Transformation and Migrant Mechanism of Sulfur and Nitrogen during Chemical Looping Combustion with CuFe2O4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coal Sample and Oxygen Carrier Particles
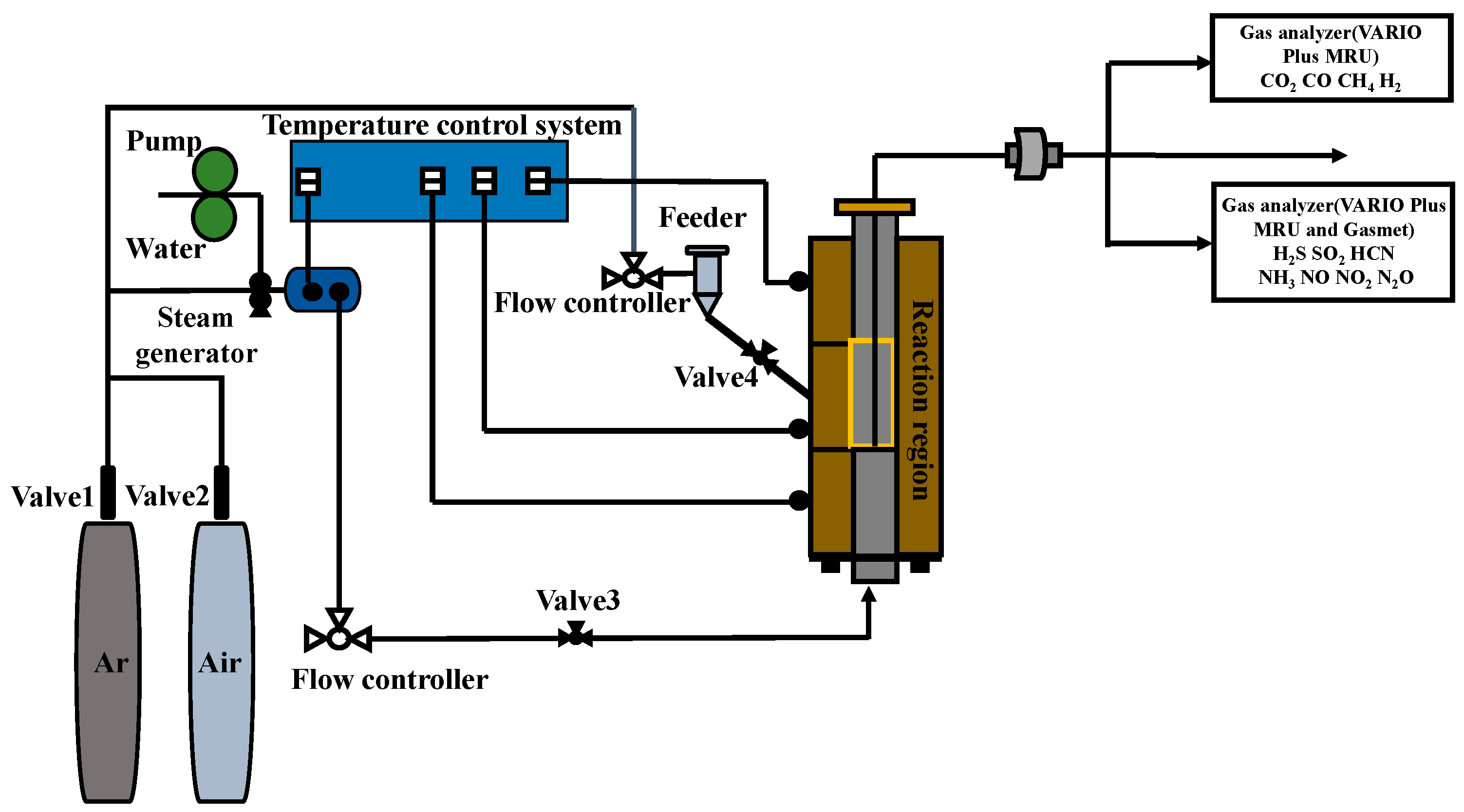
2.2. Experimental Procedure
2.3. Date Analysis
3. Results and Discussion
3.1. Thermodynamic Simulation of Fate of Sulfur and Nitrogen during CLC
