Evaluation of Different Oxygen Carriers for Chemical Looping Reforming of Toluene as Tar Model Compound in Biomass Gasification Gas: A Thermodynamic Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
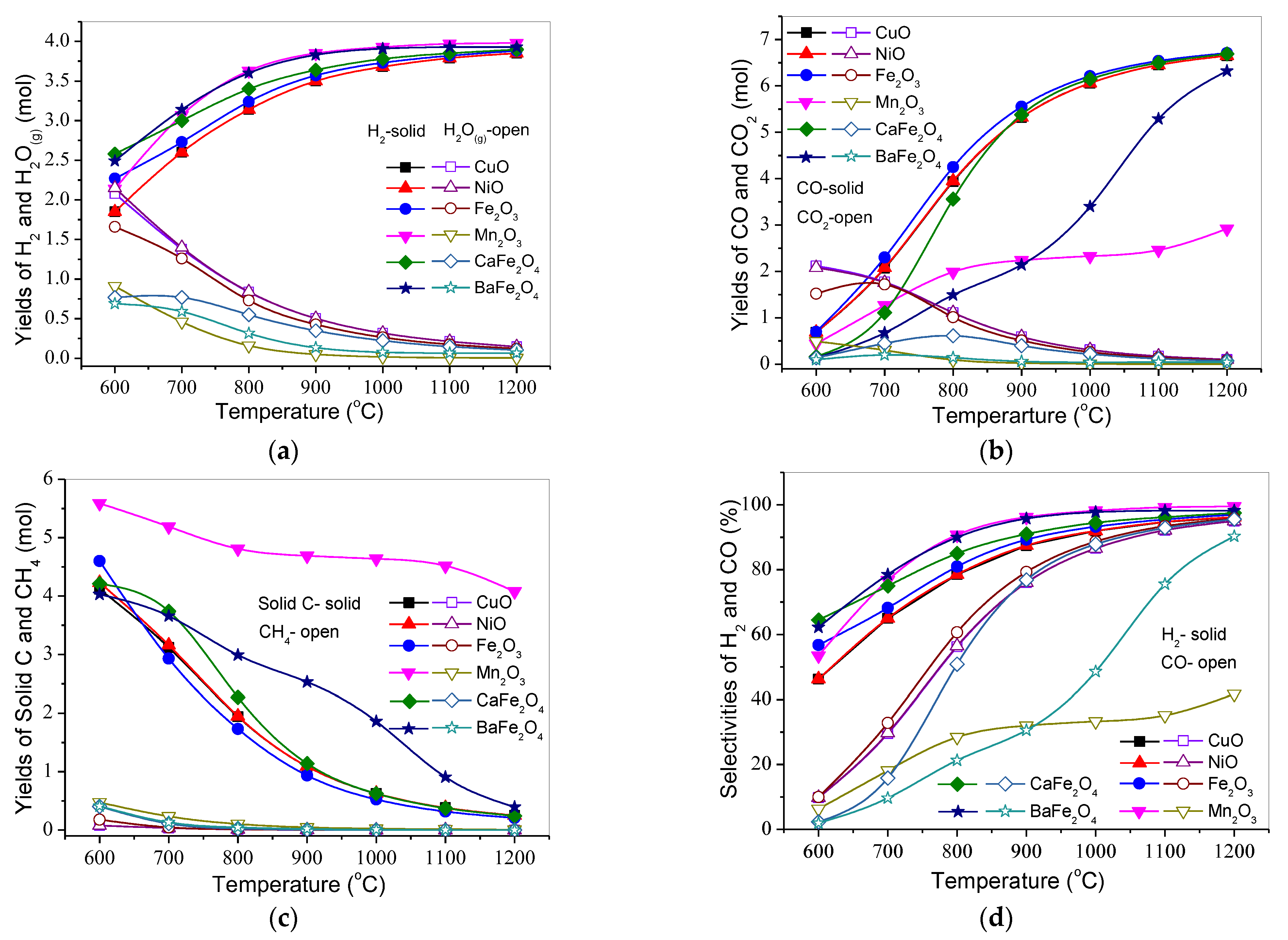
3.1. Effect of Temperature on Product Distribution and H2 and CO Selectivities
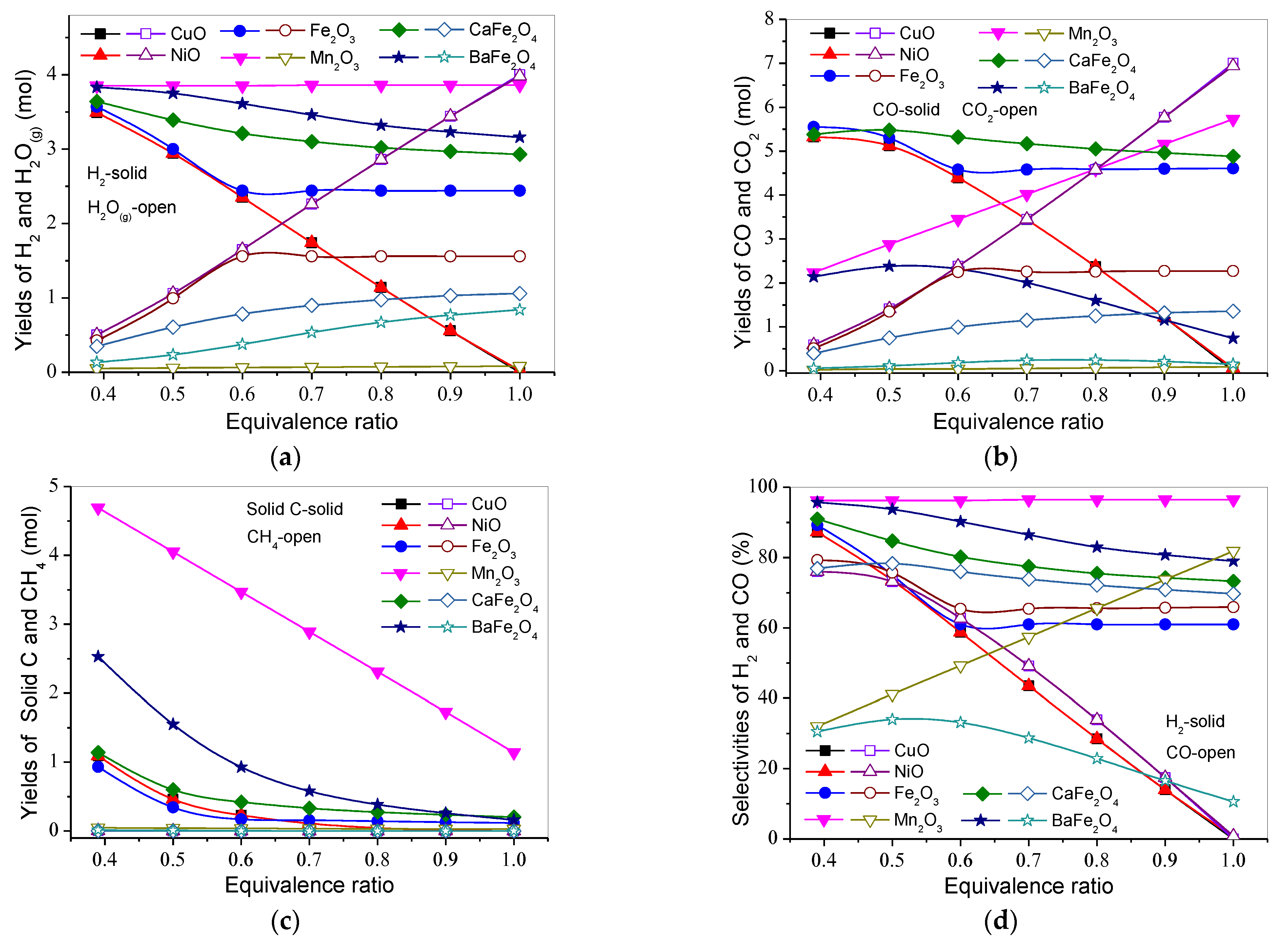
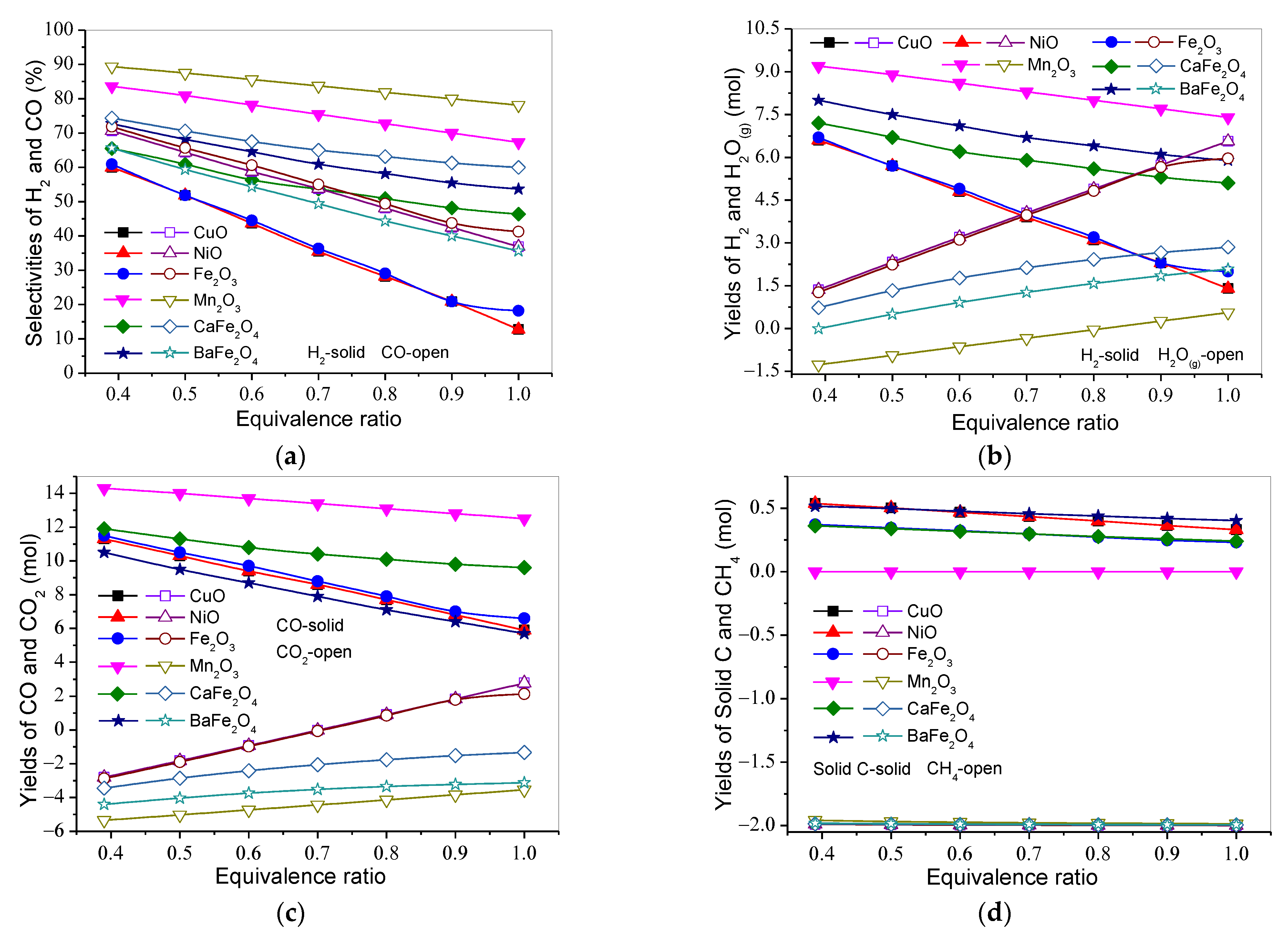
3.2. Effect of Equivalence Ratio on Product Distribution and H2 and CO Selectivities
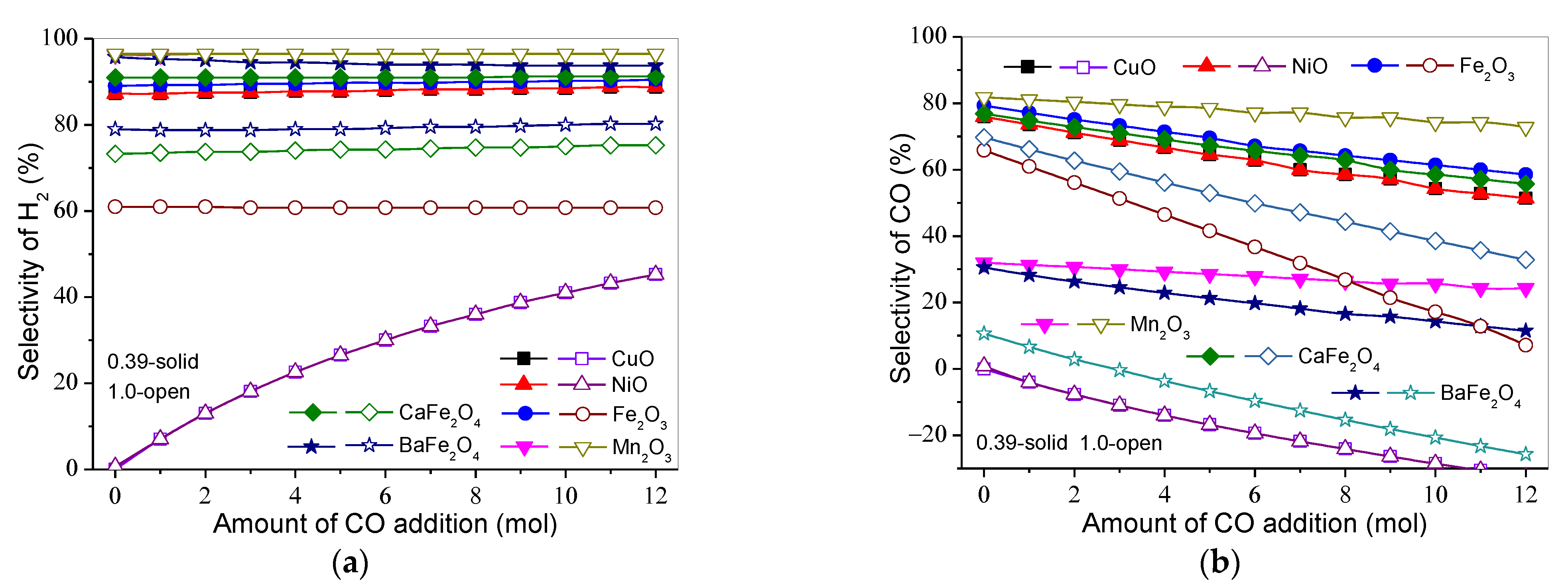
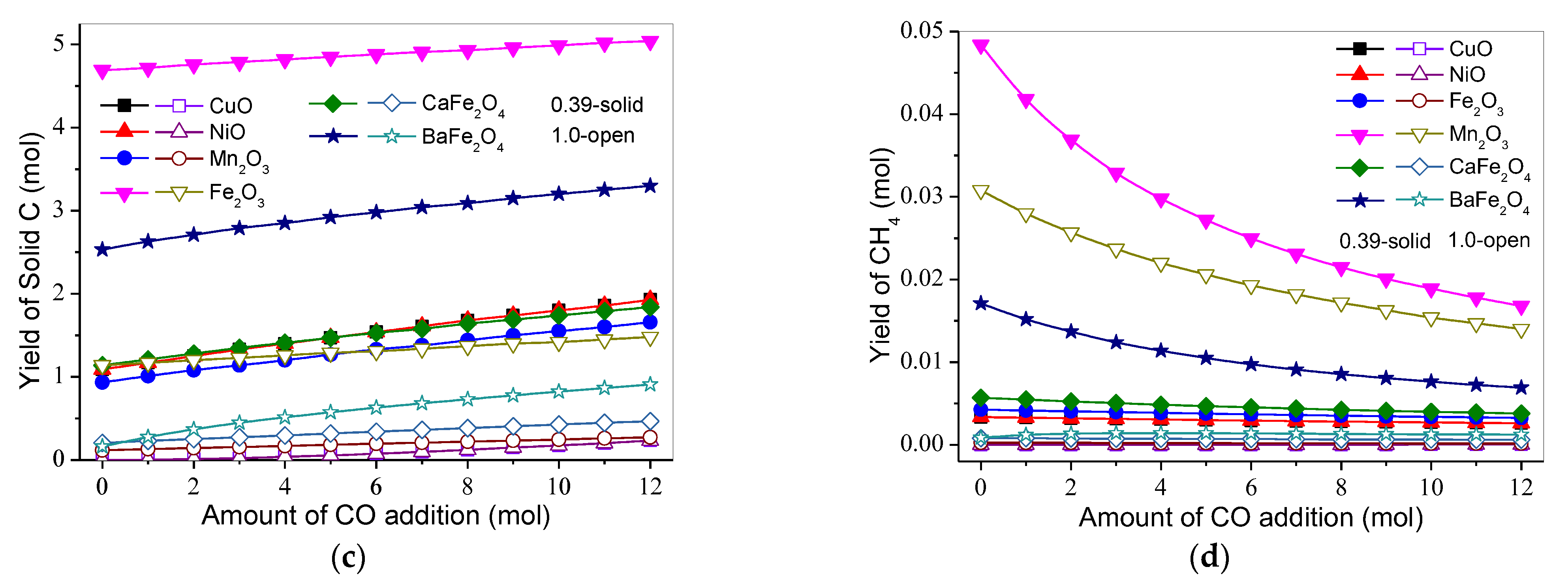
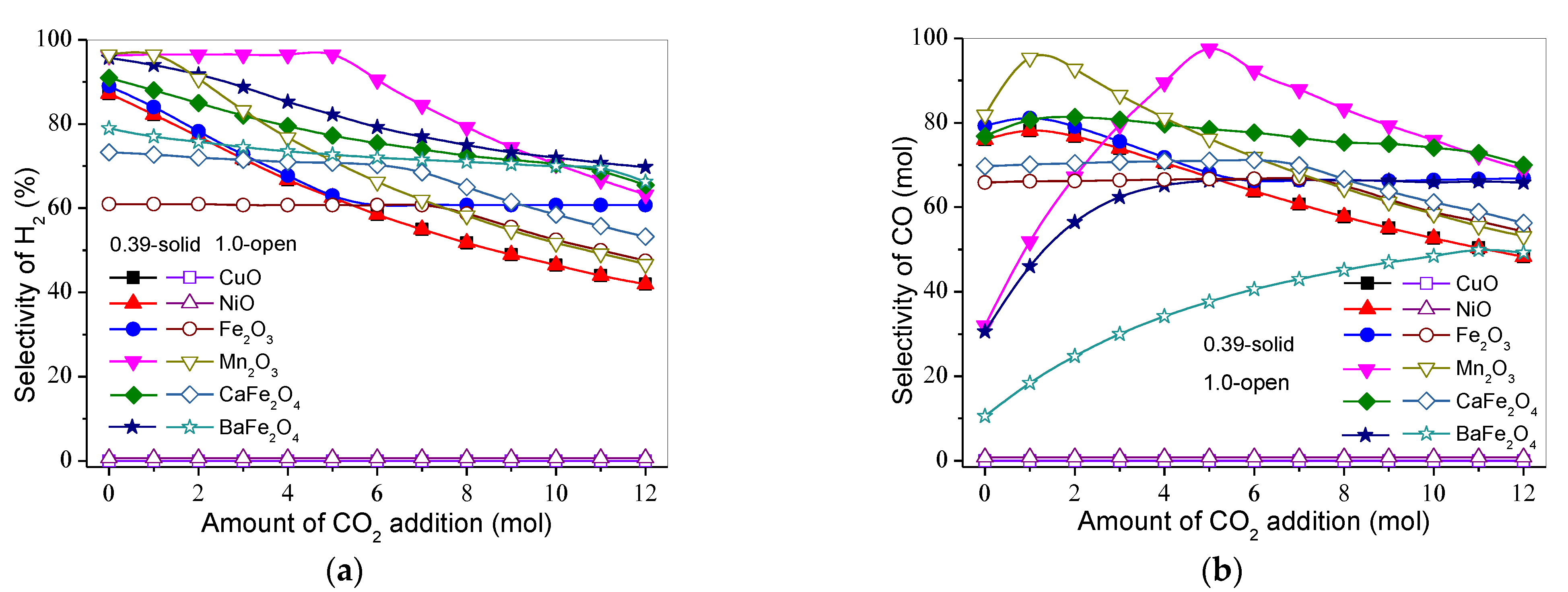
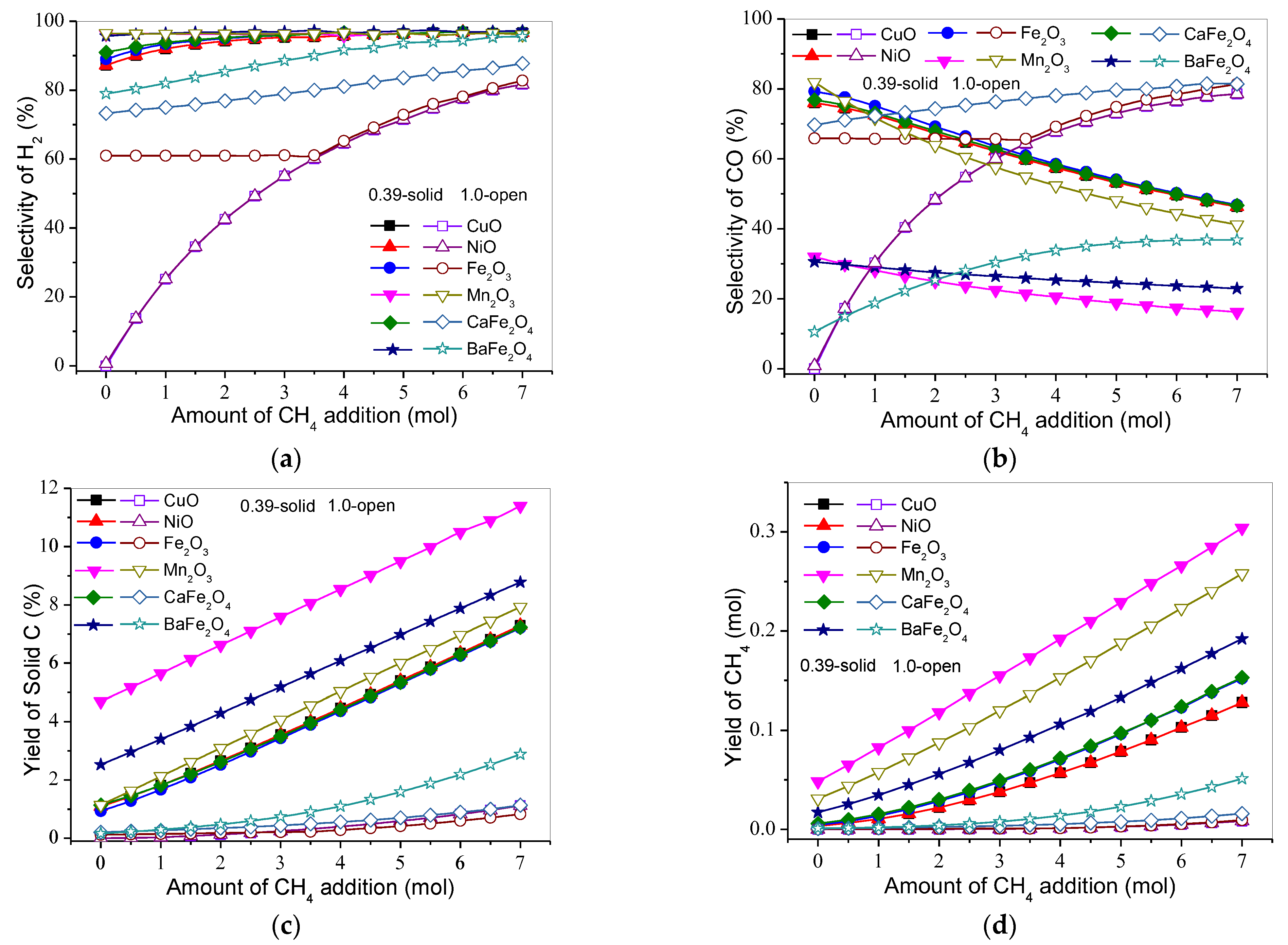
3.3. Effect of Gasification Gas Component on Product Distribution and H2 and CO Selectivities
