The Impact of Modifications in Forest Litter Inputs on Soil N2O Fluxes: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Meta-Analysis
3. Results
3.1. Effects of Litter Removal and Doubling on Soil N2O Fluxes of Forests
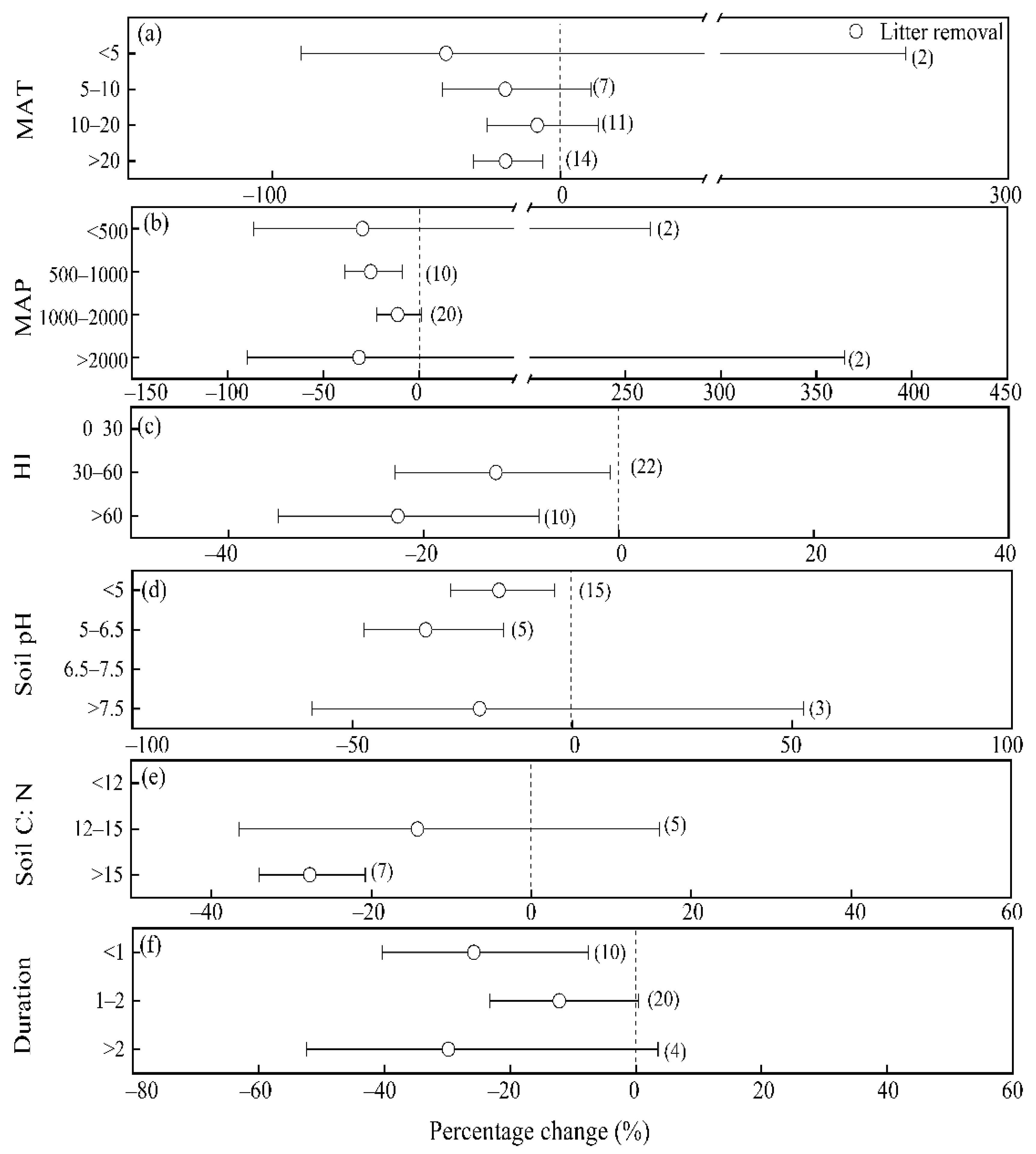
3.2. Influence of Climate Factors on Soil N2O Fluxes in Response to Litter Manipulations
3.3. Influence of Other Factors on Soil N2O Fluxes in Response to Litter Manipulations
4. Discussion
4.1. The Effect of Litter Removal and Doubling on Soil N2O Fluxes
4.2. Factors Affecting the Response of Soil N2O Fluxes to Changes in Litter Inputs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change: The physical science basis. In Working Group Į Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; pp. 915–918. [Google Scholar]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone–depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicerone, R.J. Changes in stratospheric ozone. Science 1987, 237, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. Changes in Atmospheric Constituents and in Radiative Forcing. In Climate Change 2007: The Physical Science Basis, Contribution of Working Group I to the 4th Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; p. 212. [Google Scholar]
- Zhou, W.J.; Ji, H.L.; Zhu, J.; Zhang, Y.P.; Sha, L.Q.; Liu, Y.T.; Zhang, X.; Zhao, W.; Dong, Y.X.; Bai, X.L.; et al. The effects of nitrogen fertilization on N2O emissions from a rubber plantation. Sci. Rep. 2016, 6, 28230. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.X.; Chen, G.X.; Wang, Z.P.; Cleemput, O.V.; Patrick, W.H.J. Methane and nitrous oxide emissions from a rice field in relation to soil redox and microbiological processes. Soil Sci. Soc. Am. J. 2000, 64, 2180–2186. [Google Scholar] [CrossRef]
- Song, Q.N.; Ouyang, M.; Yang, Q.P.; Lu, H.; Yang, G.Y.; Chen, F.S.; Shi, J.M. Degradation of litter quality and decline of soil nitrogen mineralization after moso bamboo (Phyllostachys pubscens) expansion to neighboring broadleaved forest in subtropical China. Plant Soil 2016, 404, 113–124. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Liu, Q.; Zheng, L.Y.; Wang, S.L.; Huang, L.J.; Jiang, J.; Wang, B.H.; Liu, X.J.; Li, X.D.; Hu, X.F.; et al. Litter removal enhances soil N2O emissions: Implications for management of leaf–harvesting Cinnamomum camphora plantations. For. Ecol. Manag. 2020, 466, 118121. [Google Scholar] [CrossRef]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. Camb. Philos. Soc. 2006, 81, 1–31. [Google Scholar] [CrossRef]
