Soil Enzyme Activity Regulates the Response of Soil C Fluxes to N Fertilization in a Temperate Cultivated Grassland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Measurement of Soil C Fluxes and Auxiliary Variables
2.3. Measurement of Soil Dissolved C and N Concentrations and pH
2.4. Measurement of Soil Enzyme Activities
2.5. Statistical Analyses
3. Results
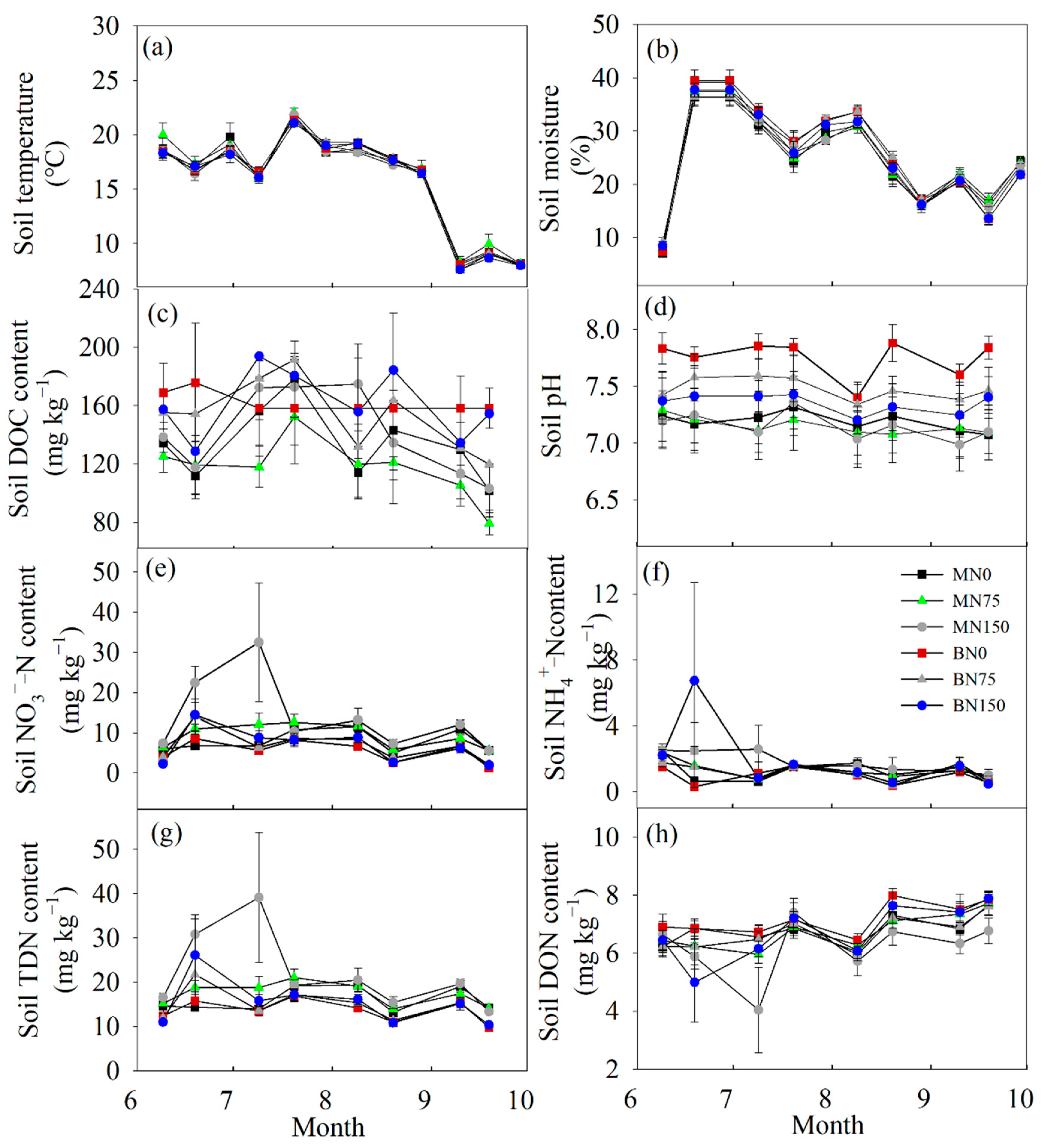
3.1. Soil General Properties
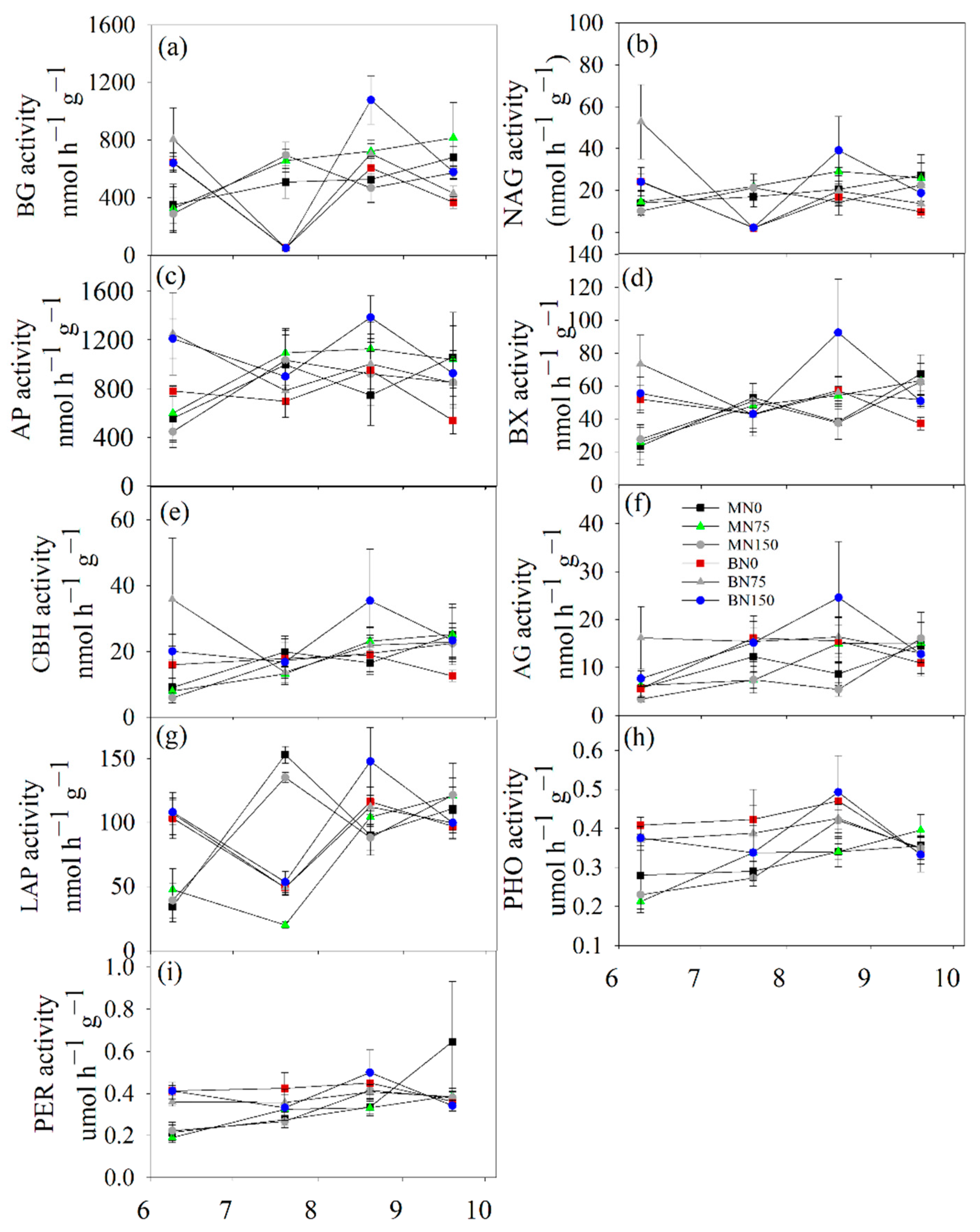
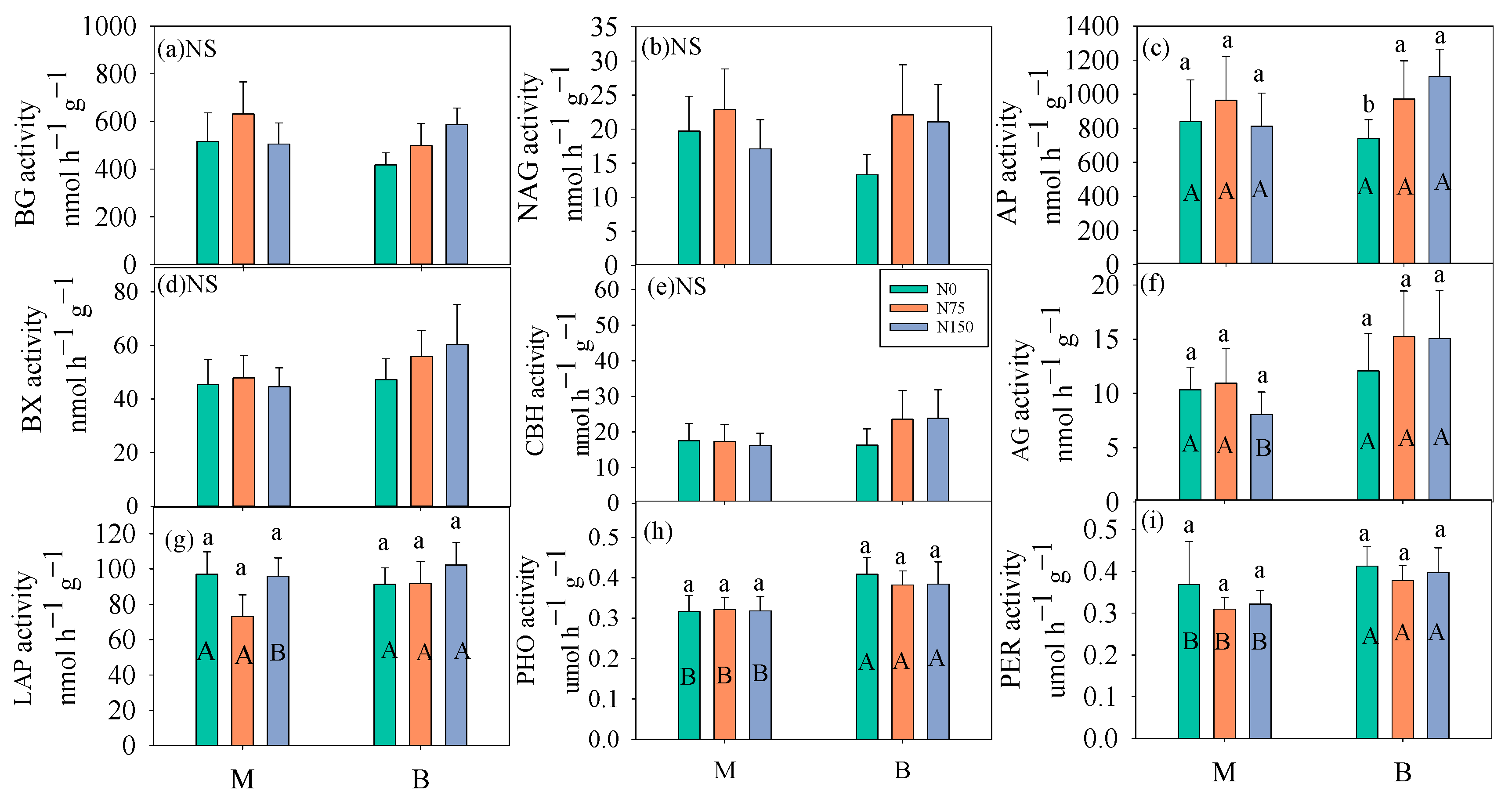
3.2. Soil Enzyme Activity
3.3. Soil C Fluxes
3.4. Relationships between Soil C Fluxes and Soil Properties
4. Discussion
4.1. The Response of Soil Main Properties to Each Treatment
4.2. The Response of Soil C Fluxes to N Fertilization
4.3. The Response of Soil C Fluxes to Enzyme Activities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.R.; Allen, R.B.; Clinton, P.W. The influence of N addition on nutrient content, leaf carbon isotope ratio, and productivity in a Nothofagus forest during stand development. Can. J. For. Res. 2004, 34, 2037–2048. [Google Scholar] [CrossRef]
- Stevens, C.J.; Dise, N.B.; Mountford, J.O.; Gowing, D.J. Impact of nitrogen deposition on the species richness of grasslands. Science 2004, 303, 1876–1879. [Google Scholar] [CrossRef] [Green Version]
- Friend, A.L.; Eide, M.R.; Hinckley, T.M. Nitrogen stress alters root proliferation in Douglas-fir seedlings. Can. J. For. Res. 1990, 20, 1524–1529. [Google Scholar] [CrossRef]
- Aerts, R.; de Caluwe, H. Nitrogen deposition effects on carbon dioxide and methane emissions from temperate peatland soils. Oikos 1999, 84, 44–54. [Google Scholar] [CrossRef]
- Sutton, M.A.; Simpson, D.; Levy, P.E.; Smith, R.I.; Reis, S.; Van Oijen, M.; De Vries, W. Uncertainties in the relationship between atmospheric nitrogen deposition and forest carbon sequestration. Glob. Chang. Biol. 2008, 14, 2057–2063. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.Q.; Canham, C.D.; Weathers, K.C.; Goodale, C.L. Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci. 2010, 3, 13–17. [Google Scholar] [CrossRef]
- Tian, H.; Lu, C.; Yang, J.; Banger, K.; Huntzinger, D.N.; Schwalm, C.R.; Michalak, A.M.; Cook, R.; Ciais, P.; Hayes, D. Global patterns and controls of soil organic carbon dynamics as simulated by multiple terrestrial biosphere models: Current status and future directions. Glob. Biogeochem. Cycles 2015, 29, 775–792. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Dong, Y.; Qi, Y.; Xiao, S.; He, Y.; Ma, T. Effects of nitrogen fertilization on soil respiration in temperate grassland in Inner Mongolia, China. Environ. Earth Sci. 2011, 62, 1163–1171. [Google Scholar] [CrossRef]
