation and regional transport impact. J. Atmos. Environ. 2018, 194, 134–145. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.M.; Song, S.; Xu, Z.R.; Xu, M.Z. Emission characteristics and hazard assessment analysis of volatile organic compounds from chemical synthesis pharmaceutical industry. J. Environ. Sci. 2014, 35, 3663–3668. [Google Scholar]
- Zhao, D.; Yuan, X.L.; Ren, A.L.; Fu, F.Y. Typical Pharmaceutical Process VOCs and Stench Pollution Characteristics and Control Techniques. J. Adv. Mater. Res. 2013, 726–731, 2017–2021. [Google Scholar]
- Ognier, S.; Cavadias, S.; Amouroux, J. Aromatic VOC removal by microparticles formation in pure nitrogen. J. High Temp. Mater. Processes 2009, 13, 107–119. [Google Scholar] [CrossRef]
- Wang, X.Q.; Lu, B.H.; Zhou, X.X. Evaluation of o-xylene and other volatile organic compounds removal using a xylene-acclimated biotrickling filter. J. Environ. Technol. 2013, 34, 2691–2699. [Google Scholar] [CrossRef]
- Yu, X.; Dang, X.Q.; Li, S.J.; Zhang, J.L.; Zhang, Q.; Cao, L. A comparison of in- and post-plasma catalysis for toluene abatement through continuous and sequential processes in dielectric barrier discharge reactors. J. Clean. Prod. 2020, 276, 124251. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Nie, G.F.; Zhu, R.Y.; Wang, J.D.; Chen, J.M.; Mao, Y.B.; Cheng, Z.W.; Anderson, W.A. Efficient degradation of chlorobenzene in a non-thermal plasma catalytic reactor supported on CeO2/HZSM-5 catalysts. J. Environ. Sci. 2016, 55, 266–273. [Google Scholar] [CrossRef]
- Shahna, F.G.; Bahrami, A.; Alimohammadi, I.; Yarahmadi, R.; Jaleh, B.; Gandomi, M.; Ebrahimi, H.; Abedi, K.A.D. Chlorobenzene degeradation by non-thermal plasma combined with EG-TiO2/ZnO as a photocatalyst: Effect of photocatalyst on CO2 selectivity and byproducts reduction. J. Hazard. Mater. 2016, 324, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Hu, F.Y.; Peng, Y.; Li, K.Z.; Bai, S.P.; Li, J.H. Non-thermal plasma catalysis for chlorobenzene removal over CoMn/TiO2 and CeMn/TiO2: Synergistic effect of chemical catalysis and dielectric constant. Chem. Eng. J. 2018, 347, 447–454. [Google Scholar] [CrossRef]
- Liu, D.; Andreasen, R.R.; Poulsen, T.G.; Feilberg, A. A comparative study of mass transfer coefficients of reduced volatile sulfur compounds for biotrickling filter packing materials. Chem. Eng. J. 2015, 260, 209–221. [Google Scholar] [CrossRef]
- Li, Y.Z.; Fan, Z.Y.; Shi, J.W.; Liu, Z.Y.; Shangguan, W.F. Post plasma-catalysis for VOCs degradation over different phase structure MnO2 catalysts. Chem. Eng. J. 2014, 241, 251–258. [Google Scholar] [CrossRef]
- Padhi, S.K.; Gokhale, S. Treatment of gaseous volatile organic compounds using a rotating biological filter. J. Bioresour. Technol. 2017, 244, 270–280. [Google Scholar] [CrossRef]
- Wang, J.D.; Chen, J.M. Removal of dichloromethane from waste gases with a bio-contact oxidation reactor. Chem. Eng. J. 2006, 123, 103–107. [Google Scholar]
- Zhou, Q.W.; Zhang, L.L.; Chen, J.M.; Xu, B.C.; Chu, G.W.; Chen, J.F. Performance and microbial analysis of two different inocula for the removal of chlorobenzene in biotrickling filters. Chem. Eng. J. 2016, 284, 174–181. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Cao, S.L.; Zhu, R.Y.; Chen, J.M.; Su, F. Analysis of characteristics and products of chlorobenzene degradation with dielectric barrier discharge. J. Environ. Sci. 2015, 36, 831–838. [Google Scholar]
- Nie, G.; Li, S.; Jiang, L.; Chen, J.; Ye, J. Chlorobenzene removal in the coupling system consisting of DBD with CuO/MnO2 and biotrickling filter. J. Acta Sci. Circumstantiae 2017, 37, 528–537. [Google Scholar]


| Treatment Technology | Advantages | Disadvantages | Application Field |
|---|---|---|---|
| Adsorption | High removal efficiency Low investment cost | Expensive of adsorbent Regular replacement | Spraying, petroleum, chemical, packaging printing, oil and gas recovery, paint, leather, etc. |
| Absorption | Mature technology Low investment cost | High cost of maintenance Wastewater treatment problems | Petrochemical, surface coating, packaging and printing, Electronics, etc. |
| Catalytic Combustion | High concentration of VOCs treatment Wide range of application | Expensive of catalyst High investment cost The formation of toxic by-products | Spraying, chemical, electronic, pharmaceutical, etc. |
| Condensation | High concentration of VOCs treatment; Recovery of single component | High investment cost Low exhaust gas flow requirement | Petrochemical; chemical industry; oil and gas recovery; Pharmaceutical, etc. |
| Membrane Separation | Recyclable components High efficiency | High cost Membrane pollution Low flux | Chemical, environmental protection, food, medicine, electronics, etc. |
| Biodegradation | Low energy consumption Low operation cost | Susceptible for activity of microorganisms High requirements for substrates | Sewage waste gas treatment, Low concentration of waste gas treatment occasions |
| Plasma Discharge | Suitable for refractory pollutants and high exhaust gas flow | Incomplete of mineralization of pollutants | Biological fermentation, chemical industry, textile printing and dyeing, etc. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Lv, J.; Xu, Q.; Cai, Y.; Yang, H.; Li, Y.; Yao, Y.; Wang, W.; Liu, N. Study of the Treatment of Organic Waste Gas Containing Benzene by a Low Temperature Plasma-Biological Degradation Method. Atmosphere 2022, 13, 622. https://doi.org/10.3390/atmos13040622
Li Y, Lv J, Xu Q, Cai Y, Yang H, Li Y, Yao Y, Wang W, Liu N. Study of the Treatment of Organic Waste Gas Containing Benzene by a Low Temperature Plasma-Biological Degradation Method. Atmosphere. 2022; 13(4):622. https://doi.org/10.3390/atmos13040622
Chicago/Turabian StyleLi, Yu, Jialin Lv, Qi Xu, Yalan Cai, Hailong Yang, Yingying Li, Yanyan Yao, Wenjuan Wang, and Nan Liu. 2022. "Study of the Treatment of Organic Waste Gas Containing Benzene by a Low Temperature Plasma-Biological Degradation Method" Atmosphere 13, no. 4: 622. https://doi.org/10.3390/atmos13040622
APA StyleLi, Y., Lv, J., Xu, Q., Cai, Y., Yang, H., Li, Y., Yao, Y., Wang, W., & Liu, N. (2022). Study of the Treatment of Organic Waste Gas Containing Benzene by a Low Temperature Plasma-Biological Degradation Method. Atmosphere, 13(4), 622. https://doi.org/10.3390/atmos13040622