- Zhang, K.R.; Zhu, Q.A.; Liu, J.X.; Wang, M.; Zhou, X.L.; Li, M.X.; Wang, K.F.; Ding, J.H.; Peng, C.H. Spatial and temporal variations of N2O emissions from global forest and grassland ecosystems. Agric. For. Meteorol. 2019, 266–267, 129–139. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization of the United Nations. The State of Forest Ecosystems; The Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Orusa, T.; Mondino, E.B. Exploring Short-Term Climate Change Effects on Rangelands and Broad-Leaved Forests by Free Satellite Data in Aosta Valley (Northwest Italy). Climate 2021, 9, 47. [Google Scholar] [CrossRef]
- DeLucia, E.H.; Hamilton, J.G.; Naidu, S.L.; Thomas, R.B.; Andrews, J.A.; Finzi, A.; Lavine, M.; Matamala, R.; Mohan, J.E.; Hendrey, G.R.; et al. Net Primary Production of a Forest Ecosystem with Experimental CO2 Enrichment. Science 1999, 284, 1177–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.L.; Liu, Q. The dynamics of forest litter and its responses to global warming. Acta Ecol. Sin. 2004, 22, 1534–1544. [Google Scholar]
- Fanin, N.; Barantal, S.; Fromin, N.; Schimann, H.; Schevin, P.; Hattenschwiler, S. Distinct microbial limitations in litter and underlying soil revealed by carbon and nutrient fertilization in a tropical rainforest. PLoS ONE 2012, 7, e49990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.; Ball, T.; Conen, F.; Dobbie, K.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2003, 54, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.Z.; Lee, X.Q.; Zhou, Z.H.; Wang, B. The effects of litter layer and soil properties on the soil–atmosphere fluxes of greenhouse gases in karst forest, southwest China. Pol. J. Ecol. 2013, 61, 79–92. [Google Scholar]
- Butterbach–Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister, B.S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Phil. Trans. R. Soc. B 2013, 368, 91–97. [Google Scholar] [CrossRef]
- Walkiewicz, A.; Rafalska, A.; Bulak, P.; Bieganowski, A.; Osborne, B. How Can Litter Modify the Fluxes of CO2 and CH4 from Forest Soils? A Mini–Review. Forests 2021, 12, 1276. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, T.X.; Wang, G.L.; Duan, L.M.; Li, D.F. Effects of rainfall and litter on soil greenhouse gas fluxes in artificial poplar forest. J. Agro-Environ. Sci. 2019, 38, 1398–1407. [Google Scholar]
- Zhang, R.T.; Ni, H.W.; Liu, Y.N.; Fu, X.Y.; Wang, J.B. Response of greenhouse gas emission in the growing season to add and remove litter in Calamagrostis angustifolia Wetlands of Sanjiang Plain. Acta Sci. Circumstantiae 2020, 40, 1467–1475. [Google Scholar]
- Leitner, S.; Sae–Tun, O.; Kranzinger, L.; Zechmeister–Boltenstern, S.; Zimmermann, M. Contribution of litter layer to soil greenhouse gas emissions in a temperate beech forest. Plant Soil 2016, 403, 455–469. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.D.; Wang, H.M.; Wang, Z.L.; Ma, Z.Q.; Dai, X.Q.; Wen, X.F.; Liu, Y.F. Effect of litter layer on soil–atmosphere N2O flux of a subtropical pine plantation in China. Atmos. Environ. 2014, 82, 106–112. [Google Scholar] [CrossRef]