- Yamulki, S.; Jarvis, S. Short-term effects of tillage and compaction on nitrous oxide, nitric oxide, nitrogen dioxide, methane and carbon dioxide fluxes from grassland. Biol. Fertil. Soils 2002, 36, 224–231. [Google Scholar] [CrossRef]
- Khalil, M.; Rasmussen, R. Atmospheric methane: Trends over the last 10,000 years. Atmos. Environ. 1987, 21, 2445–2452. [Google Scholar] [CrossRef]
- Dutaur, L.; Verchot, L.V. A global inventory of the soil CH4 sink. Glob. Biogeochem. Cycles 2007, 21, GB4013. [Google Scholar] [CrossRef]
- Chen, W.; Wolf, B.; Zheng, X.; Yao, Z.; BUTTERBACH-BAHL, K.; Brueggemann, N.; Liu, C.; Han, S.; Han, X. Annual methane uptake by temperate semiarid steppes as regulated by stocking rates, aboveground plant biomass and topsoil air permeability. Glob. Chang. Biol. 2011, 17, 2803–2816. [Google Scholar] [CrossRef]
- Rogger, J.; Hörtnagl, L.; Buchmann, N.; Eugster, W. Carbon dioxide fluxes of a mountain grassland: Drivers, anomalies and annual budgets. Agric. For. Meteorol. 2022, 314, 108801. [Google Scholar] [CrossRef]
- Poeplau, C.; Zopf, D.; Greiner, B.; Geerts, R.; Korvaar, H.; Thumm, U.; Don, A.; Heidkamp, A.; Flessa, H. Why does mineral fertilization increase soil carbon stocks in temperate grasslands? Agric. Ecosyst. Environ. 2018, 265, 144–155. [Google Scholar] [CrossRef]
- Scurlock, J.; Hall, D. The global carbon sink: A grassland perspective. Glob. Chang. Biol. 1998, 4, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Soussana, J.F.; Loiseau, P.; Vuichard, N.; Ceschia, E.; Balesdent, J.; Chevallier, T.; Arrouays, D. Carbon cycling and sequestration opportunities in temperate grasslands. Soil Use Manag. 2004, 20, 219–230. [Google Scholar] [CrossRef]
- Collins, S.L.; Sinsabaugh, R.L.; Crenshaw, C.; Green, L.; Porras-Alfaro, A.; Stursova, M.; Zeglin, L.H. Pulse dynamics and microbial processes in aridland ecosystems. J. Ecol. 2008, 96, 413–420. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, L.; Li, L.; Li, A.; Wu, M.; Wan, S. Diversity-dependent stability under mowing and nutrient addition: Evidence from a 7-year grassland experiment. Ecol. Lett. 2012, 15, 619–626. [Google Scholar] [CrossRef]
- Xu, L.; Xu, X.; Tang, X.; Xin, X.; Ye, L.; Yang, G.; Tang, H.; Lv, S.; Xu, D.; Zhang, Z. Managed grassland alters soil N dynamics and N2O emissions in temperate steppe. J. Environ. Sci. 2018, 66, 20–30. [Google Scholar] [CrossRef]