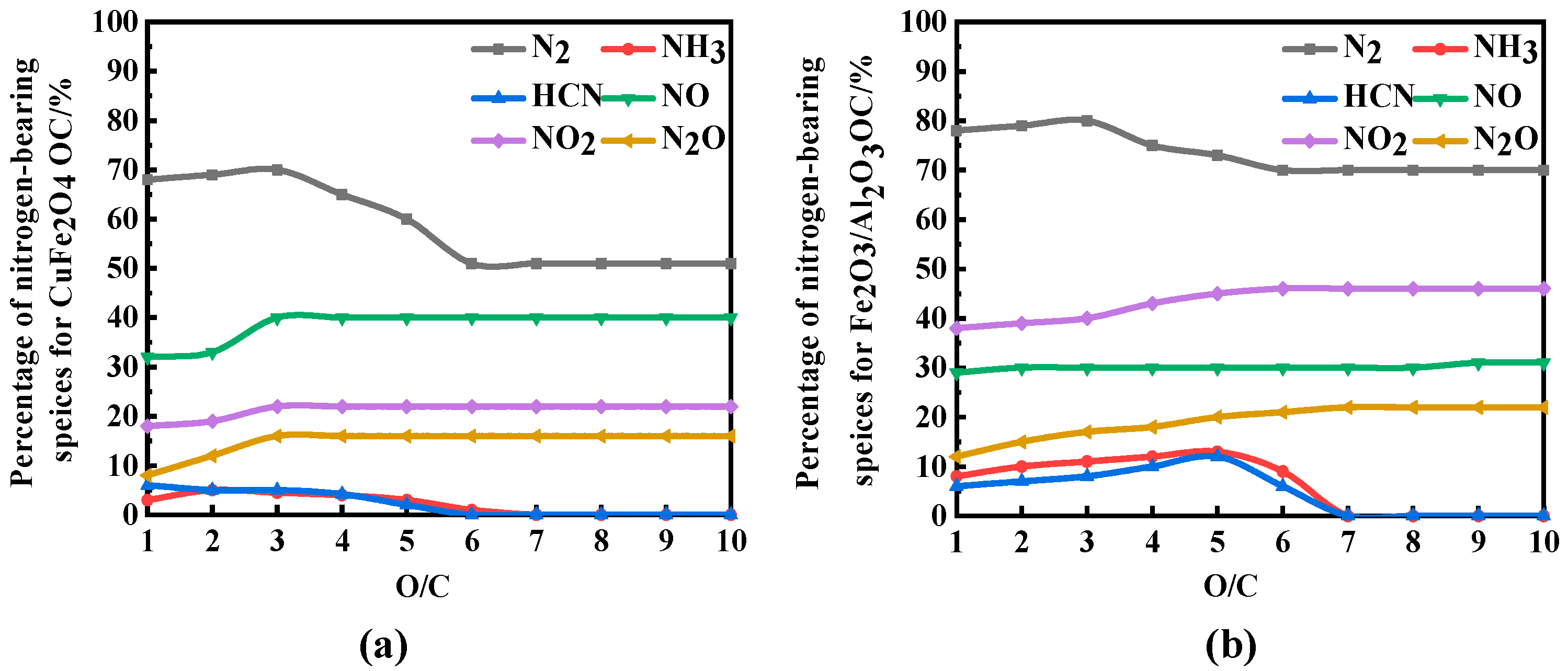
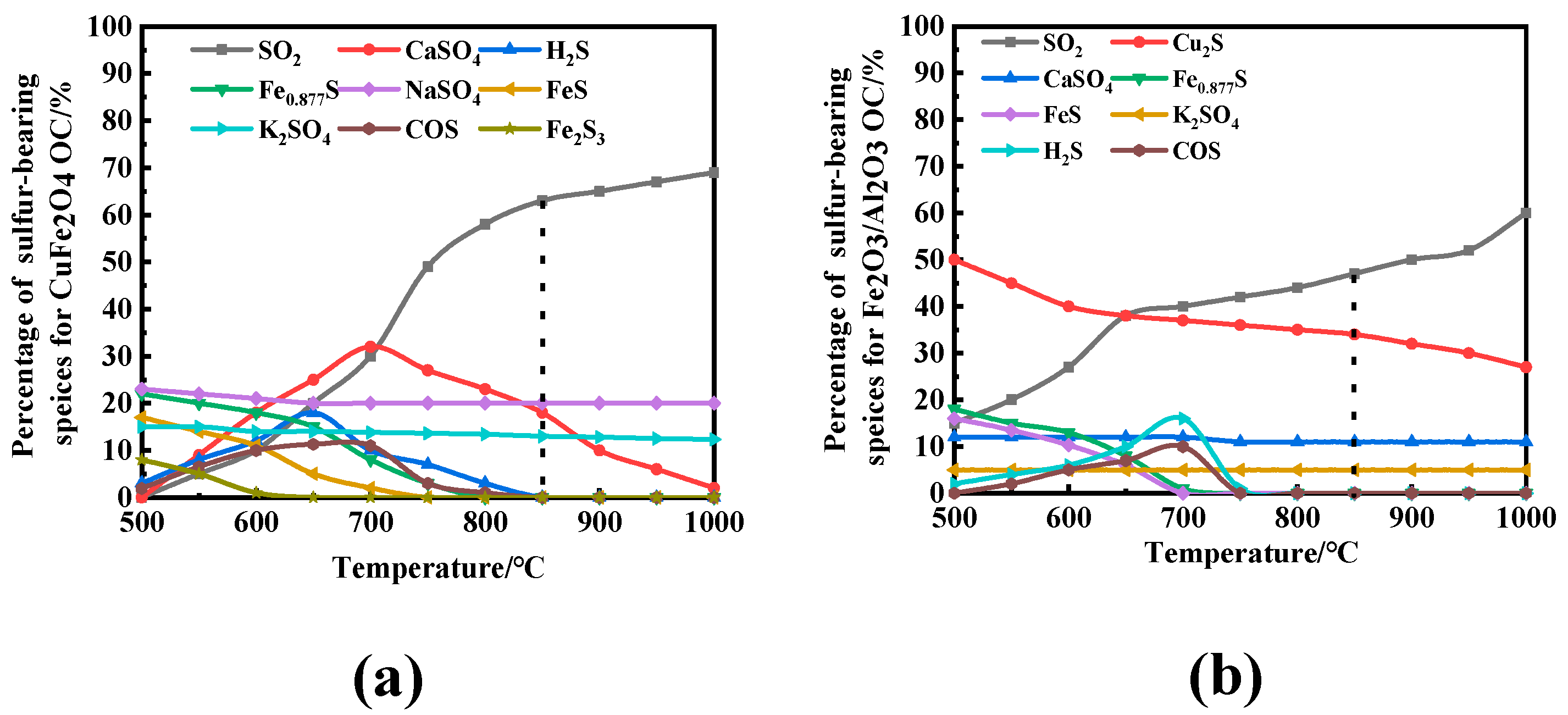
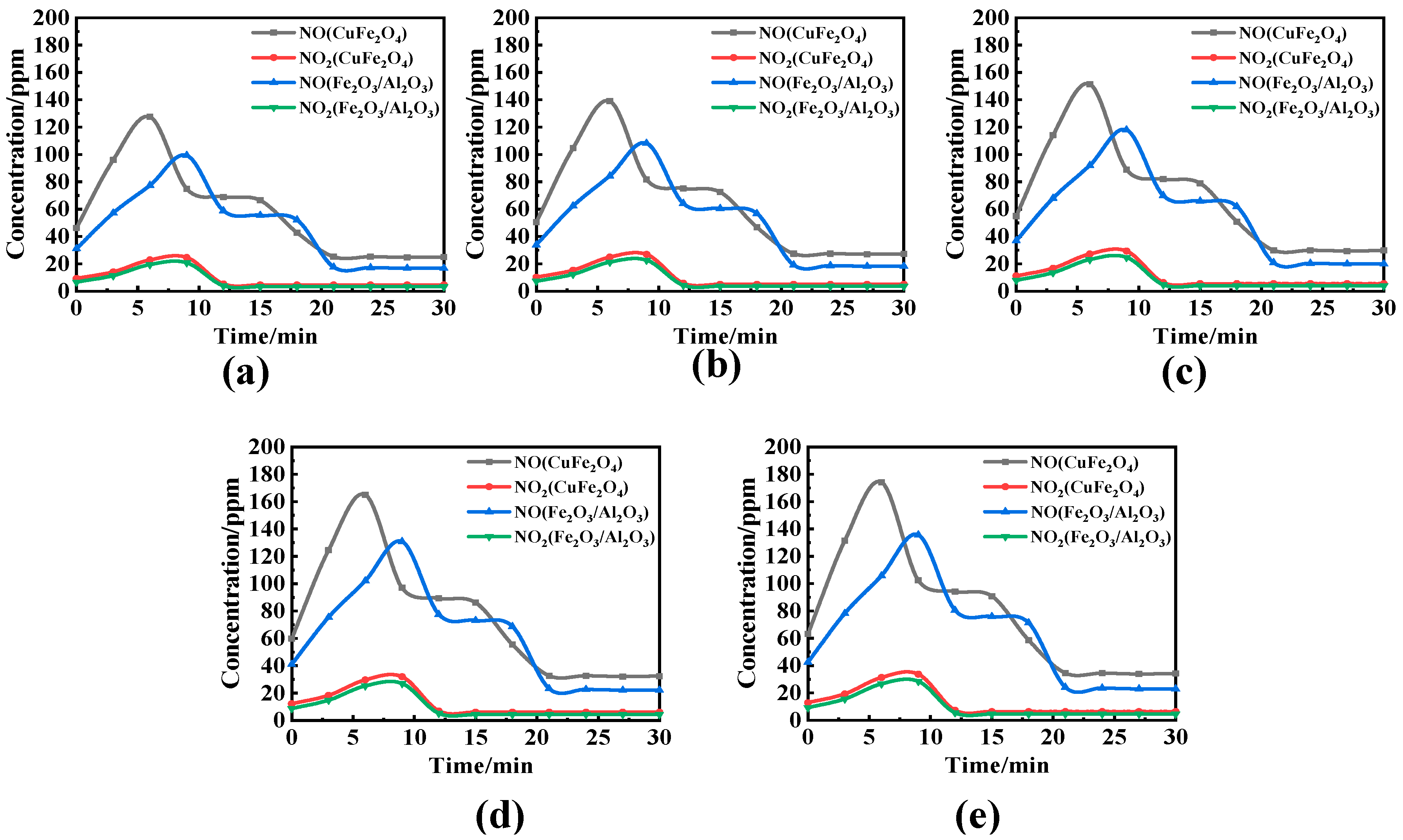
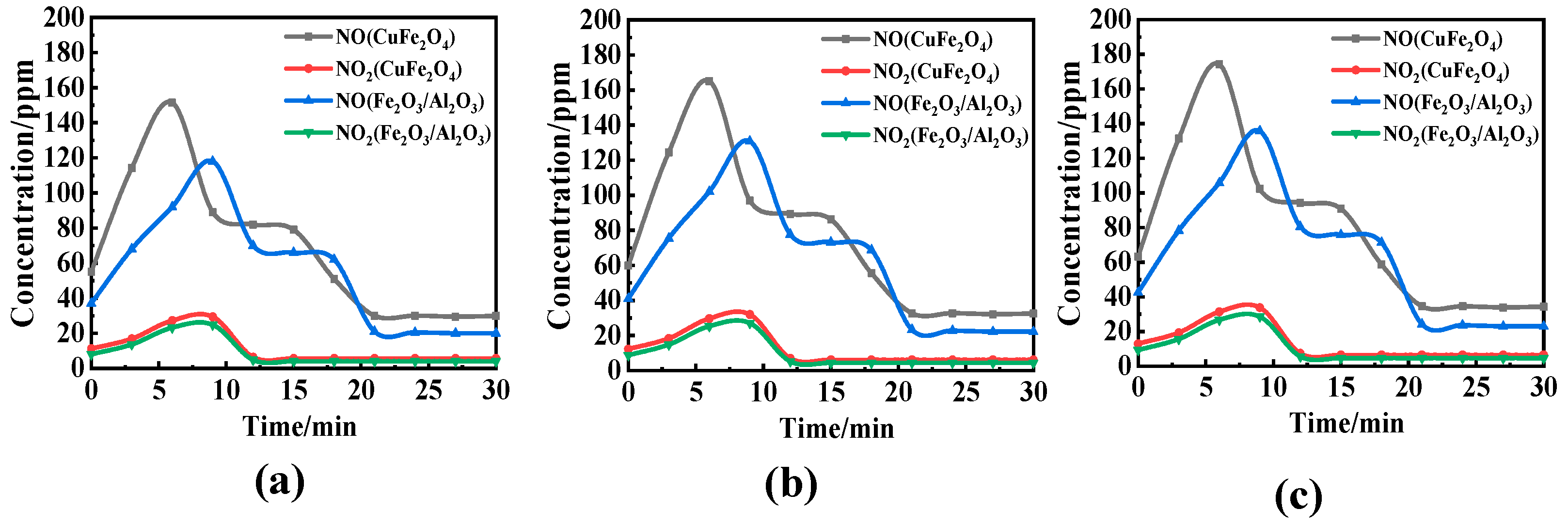
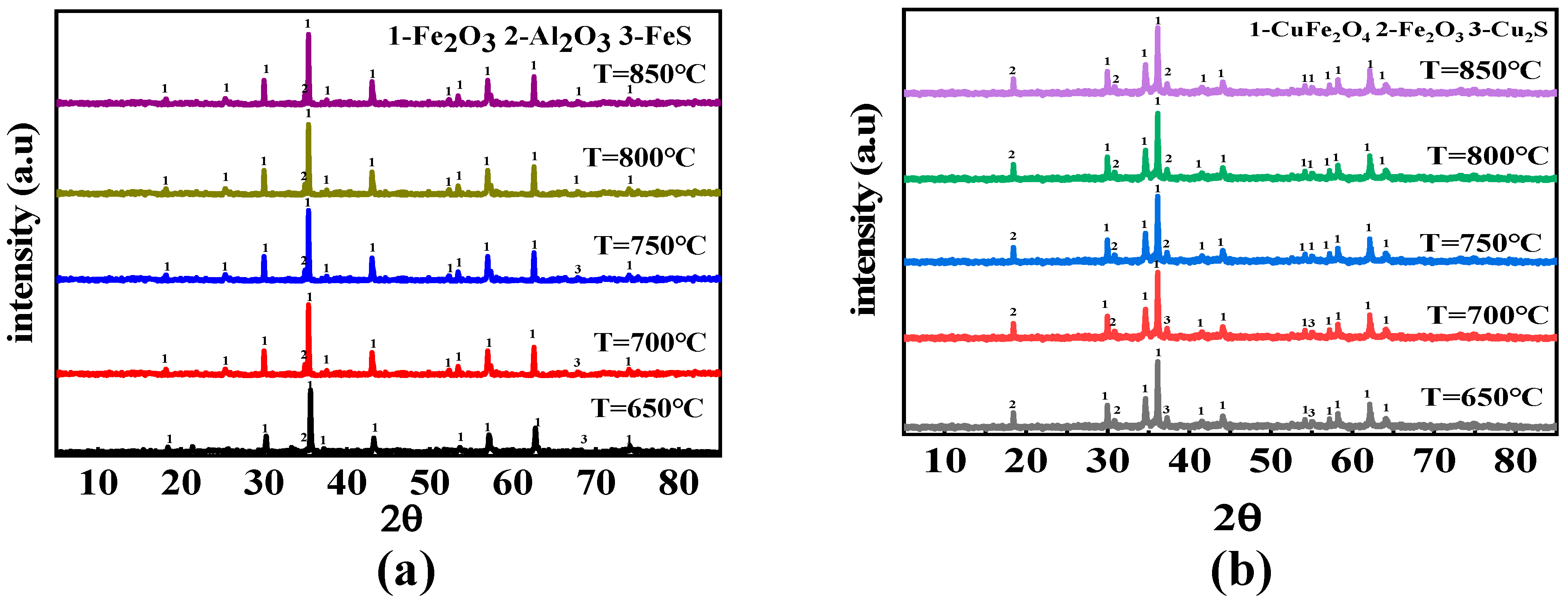
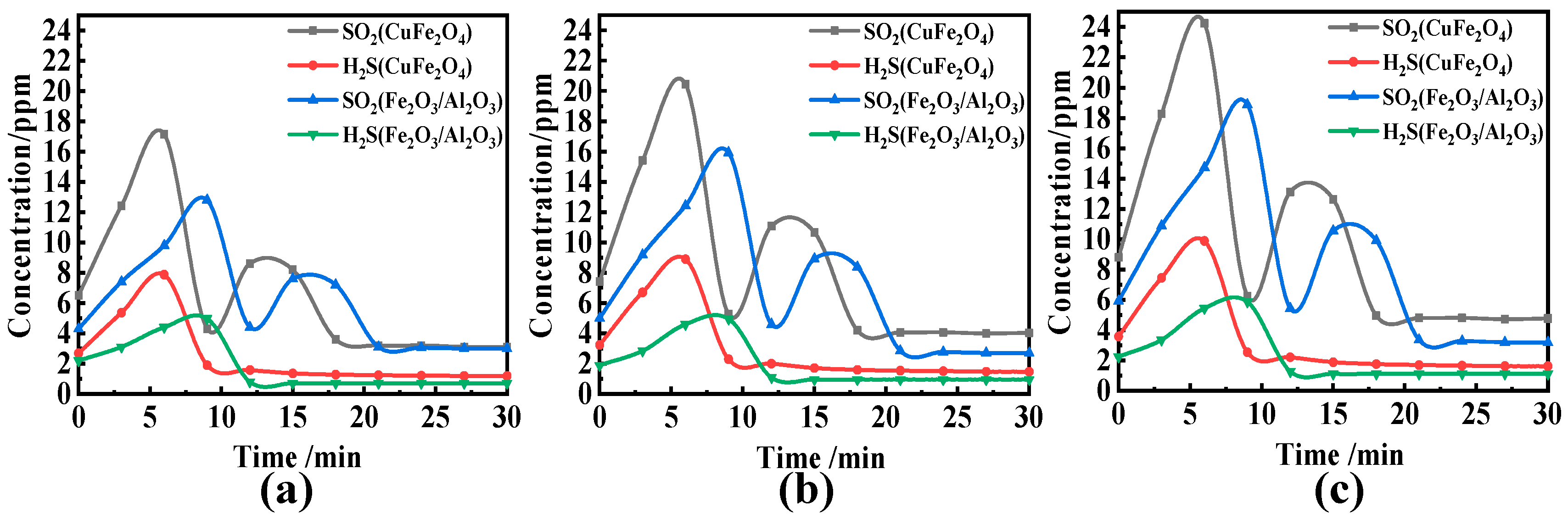
3.2. Effect of Reaction Temperature and O/C on the Migration and Transformation Mechanism of Sulfur and Nitrogen during CLC