3.3.1. H2
3.3.2. CO
3.3.3. CO2
3.3.4. H2O(g)
3.3.5. CH4
3.4. Effect of Simulated Gasification Gas on Product Distribution and H2 and CO Selectivities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.; Wang, J.; Bhattacharyya, D.; Jiang, Y.; De Vallance, D. Economic and environmental analyses of coal and biomass to liquid fuels. Energy 2017, 141, 76–86. [Google Scholar] [CrossRef]
- Lozano, F.J.; Lozano, R. Assessing the potential sustainability benefits of agricultural residues: Biomass conversion to syngas for energy generation or to chemicals production. J. Clean. Prod. 2018, 172, 4162–4169. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Rosen, M.A.; Tyagi, S.K. Global challenges in the sustainable development of biomass gasification: An overview. Renew. Sustain. Energy Rev. 2017, 80, 23–43. [Google Scholar] [CrossRef]
- Rios, M.L.V.; González, A.M.; Lora, E.E.S.; del Olmo, O.A.A. Reduction of tar generated during biomass gasification: A review. Biomass Bioener. 2018, 108, 345–370. [Google Scholar] [CrossRef]
- Liu, S.Y.; Mei, D.H.; Nahil, M.A.; Gadkari, S.; Gu, S.; Williams, P.T.; Tu, X. Hybrid plasma-catalytic steam reforming of toluene as a biomass tar model compound over Ni/Al2O3 catalysts. Fuel Process. Technol. 2017, 166, 269–275. [Google Scholar] [CrossRef]
- Anis, S.; Zainal, Z.A. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods: A review. Renew. Sustain. Energy Rev. 2011, 15, 2355–2377. [Google Scholar] [CrossRef]