- Jones, S.K.; Helfter, C.; Anderson, M.; Coyle, M.; Campbell, C.; Famulari, D.; Marco, C.D.; Dijk, N.v.; Tang, Y.S.; Topp, C.F. The nitrogen, carbon and greenhouse gas budget of a grazed, cut and fertilised temperate grassland. Biogeosciences 2017, 14, 2069–2088. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.; Rees, R.; Skiba, U.; Ball, B. Greenhouse gas emissions from a managed grassland. Glob. Planet. Chang. 2005, 47, 201–211. [Google Scholar] [CrossRef]
- Ambus, P.; Robertson, G. The effect of increased N deposition on nitrous oxide, methane and carbon dioxide fluxes from unmanaged forest and grassland communities in Michigan. Biogeochemistry 2006, 79, 315–337. [Google Scholar] [CrossRef]
- Jiang, C.; Yu, G.; Fang, H.; Cao, G.; Li, Y. Short-term effect of increasing nitrogen deposition on CO2, CH4 and N2O fluxes in an alpine meadow on the Qinghai-Tibetan Plateau, China. Atmos. Environ. 2010, 44, 2920–2926. [Google Scholar] [CrossRef]
- Li, K.; Gong, Y.; Song, W.; He, G.; Hu, Y.; Tian, C.; Liu, X. Responses of CH4, CO2 and N2O fluxes to increasing nitrogen deposition in alpine grassland of the Tianshan Mountains. Chemosphere 2012, 88, 140–143. [Google Scholar] [CrossRef]
- Kammann, C.; Grünhage, L.; Jäger, H.-J.; Wachinger, G. Methane fluxes from differentially managed grassland study plots: The important role of CH4 oxidation in grassland with a high potential for CH4 production. Environ. Pollut. 2001, 115, 261–273. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Hu, H.-W.; Han, H.-Y.; Du, Y.; Wan, S.-Q.; Xu, Z.-W.; Chen, B.-D. Abundance and community structure of ammonia-oxidizing Archaea and Bacteria in response to fertilization and mowing in a temperate steppe in Inner Mongolia. FEMS Microbiol. Ecol. 2014, 89, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Carreiro, M.; Sinsabaugh, R.; Repert, D.; Parkhurst, D. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 2000, 81, 2359–2365. [Google Scholar] [CrossRef]
- Neff, J.C.; Townsend, A.R.; Gleixner, G.; Lehman, S.J.; Turnbull, J.; Bowman, W.D. Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 2002, 419, 915–917. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Zeglin, L.H.; Stursova, M.; Sinsabaugh, R.L.; Collins, S.L. Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 2007, 154, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Keeler, B.L.; Hobbie, S.E.; Kellogg, L.E. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: Implications for litter and soil organic matter decomposition. Ecosystems 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, T.; Guo, R.; Cao, H.; Shi, L.; Guo, J.; Sun, W. Response of soil enzyme activity to warming and nitrogen addition in a meadow steppe. Soil Res. 2015, 53, 242–252. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Gao, Y.; Xing, F.; Sun, S.; Zhang, T.; Zhu, X.; Chen, C.; Li, Z. Responses of soil extracellular enzyme activities and microbial community properties to interaction between nitrogen addition and increased precipitation in a semi-arid grassland ecosystem. Sci. Total Environ. 2020, 703, 134691. [Google Scholar] [CrossRef]
- Xu, M.; Xu, L.; Fang, H.; Cheng, S.; Yu, G.; Yang, Y.; Lu, M. Alteration in enzymatic stoichiometry controls the response of soil organic carbon dynamic to nitrogen and water addition in temperate cultivated grassland. Eur. J. Soil Biol. 2020, 101, 103248. [Google Scholar] [CrossRef]
- Kardol, P.; Cregger, M.A.; Campany, C.E.; Classen, A.T. Soil ecosystem functioning under climate change: Plant species and community effects. Ecology 2010, 91, 767–781. [Google Scholar] [CrossRef]
- Liu, W.; Tian, R.; Peng, Z.; Yang, S.; Liu, X.; Yang, Y.; Zhang, W.; Liu, L. Nonlinear responses of the Vmax and Km of hydrolytic and polyphenol oxidative enzymes to nitrogen enrichment. Soil Biol. Biochem. 2020, 141, 107656. [Google Scholar] [CrossRef]
- Koch, O.; Tscherko, D.; Kandeler, E. Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Glob. Biogeochem. Cycles 2007, 21, GB4017. [Google Scholar] [CrossRef]
- Chen, S.; Hao, T.; Goulding, K.; Misselbrook, T.; Liu, X. Impact of 13-years of nitrogen addition on nitrous oxide and methane fluxes and ecosystem respiration in a temperate grassland. Environ. Pollut. 2019, 252, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Cheng, S.; Yu, G.; Cooch, J.; Wang, Y.; Xu, M.; Li, L.; Dang, X.; Li, Y. Low-level nitrogen deposition significantly inhibits methane uptake from an alpine meadow soil on the Qinghai–Tibetan Plateau. Geoderma 2014, 213, 444–452. [Google Scholar] [CrossRef]