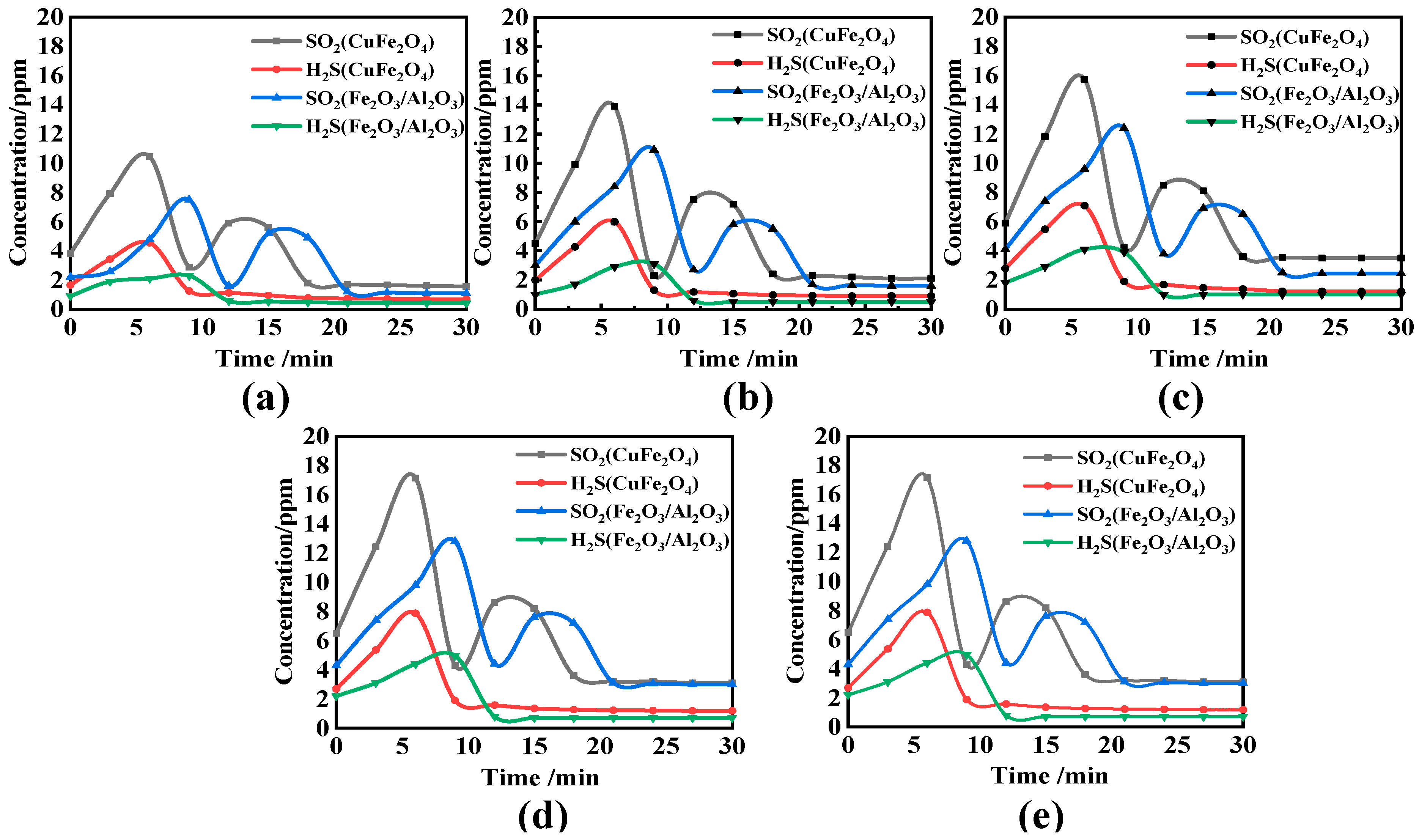
3.3. Transformation and Migrant Behavior of Sulfur and Nitrogen in Cyclic Experiment
3.4. Reaction Characteristics of CLC
4. Conclusions
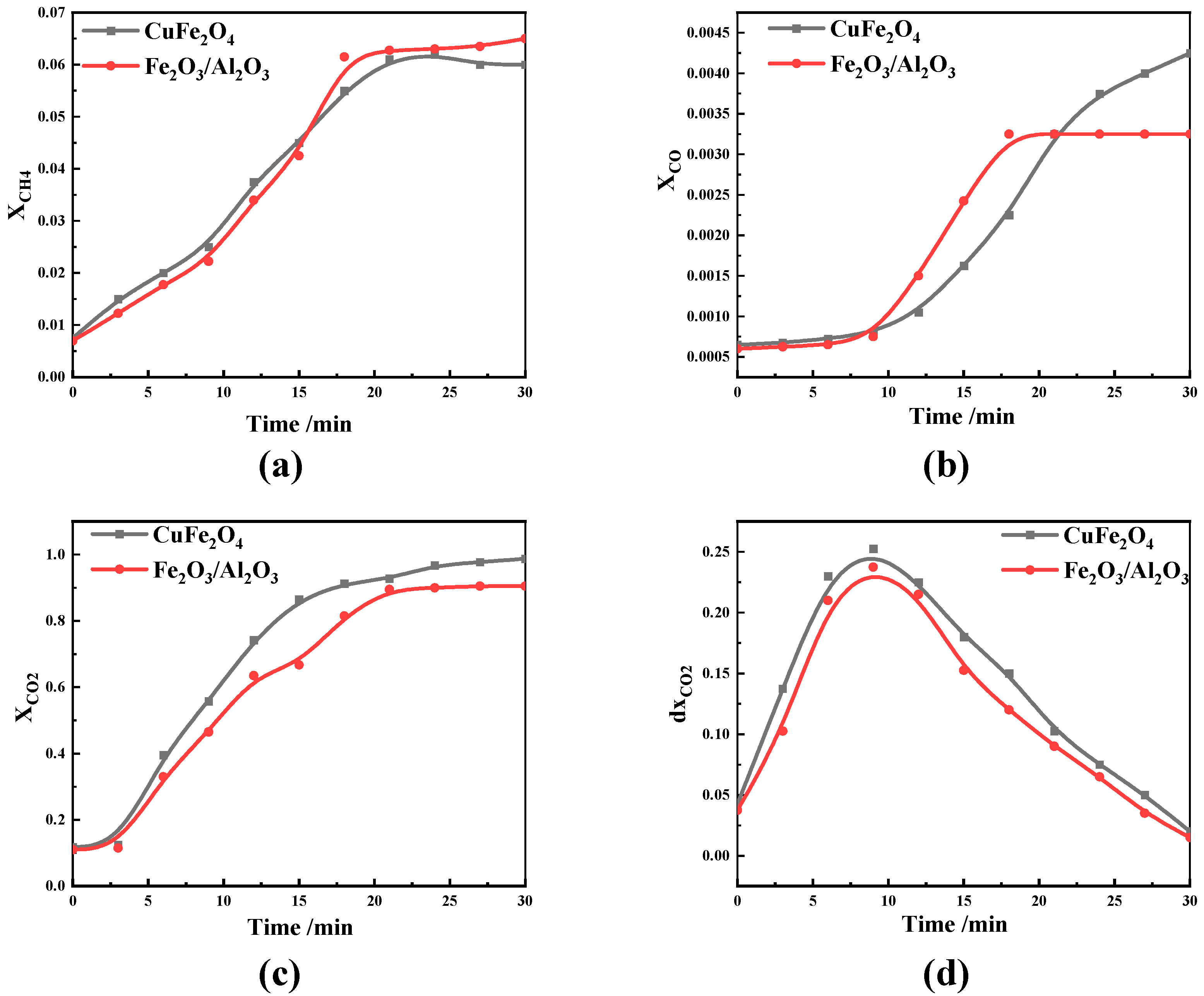
- Different OC have different effects on CO, CH4 and CO2 at the same O/C and reaction temperature. CuFe2O4 contains both iron and copper phases, and so has the dual advantages of Fe2O3 and CuO. It can simultaneously release molecular oxygen and lattice oxygen, and has good oxygen release characteristics and reaction characteristics. Compared with iron-based OC, it is easier to obtain a high concentration of CO, which is more suitable for CLC.
- H2S increased first and then decreased in the reaction process, and a lot was released in the coal pyrolysis stage, while SO2 showed a bimodal release trend. Compared with solid–solid reactions, CuFe2O4 can release molecular oxygen. A gas–solid reaction is more likely to occur. Therefore, CuFe2O4 can promote the release of H2S and SO2, and its promotion effect on H2S and SO2 is still obvious with the increase in O/C and reaction temperature. Based on the thermodynamic simulation and experimental results, when the reaction temperature is higher than 800 °C, CuFe2O4 can resist sulfur poisoning. Therefore, an appropriate reaction temperature can not only inhibit OC particles sulfur poisoning, but also promote combustion characteristics.