- Wieder, W.R.; Cleveland, C.C.; Townsend, A.R. Throughfall exclusion and leaf litter addition drive higher rates of soil nitrous oxide emissions from a lowland wet tropical forest. Glob. Chang. Biol. 2011, 17, 3195–3207. [Google Scholar] [CrossRef]
- Vasconcelos, S.S.; Zarin, D.J.; Capanu, M.; Littell, R.; Davidson, E.A.; Ishida, F.Y.; Santos, E.B.; Araujo, M.M.; Aragao, D.V.; Rangel–Vascolencos, L.G.T.; et al. Moisture and substrate availability constrain soil trace gas fluxes in an eastern Amazonian regrowth forest. Glob. Biogeochem. Cycles 2004, 18, GB2009. [Google Scholar] [CrossRef]
- Tang, X.L.; Liu, S.G.; Zhou, G.Y.; Zhang, D.Q.; Zhou, C.Y. Soil–atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Glob. Chang. Biol. 2006, 12, 546–560. [Google Scholar] [CrossRef]
- Yan, Y.P.; Sha, L.Q.; Cao, M.; Zheng, Z.; Tang, J.W.; Wang, Y.H.; Zhang, Y.P.; Wang, R.; Liu, G.R.; Wang, Y.S.; et al. Fluxes of CH4 and N2O from soil under a tropical seasonal rain forest in Xishuangbanna, Southwest China. J. Environ. Sci. 2008, 20, 207–215. [Google Scholar] [CrossRef]
- Gao, J.B.; Zhou, W.J.; Liu, Y.T.; Zhu, J.; Sha, L.Q.; Song, Q.H.; Ji, H.L.; Lin, Y.X.; Fei, X.H.; Bai, X.L.; et al. Effects of Litter Inputs on N2O Emissions from a Tropical Rainforest in Southwest China. Ecosystems 2018, 21, 1013–1026. [Google Scholar] [CrossRef]
- Yan, W.D.; Chen, X.Y.; Tian, D.L.; Peng, Y.Y.; Wang, G.J.; Zheng, W. Impacts of changed litter inputs on soil CO2 efflux in three forest types in central south China. Chin. Sci. Bull. 2013, 58, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A meta–analysis and review of plant–growth response to humic substances: Practical implications for agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar]
- Wang, S.G.; Auge, R.M.; Toler, H.D. Arbuscular mycorrhiza formation and its function under elevated atmospheric O3: A meta-analysis. Environ. Pollut. 2017, 226, 104–117. [Google Scholar] [CrossRef]
- Liao, C.Z.; Peng, R.H.; Luo, Y.Q.; Zhou, X.H.; Wu, X.W.; Fang, C.M.; Chen, J.K.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef]
- Wallace, B.C.; Lajeunesse, M.J.; Dietz, G.; Dahabreh, I.J.; Trikalinos, T.A.; Schmid, C.H.; Gurevitch, J. Open MEE: Intuitive, opensource software for meta–analysis in ecology and evolutionary biology. Methods Ecol. Evol. 2017, 8, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Huang, C.B.; Dong-Gill, K.; Shangguan, Z.P.; Wang, K.B.; Song, X.Z.; Peng, C.H. Soil GHG fluxes are altered by N deposition: New data indicate lower N stimulation of the N2O flux and greater stimulation of the calculated C pools. Glob. Chang. Biol. 2020, 26, 2613–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Deng, S.N.; Ren, Y.Y.; Liang, T.; Yu, K.K.; Zou, J.L.; Liu, F. Response of soil respiration to surface litter input based on a meta–analysis. Ecol. Environ. Sci. 2020, 29, 447–456. [Google Scholar]