- Lu, M.; Yang, Y.; Luo, Y.; Fang, C.; Zhou, X.; Chen, J.; Yang, X.; Li, B. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis. New Phytol. 2011, 189, 1040–1050. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, S.; Fang, H.; Yu, G.; Xu, X.; Xu, M.; Wang, L.; Li, X.; Si, G.; Geng, J. Contrasting effects of ammonium and nitrate inputs on soil CO2 emission in a subtropical coniferous plantation of southern China. Biol. Fertil. Soils 2015, 51, 815–825. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, W.; Yang, H.; Yang, H.; Chen, G.; Luo, Y.; Fang, H.; Li, S. Effects of nitrogen addition on soil inorganic N content and soil N mineralization of a cold-temperate coniferous forest in Great Xing’an Mountains. Acta Ecol. Sin. 2015, 35, 130–136. [Google Scholar] [CrossRef]
- Malik, A.; Gleixner, G. Importance of microbial soil organic matter processing in dissolved organic carbon production. FEMS Microbiol. Ecol. 2013, 86, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huygens, D.; Díaz, S.; Urcelay, C.; Boeckx, P. Microbial recycling of dissolved organic matter confines plant nitrogen uptake to inorganic forms in a semi-arid ecosystem. Soil Biol. Biochem. 2016, 101, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Ladd, J. Properties of proteolytic enzymes extracted from soil. Soil Biol. Biochem. 1972, 4, 227–237. [Google Scholar] [CrossRef]
- Hofmockel, K.S.; Fierer, N.; Colman, B.P.; Jackson, R.B. Amino acid abundance and proteolytic potential in North American soils. Oecologia 2010, 163, 1069–1078. [Google Scholar] [CrossRef]
- Yahdjian, L.; Gherardi, L.; Sala, O.E. Nitrogen limitation in arid-subhumid ecosystems: A meta-analysis of fertilization studies. J. Arid Environ. 2011, 75, 675–680. [Google Scholar] [CrossRef]
- Hawkins, H.-J.; Wolf, G.; Stock, W.D. Cluster roots of Leucadendron laureolum (Proteaceae) and Lupinus albus (Fabaceae) take up glycine intact: An adaptive strategy to low mineral nitrogen in soils? Ann. Bot. 2005, 96, 1275–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Carrillo, Y.; Pendall, E.; Dijkstra, F.A.; Evans, R.D.; Morgan, J.A.; Williams, D.G. Soil microbes compete strongly with plants for soil inorganic and amino acid nitrogen in a semiarid grassland exposed to elevated CO2 and warming. Ecosystems 2015, 18, 867–880. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.; He, H.; Cheng, W.; Bai, Z.; Wang, Y.; Zhang, X. Factors controlling soil organic carbon stability along a temperate forest altitudinal gradient. Sci. Rep. 2016, 6, 18783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, I.; Lee, S.; Hong, J.-H.; Kang, H. Methane oxidation rates in forest soils and their controlling variables: A review and a case study in Korea. Ecol. Res. 2006, 21, 849–854. [Google Scholar] [CrossRef]