- Pallozzi, V.; Di Carlo, A.; Bocci, E.; Carlini, M. Combined gas conditioning and cleaning for reduction of tars in biomass gasification. Biomass Bioenergy 2018, 109, 85–90. [Google Scholar] [CrossRef]
- Guan, G.; Kaewpanha, M.; Hao, X.; Abudula, A. Catalytic steam reforming of biomass tar: Prospects and challenges. Renew. Sustain. Energy Rev. 2016, 58, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Oh, G.; Kim, K.; Seo, M.W.; Ra, H.W.; Mun, T.Y.; Lee, J.G.; Yoon, S.J. Deactivation characteristics of Ni and Ru catalysts in tar steam reforming. Renew. Energy 2017, 105, 76–83. [Google Scholar] [CrossRef]
- Mattisson, T.; Keller, M.; Linderholm, C.; Moldenhauer, P.; Rydén, M.; Leion, H.; Lyngfelt, A. Chemical-looping technologies using circulating fluidized bed systems: Status of development. Fuel Process. Technol. 2018, 172, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kannari, N.; Satomi, C.; Oyama, Y.; Takarada, T. Durability studies of limonite ore for catalytic decomposition of phenol as a model biomass tar in a fluidized bed. Biomass Bioenergy 2017, 107, 86–92. [Google Scholar] [CrossRef]
- Ryden, M.; Lyngfelt, A.; Mattisson, T. Synthesis gas generation by chemical-looping reforming in a continuously operating laboratory reactor. Fuel 2006, 85, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Wei, Y.; Li, H.; Wang, H. Synthesis gas generation by chemical-looping reforming using Ce-based oxygen carriers modified with Fe, Cu, and Mn oxides. Energy Fuel 2009, 23, 2095–2102. [Google Scholar] [CrossRef]