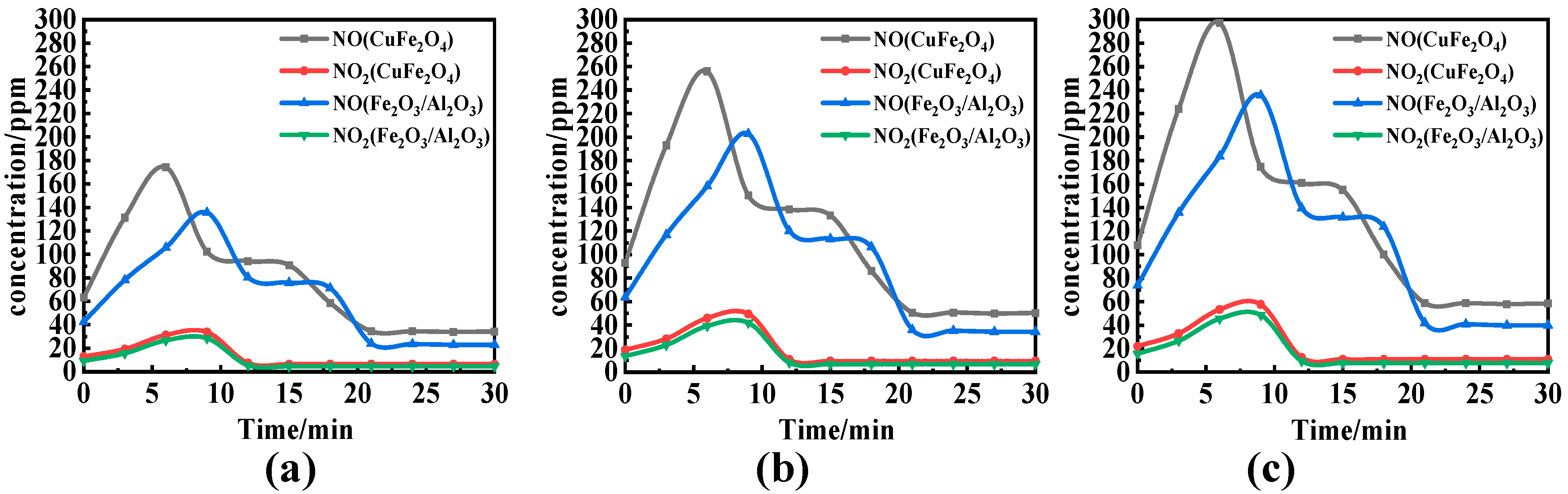
- Considering nitrogen in coal, the content of NOx in flue gas increased significantly when OC released molecular oxygen, and the content of NO and NO2 increased with the increase in reaction temperature. The effect of O/C on NOx in flue gas was not obvious, and with the increase in O/C the content of NOx did not change obviously. Therefore, the oxygen release mode of OC only affects the conversion of the fuel nitrogen valence state. The oxygen decoupling oxygen carrier can convert fuel nitrogen into NO and NO2, so that CLC is similar to the traditional combustion process. Iron-based OC can convert fuel nitrogen into N2 instead of releasing fuel nitrogen in the form of NOx. Therefore, Fe2O3/Al2O3 can release lattice oxygen and is more suitable for CLC with a high content nitrogen of coal, which can convert fuel nitrogen into N2 release and reduce NOx release.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saqline, S.; Chua, Z.Y.; Liu, W. Coupling chemical looping combustion of solid fuels with advanced steam cycles for CO2 capture: A process modelling study. Energy Convers. Manag. 2021, 244, 114455. [Google Scholar] [CrossRef]
- Abuelgasim, S.; Wang, W.; Abdalazeez, A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress. Sci. Total Environ. 2021, 764, 142892. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tian, X.; Ma, J.; Chen, X.; Su, M.; Zheng, C.; Wang, Y. Chemical looping combustion of coal in China: Comprehensive progress, Remaining Challenges, and Potential Opportunities. Energy Fuels 2020, 34, 6696–6734. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Abad, A.; Gayán, P.; García-Labiano, F.; Luis, F.; Adánez, J. The fate of sulphur in the Cu-based Chemical Looping with Oxygen Uncoupling (CLOU) Process. Appl. Energy 2014, 113, 1855–1862. [Google Scholar] [CrossRef] [Green Version]
- Linderholm, C.; Schmitz, M. Chemical-looping combustion of solid fuels in a 100 kW dual circulating fluidized bed system using iron ore as oxygen carrier. J. Environ. Chem. Eng. 2016, 4, 1029–1039. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A.; Mendiara, T.; Gayán, P.; De Diego, L.F.; García-Labiano, F. Chemical looping combustion of solid fuels. Prog. Energy Combust. Sci. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, X.; Ma, J.; Su, M.; Wang, B.; Mei, D. Development of tailor-made oxygen carriers and reactors for chemical looping processes at Huazhong University of Science & Technology. Int. J. Greenh. Gas Control 2020, 93, 102898. [Google Scholar]
- Wang, P.; Howard, B.; Means, N. Investigation of reactivities of bimetallic Cu-Fe oxygen carriers with coal in high temperature in-situ gasification chemical-looping combustion (iG-CLC) and chemical-looping with oxygen uncoupling (CLOU) using a fixed bed reactor. Fuel 2021, 285, 119012. [Google Scholar] [CrossRef]
- Zhu, X.; Shen, T.; Bollas, G.; Shen, L. Design and operation of a multi-stage reactor system for chemical looping combustion process. Fuel Process. Technol. 2021, 215, 106748. [Google Scholar] [CrossRef]
- Wen, C.; Yu, D.; Wang, J.; Wu, J.; Yao, H.; Xu, M. Effect of the devolatilization process on PM10 Formation during oxy-fuel combustion of a typical bituminous coal. Energy Fuels 2014, 28, 5682–5689. [Google Scholar] [CrossRef]
- Song, T.; Shen, L.; Xiao, J.; Chen, D.; Gu, H.; Zhang, S. Nitrogen transfer of fuel-N in chemical looping combustion. Combust. Flame 2012, 159, 1286–1295. [Google Scholar] [CrossRef]
- Izquierdo, M.T.; García-Labiano, F.; Abad, A.; Cabello, A.; Gayán, P.; de Diego, L.F.; Adánez, J. On the optimization of physical and chemical stability of a Cu/Al2O3 impregnated oxygen carrier for chemical looping combustion. Fuel Process. Technol. 2021, 215, 106740. [Google Scholar] [CrossRef]
- Pachler, R.F.; Mayer, K.; Penthor, S.; Kollerits, M.; Hofbauer, H. Fate of sulfur in chemical looping combustion of gaseous fuels using a copper-based oxygen carrier. Int. J. Greenh. Gas Control 2018, 71, 86–94. [Google Scholar] [CrossRef]
- Wang, B.; Yan, R.; Zhao, H.; Zheng, Y.; Liu, Z.; Zheng, C. Investigation of chemical looping combustion of coal with CuFe2O4 oxygen carrier. Energy Fuels 2011, 25, 3344–3354. [Google Scholar] [CrossRef]