- Chen, X.; Chen, H.Y.H. Global effects of plant litter alterations on soil CO2 to the atmosphere. Glob. Chang. Biol. 2018, 24, 3462–3471. [Google Scholar] [CrossRef]
- Xiao, R.H.; Man, X.L.; Duan, B.X.; Cai, T.J. Short–Term Litter Manipulations have Strong Impact on Soil Nitrogen Dynamics in Larix gmelinii Forest of Northeast China. Forests 2020, 11, 1205. [Google Scholar] [CrossRef]
- Breithaupt, J.L.; Duga, E.; Witt, M.; Filyaw, R.; Friedland, N.; Donnelly, M.J.; Walters, L.J.; Chambers, L.G. Carbon and nutrient fluxes from seagrass and mangrove wrack are mediated by soil interactions. Estuar. Coast. Shelf Sci. 2019, 229, 106409. [Google Scholar] [CrossRef]
- Jia, B.R.; Xu, Z.Z.; Zhou, G.S.; Yin, X.J. Statistical characteristics of forest litterfall in China. Sci. China Life Sci. 2018, 61, 358–360. [Google Scholar] [CrossRef]
- Hu, X.K.; Liu, L.L.; Zhu, B.; Du, E.Z.; Hu, X.Y.; Li, P.; Zhou, Z.; Ji, C.J.; Zhu, J.L.; Shen, H.H.; et al. Asynchronous responses of soil carbon dioxide, nitrous oxide emissions and net nitrogen mineralization to enhanced fine root input. Soil Biol. Biochem. 2016, 92, 67–78. [Google Scholar] [CrossRef]
- Eickenscheidt, N.; Brumme, R. Regulation of N2O and NOx emission patterns in six acidtemperate beech forest soils by soil gas diffusivity, N turnover, and atmospheric NOx concentrations. Plant Soil. 2013, 369, 515–529. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.X.; Sun, Z.L.; Yin, X.; Ma, Y.L.; Ju, X.T.; Zhang, L.J. Influence of solute carbon on N2O and CO2 emissions from soil of typical farm–land in north China. J. Soil Water Conserv. 2013, 27, 222–227. [Google Scholar]
- Tian, Y.N.; Zhang, S.Q.; Lin, S.; Shaaban, M.; He, Z.L. Influence of Soluble Carbon and Nitrogen Additions on N2O and CO2 Emissions from Two Soils with Different Organic Carbon Content. J. Agro-Environ. Sci. 2015, 34, 2410–2417. [Google Scholar]
- Liu, H.; Zhao, P.; Lin, Y.B.; Rao, X.Q. CH4 and N2O Fluxes from Soil Surface of 2 Land Use in a Hilly Area of South China. J. Trop. Subtrop. Bot. 2008, 16, 304–314. [Google Scholar]
- Zhang, C.L.; Xu, J.M. Effect of organic and inorganic fertilizer application on the bioindicators of soil quality. J. Guangxi Agric. Biol. Sci. 2004, 23, 81–85. [Google Scholar]
- Lohnis, F. Nitrogen availability of green manures. Soil Sci. 1926, 22, 253–290. [Google Scholar] [CrossRef]
- Chen, H.H.; Li, X.C.; Hu, F.; Shi, W. Soil nitrous oxide emissions following crop residue addition: A meta analysis. Glob. Chang. Biol. 2013, 19, 2956–2964. [Google Scholar] [CrossRef]
- Liu, R.P.; Mao, Z.J.; Li, X.H.; Sun, T.; Li, N.; Lü, H.L.; Liu, C.Z. Effects of simulated temperature increase and vary little quality on litter decomposition. Acta Ecol. Sin. 2013, 33, 5661–5667. [Google Scholar]