- Fang, H.; Yu, G.; Cheng, S.; Zhu, T.; Wang, Y.; Yan, J.; Wang, M.; Cao, M.; Zhou, M. Effects of multiple environmental factors on CO2 emission and CH4 uptake from old-growth forest soils. Biogeosciences 2010, 7, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Nyerges, G.; Stein, L.Y. Ammonia cometabolism and product inhibition vary considerably among species of methanotrophic bacteria. FEMS Microbiol. Lett. 2009, 297, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Whalen, S.; Reeburgh, W. Effect of nitrogen fertilization on atmospheric methane oxidation in boreal forest soils. Chemosphere-Glob. Chang. Sci. 2000, 2, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Nanba, K.; King, G.M. Response of atmospheric methane consumption by Maine forest soils to exogenous aluminum salts. Appl. Environ. Microbiol. 2000, 66, 3674–3679. [Google Scholar] [CrossRef] [Green Version]
- Tamai, N.; Takenaka, C.; Ishizuka, S. Water-soluble Al inhibits methane oxidation at atmospheric concentration levels in Japanese forest soil. Soil Biol. Biochem. 2007, 39, 1730–1736. [Google Scholar] [CrossRef]
- Xu, X.; Inubushi, K. Effects of N sources and methane concentrations on methane uptake potential of a typical coniferous forest and its adjacent orchard soil. Biol. Fertil. Soils 2004, 40, 215–221. [Google Scholar] [CrossRef]
- Acton, S.; Baggs, E. Interactions between N application rate, CH4 oxidation and N2O production in soil. Biogeochemistry 2011, 103, 15–26. [Google Scholar] [CrossRef]
- Jassal, R.S.; Black, T.A.; Roy, R.; Ethier, G. Effect of nitrogen fertilization on soil CH4 and N2O fluxes, and soil and bole respiration. Geoderma 2011, 162, 182–186. [Google Scholar] [CrossRef]
- Barton, L.; Thamo, T.; Engelbrecht, D.; Biswas, W.K. Does growing grain legumes or applying lime cost effectively lower greenhouse gas emissions from wheat production in a semi-arid climate? J. Clean. Prod. 2014, 83, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tong, X.-j.; Yu, Q. Methane uptake and oxidation by unsaturated soil. Acta Ecol. Sin 2005, 25, 141–147. [Google Scholar]
- Raji, S.G.; Dörsch, P. Effect of legume intercropping on N2O emissions and CH4 uptake during maize production in the Great Rift Valley, Ethiopia. Biogeosciences 2020, 17, 345–359. [Google Scholar] [CrossRef] [Green Version]