- Fan, B.; Wen, C.; Zeng, X.; Wu, J.; Yu, X. Emission Behaviors of Inorganic Ultrafine Particles during Zhundong Coal Oxy-Fuel Combustion with Characterized Oxygen Input Fractions Comparable to Air Combustion. Appl. Sci. 2018, 8, 1486. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Zhou, L.; Cai, J.; Zhang, H.; Wang, C. Migration of sulfur in in-situ gasification chemical looping combustion of Beisu coal with iron-and copper-based oxygen carriers. Chin. J. Chem. Eng. 2021, 35, 247–255. [Google Scholar]
- Mendiara, T.; Izquierdo, M.T.; Abad, A.; De Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Release of pollutant components in CLC of lignite. Int. J. Greenh. Gas Control 2014, 22, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Mei, D.; Wang, C.; Tian, X.; Liu, Z.; Zhao, H. Sulfur fate during in-situ gasification chemical looping combustion (iG-CLC) of coal. Chem. Eng. J. 2021, 406, 126773. [Google Scholar] [CrossRef]
- Song, T.; Shen, T.; Shen, L.; Xiao, J.; Gu, H.; Zhang, S. Evaluation of hematite oxygen carrier in chemical-looping combustion of coal. Fuel 2013, 104, 244–252. [Google Scholar] [CrossRef]
- Pérez-Vega, R.; Adánez-Rubio, I.; Gayán, P.; Izquierdo, M.T.; Abad, A.; García-Labiano, F.; Luis, F.; Adánez, J. Sulphur, nitrogen and mercury emissions from coal combustion with CO2 capture in chemical looping with oxygen uncoupling (CLOU). Int. J. Greenh. Gas Control 2016, 46, 28–38. [Google Scholar] [CrossRef]
- Ishida, M.; Jin, H. A novel chemical-looping combustor without NOx formation. Ind. Eng. Chem. Res. 1996, 35, 2469–2472. [Google Scholar] [CrossRef]
- An, M.; Ma, J.; Guo, Q. Transformation and migration of mercury during chemical-looping gasification of coal. Ind. Eng. Chem. Res. 2019, 58, 20481–20490. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Gayán, P.; García-Labiano, F.; Luis, F.; Adánez, J.; Abad, A. Development of CuO-based oxygen-carrier materials suitable for Chemical-Looping with Oxygen Uncoupling (CLOU) process. Energy Procedia 2011, 4, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Luo, M.; Zhou, L.; Zhang, H. Sulfur Transformation Behavior of Inorganic Sulfur-Containing Compounds in Chemical Looping Combustion. Energy Fuels 2020, 34, 3969–3975. [Google Scholar] [CrossRef]
- Ma, J.; Tian, X.; Wang, C.; Zhao, H.; Liu, Z.; Zheng, C. Fate of fuel-nitrogen during in situ gasification chemical looping combustion of coal. Fuel Process. Technol. 2021, 215, 106710. [Google Scholar] [CrossRef]
- Chung, C.; Pottimurthy, Y.; Xu, M.; Hsieh, T.; Xu, D.; Zhang, Y.; Chen, Y.; He, P.; Pickarts, M.; Fan, L.; et al. Fate of sulfur in coal-direct chemical looping systems. Appl. Energy 2017, 208, 678–690. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Zhong, Z.; Niu, X.; Ge, H.; Zhou, Y.; Xiao, S.; Jiang, S. NO release during chemical looping combustion with iron ore as an oxygen carrier. Chem. Eng. J. 2015, 264, 211–220. [Google Scholar] [CrossRef]
- Chen, P.; Gu, M.; Chen, G.; Huang, X.; Lin, Y. The effect of metal calcium on nitrogen migration and transformation during coal pyrolysis: Mass spectrometry experiments and quantum chemical calculations. Fuel 2020, 264, 116814. [Google Scholar] [CrossRef]




| Sample | Proximate Analysis wt.% | Ultimate Analysis wt.% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | A | V | FC | C | H | N | S | O | |
| NX | 5.18 | 4.56 | 26.95 | 63.31 | 77.60 | 5.16 | 1.46 | 0.40 | 10.77 |
| Sample | SBET/(m2/g) of CuFe2O4 | SBET/(m2/g) of Fe2O3/Al2O3 |
|---|---|---|
| Fresh | 2.339 | 1.872 |
| After 1st | 5.101 | 4.446 |
| After 5th | 5.576 | 4.782 |
| After 10th | 6.475 | 5.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Han, Z.; Hu, C.; Ma, J.; Guo, Q. Transformation and Migrant Mechanism of Sulfur and Nitrogen during Chemical Looping Combustion with CuFe2O4. Atmosphere 2022, 13, 786. https://doi.org/10.3390/atmos13050786
Li H, Han Z, Hu C, Ma J, Guo Q. Transformation and Migrant Mechanism of Sulfur and Nitrogen during Chemical Looping Combustion with CuFe2O4. Atmosphere. 2022; 13(5):786. https://doi.org/10.3390/atmos13050786
Chicago/Turabian StyleLi, Haichuan, Ziheng Han, Chenye Hu, Jingjing Ma, and Qingjie Guo. 2022. "Transformation and Migrant Mechanism of Sulfur and Nitrogen during Chemical Looping Combustion with CuFe2O4" Atmosphere 13, no. 5: 786. https://doi.org/10.3390/atmos13050786
APA StyleLi, H., Han, Z., Hu, C., Ma, J., & Guo, Q. (2022). Transformation and Migrant Mechanism of Sulfur and Nitrogen during Chemical Looping Combustion with CuFe2O4. Atmosphere, 13(5), 786. https://doi.org/10.3390/atmos13050786