- Werner, C.; Butterbach–Bahl, K.; Haas, E.; Hickler, T.; Kiese, R. A global inventory of N2O emissions from tropical rainforest soils using a detailed biogeochemical model. Glob. Biogeochem. Cycle 2007, 21, GB3010. [Google Scholar] [CrossRef]
- Tang, J.W.; Cao, M.; Zhang, J.H.; Li, M.H. Litterfall production, decomposition and nutrient use efficiency varies with tropical forest types in Xishuangbanna, SW China: A 10–year study. Plant Soil 2010, 335, 271–288. [Google Scholar] [CrossRef]
- Zinke, P.J.; Stangenberger, A.G.; Post, W.M.; Emanuel, W.R.; Olson, J.S. Worldwide Organic Soil Carbon and Nitrogen Data ORNL/TM-8857; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1984. [Google Scholar]
- Zhang, W.; Mo, J.M.; Yu, G.R.; Fang, Y.T.; Li, D.J.; Lu, X.K.; Wang, H. Emissions of nitrous oxide from three tropical forests in Southern China in response to simulated nitrogen deposition. Plant Soil 2008, 306, 221–236. [Google Scholar] [CrossRef]
- Post, W.M.; Pastor, J.; Zinke, P.J.; Stangenberger, A.G. Global patterns of soil nitrogen storage. Nature 1985, 317, 613–616. [Google Scholar] [CrossRef]
- Chen, S.D. Effects of Simulated Nitrogen Deposition on N2O Emission from Midsubtropical Forest Soils; Fujian Normal University: Fuzhou, China, 2012. [Google Scholar]
- Du, E.Z.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.H.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Zhong, H.T.; Smith, C.; Robinson, B.; Kim, Y.N.; Dickinson, N. Plant litter variability and soil N mobility. Soil Res. 2016, 55, 253–263. [Google Scholar] [CrossRef]
- Peichl, M.; Arain, M.A.; Ullah, S.; Moore, T. Carbon dioxide, methane, and nitrous oxide exchanges in an age–sequence of temperate pine forests. Glob. Chang. Biol. 2010, 16, 2198–2212. [Google Scholar] [CrossRef]
- Zhang, Z.F. Study on Decomposition Characteristics of Litter and Its Effect on Soil; South China Agricultural University: Guangzhou, China, 2006. [Google Scholar]
- Ge, S.F.; Xu, H.G.; Ji, M.M.; Jiang, Y.M. Effects of soil C:N on growth and distribution of nitrogen and carbon of Malus hupehensis seedlings. Chin. J. Plant Ecol. 2013, 37, 942–949. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Meng, D.; Osborne, B.; Fan, Y.; Zou, J. The Impact of Modifications in Forest Litter Inputs on Soil N2O Fluxes: A Meta-Analysis. Atmosphere 2022, 13, 742. https://doi.org/10.3390/atmos13050742
Zhou Y, Meng D, Osborne B, Fan Y, Zou J. The Impact of Modifications in Forest Litter Inputs on Soil N2O Fluxes: A Meta-Analysis. Atmosphere. 2022; 13(5):742. https://doi.org/10.3390/atmos13050742
Chicago/Turabian StyleZhou, Yuting, Delong Meng, Bruce Osborne, Yue Fan, and Junliang Zou. 2022. "The Impact of Modifications in Forest Litter Inputs on Soil N2O Fluxes: A Meta-Analysis" Atmosphere 13, no. 5: 742. https://doi.org/10.3390/atmos13050742