- Naudin, C.; Corre-Hellou, G.; Pineau, S.; Crozat, Y.; Jeuffroy, M.-H. The effect of various dynamics of N availability on winter pea–wheat intercrops: Crop growth, N partitioning and symbiotic N2 fixation. Field Crops Res. 2010, 119, 2–11. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, L.; Fang, H.; Yu, G.; Yang, X.; Li, X.; Si, G.; Geng, J.; He, S.; Yu, G. Nonlinear responses of soil nitrous oxide emission to multi-level nitrogen enrichment in a temperate needle-broadleaved mixed forest in Northeast China. Catena 2016, 147, 556–563. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Saiya-Cork, K.; Sinsabaugh, R.; Zak, D. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Bodelier, P.L.; Frenzel, P. Contribution of methanotrophic and nitrifying bacteria to CH4 and NH4+ oxidation in the rhizosphere of rice plants as determined by new methods of discrimination. Appl. Environ. Microbiol. 1999, 65, 1826–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Meng, H.; Gu, J.-D. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Floch, C.; Alarcon-Gutiérrez, E.; Criquet, S. ABTS assay of phenol oxidase activity in soil. J. Microbiol. Methods 2007, 71, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Gu, J.-D. Seasonal variability of extracellular enzymes involved in carbon mineralization in sediment of a subtropical mangrove wetland. Geomicrobiol. J. 2015, 32, 68–76. [Google Scholar] [CrossRef]
- Whitaker, J.; Ostle, N.; Nottingham, A.T.; Ccahuana, A.; Salinas, N.; Bardgett, R.D.; Meir, P.; McNamara, N.P. Microbial community composition explains soil respiration responses to changing carbon inputs along an A ndes-to-A mazon elevation gradient. J. Ecol. 2014, 102, 1058–1071. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-R.; Delgado-Baquerizo, M.; Wang, J.-T.; Hu, H.-W.; Yang, Z.; He, J.-Z. New insights into the role of microbial community composition in driving soil respiration rates. Soil Biol. Biochem. 2018, 118, 35–41. [Google Scholar] [CrossRef] [Green Version]
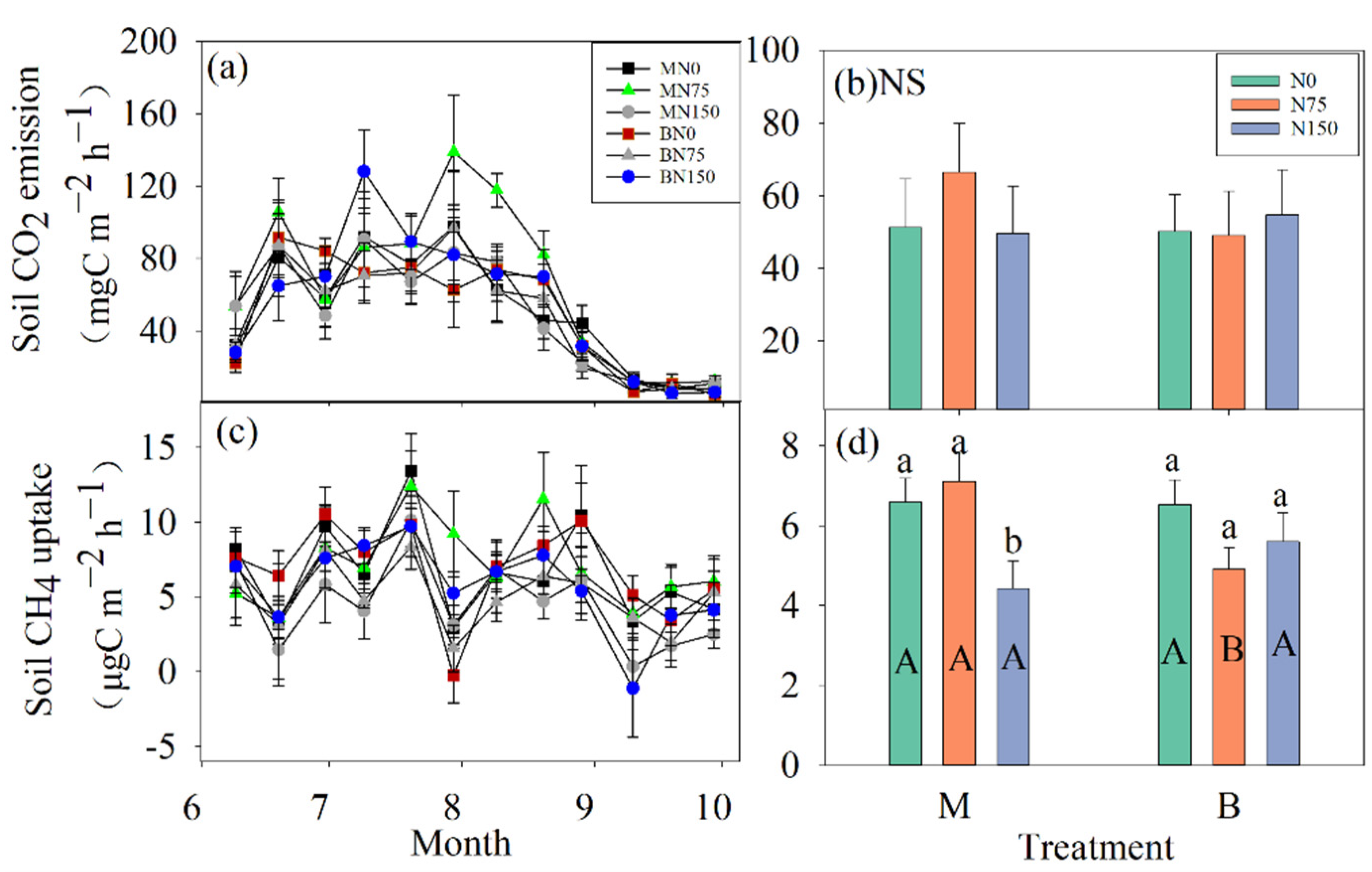
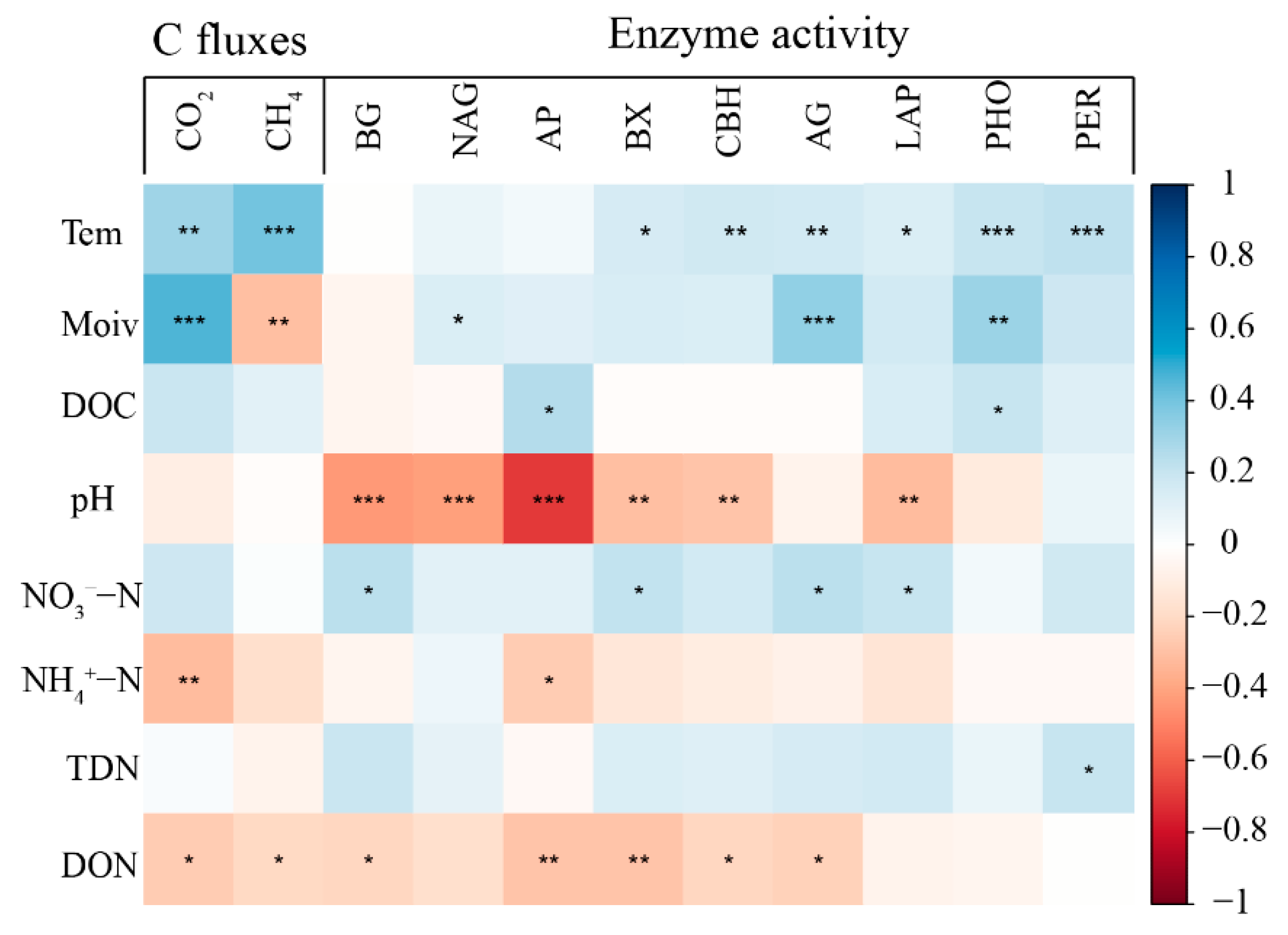
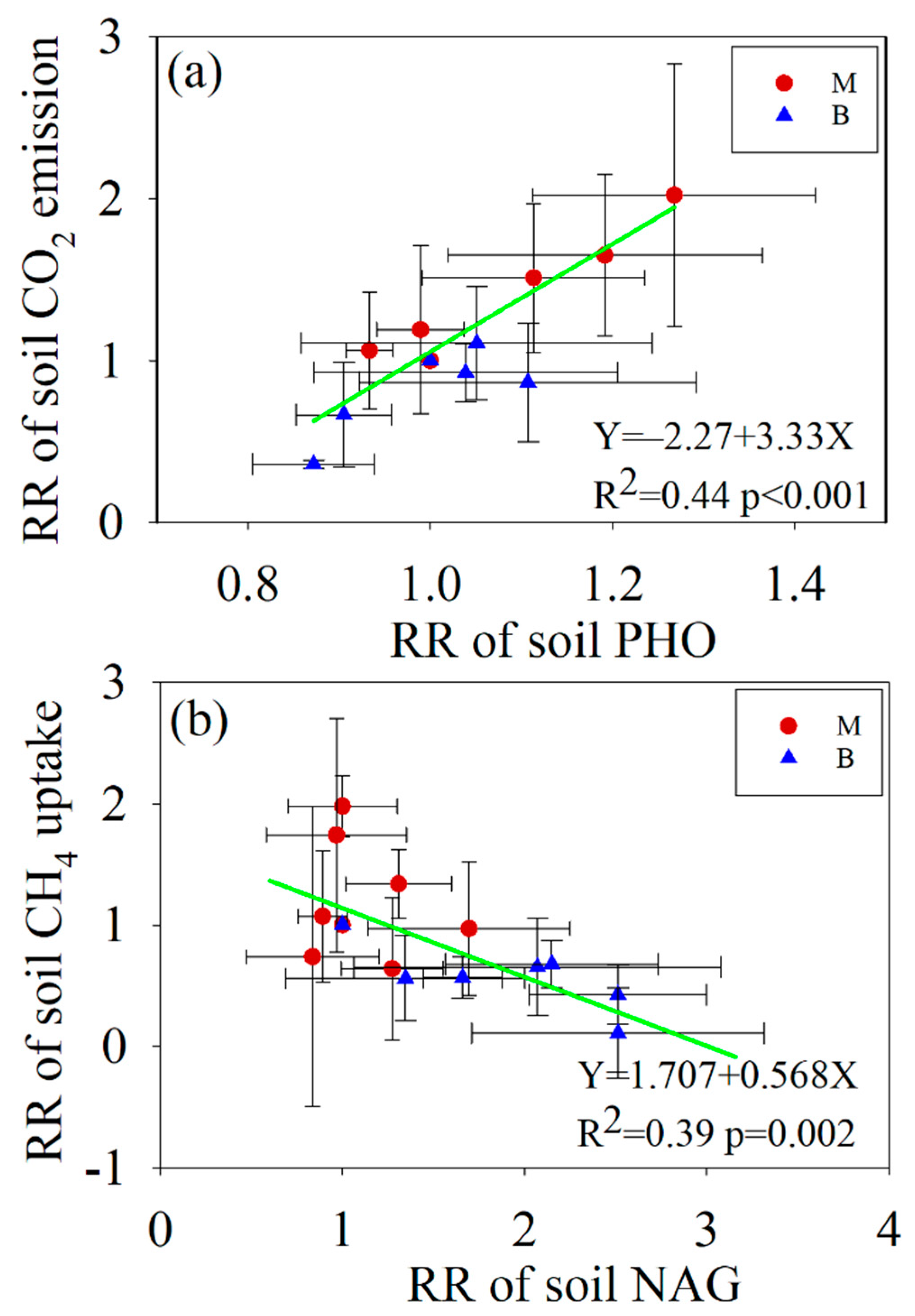
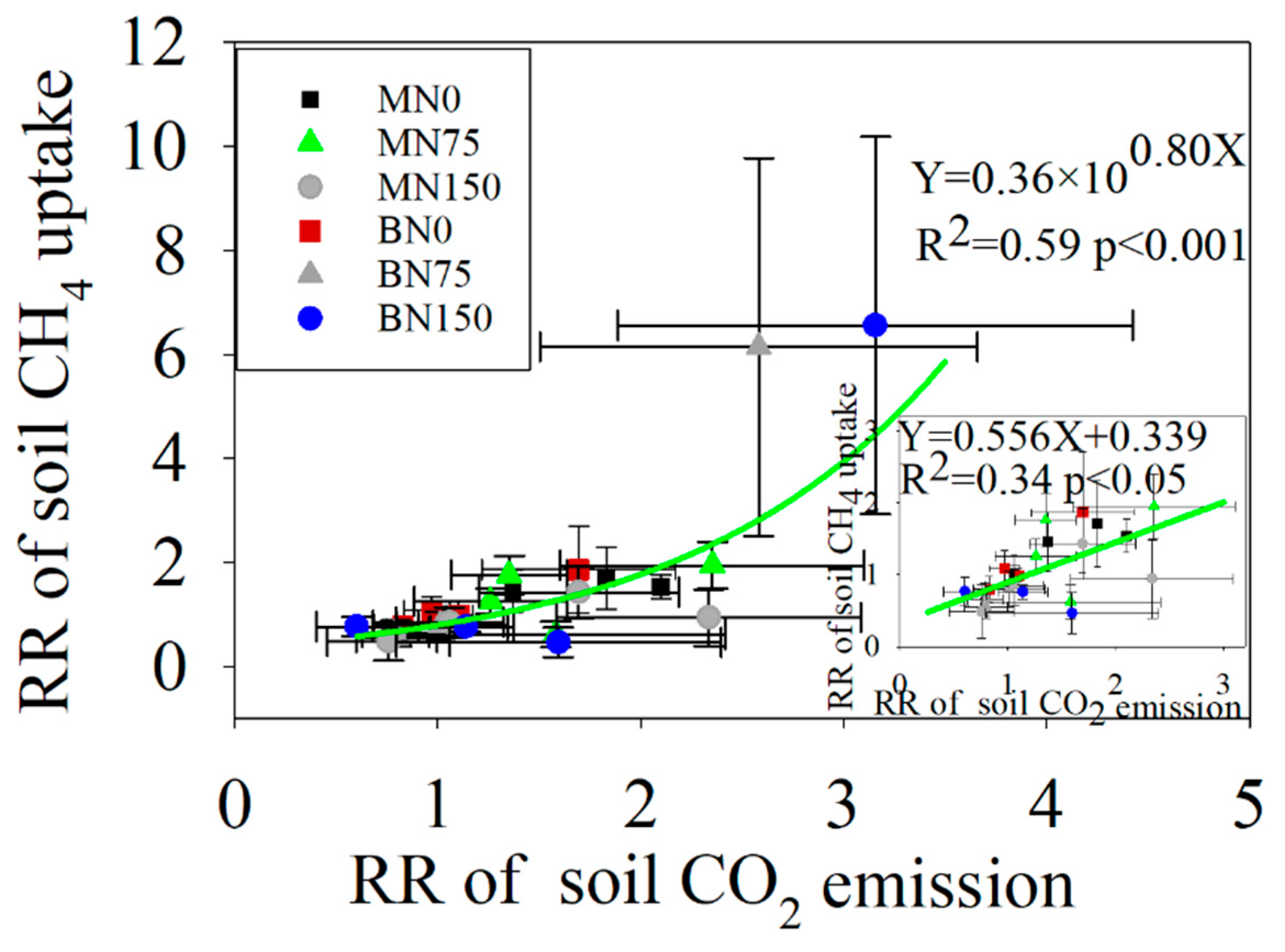
| Treatment | Ts † | Ms † | DOC | pH | NO3−–N | NH4+–N | TDN | DON |
|---|---|---|---|---|---|---|---|---|
| (℃) | (%) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | ||
| MN0 | 15.81 ± 1.10 aA | 25.52 ± 1.10 aA | 136.22 ± 12.52 aA | 7.76 ± 0.07 aA | 7.67 ± 0.49 bA | 1.23 ± 0.09 aA | 15.75 ± 0.49 bA | 6.85 ± 0.19 aA |
| MN75 | 16.07 ± 1.13 aA | 23.65 ± 1.07 aB | 103.06 ± 8.64 bB | 7.41 ± 0.10 bA | 8.96 ± 0.72 abA | 1.30 ± 0.13 aA | 17.01 ± 0.78 abA | 6.80 ± 0.20 aA |
| MN150 | 15.62 ± 1.11 aA | 24.30 ± 1.19 aA | 140.56 ± 11.66 aA | 7.24 ± 0.10 bA | 10.26 ± 1.02 aA | 1.15 ± 0.24 aA | 19.38 ± 1.44 aA | 6.80 ± 0.23 aA |
| BN0 | 15.79 ± 1.11 aA | 26.61 ± 1.59 aA | 152.91 ± 17.60 aA | 7.39 ± 0.13 aA | 5.29 ± 0.51 aB | 0.93 ± 0.21 aA | 13.80 ± 0.74 aB | 7.27 ± 0.22 aA |
| BN75 | 15.71 ± 1.13 aA | 26.19 ± 1.24 aA | 160.76 ± 20.58 aA | 7.05 ± 0.17 aB | 6.27 ± 0.69 aB | 1.05 ± 0.18 aA | 14.19 ± 0.70 aB | 6.87 ± 0.16 abA |
| BN150 | 15.61 ± 1.14 aA | 25.70 ± 1.43 aA | 165.32 ± 17.29 aA | 7.29 ± 0.13 aA | 5.83 ± 0.57 aB | 1.15 ± 0.16 aA | 13.56 ± 0.60 aB | 6.58 ± 0.19 bA |
| Extracellular Enzyme Activity | C Fluxes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of Variation | BG | NAG | AP | BX | CBH | AG | LAP | PHO | PER | CO2 | CH4 |
| Between subjects | |||||||||||
| Pasture | 0.396 | 0.753 | 0.653 | 0.090 | 0.128 | 0.019 | 0.426 | 0.007 | 0.041 | 0.383 | 0.140 |
| Nitrogen | 0.356 | 0.373 | 0.553 | 0.528 | 0.502 | 0.629 | 0.242 | 0.911 | 0.476 | 0.499 | 0.561 |
| Nitrogen × Pasture | 0.286 | 0.472 | 0.546 | 0.503 | 0.349 | 0.457 | 0.468 | 0.849 | 0.914 | 0.291 | 0.362 |
| within subjects (Multivariate) | |||||||||||
| Month | <0.001 | <0.001 | 0.049 | 0.027 | 0.073 | 0.013 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| Month × Pasture | <0.001 | 0.001 | 0.001 | 0.001 | 0.175 | 0.050 | <0.001 | 0.003 | 0.016 | 0.766 | 0.764 |
| Month × Nitrogen | 0.794 | 0.63 | 0.874 | 0.904 | 0.551 | 0.675 | 0.01 | 0.282 | 0.585 | 0.705 | 0.104 |
| Month × Pasture × Nitrogen | 0.063 | 0.075 | 0.441 | 0.585 | 0.742 | 0.690 | 0.003 | 0.959 | 0.855 | 0.019 | 0.361 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Fang, H.; Cheng, S.; Xu, L.; Lu, M.; Guo, Y.; Li, Y.; Zhou, Y. Soil Enzyme Activity Regulates the Response of Soil C Fluxes to N Fertilization in a Temperate Cultivated Grassland. Atmosphere 2022, 13, 777. https://doi.org/10.3390/atmos13050777
Yang Y, Fang H, Cheng S, Xu L, Lu M, Guo Y, Li Y, Zhou Y. Soil Enzyme Activity Regulates the Response of Soil C Fluxes to N Fertilization in a Temperate Cultivated Grassland. Atmosphere. 2022; 13(5):777. https://doi.org/10.3390/atmos13050777
Chicago/Turabian StyleYang, Yan, Huajun Fang, Shulan Cheng, Lijun Xu, Mingzhu Lu, Yifan Guo, Yuna Li, and Yi Zhou. 2022. "Soil Enzyme Activity Regulates the Response of Soil C Fluxes to N Fertilization in a Temperate Cultivated Grassland" Atmosphere 13, no. 5: 777. https://doi.org/10.3390/atmos13050777