- Mihai, O.; Chen, D.; Holmen, A. Catalytic consequence of oxygen of lanthanum ferrite perovskite in chemical looping reforming of methane. Ind. Eng. Chem. Res. 2011, 50, 2613–2621. [Google Scholar] [CrossRef]
- He, F.; Li, X.; Zhao, K.; Huang, Z.; Wei, G.; Li, H. The use of La1-xSrxFeO3 perovskite type oxides as oxygen carriers in chemical-looping reforming of methane. Fuel 2013, 108, 465–473. [Google Scholar] [CrossRef]
- Plou, J.; Durán, P.; Herguido, J.; Peña, J.A. Purified hydrogen from synthetic biogas by joint methane dry reforming and steam-iron process: Behaviour of metallic oxides and coke formation. Fuel 2014, 118, 100–106. [Google Scholar] [CrossRef]
- Forutan, H.R.; Karimi, E.; Hafizi, A.; Rahimpour, M.R.; Keshavarz, P. Expert representation chemical looping reforming: A comparative study of Fe, Mn, Co and Cu as oxygen carriers supported on Al2O3. J. Ind. Eng. Chem. 2015, 21, 900–911. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, L.; Xu, D.; Kathe, M.; Chung, E.; Deshpande, N.; Qin, L.; Qin, L.; Majumder, A.; Hsieh, T.-L.; et al. Shale gas-to-syngas chemical looping process for stable shale gas conversion to high purity syngas with a H2:CO ratio of 2:1. Energy Environ. Sci. 2014, 7, 4104–4117. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Lind, F.; Seemann, M.; Thunmani, H. Continuous catalytic tar reforming of biomass derived raw gas with simultaneous catalyst regeneration. Ind. Eng. Chem. Res. 2011, 50, 11553–11562. [Google Scholar] [CrossRef]
- Mendiara, T.; Johansen, J.M.; Utrilla, R.; Geraldo, P.; Jensen, A.D.; Glarborg, P. Evaluation of different oxygen carriers for biomass tar reforming (I): Carbon deposition in experiments with toluene. Fuel 2011, 90, 1049–1060. [Google Scholar] [CrossRef] [Green Version]
- Mendiara, T.; Johansen, J.M.; Utrilla, R.; Jensen, A.D.; Glarborg, P. Evaluation of different oxygen carriers for biomass tar reforming (II): Carbon deposition in experiments with methane and other gases. Fuel 2011, 90, 1370–1382. [Google Scholar] [CrossRef] [Green Version]
- Berguerand, N.; Lind, F.; Israelsson, M.; Seemann, M.; Biollaz, S.; Thunman, H. Use of nickel oxide as a catalyst for tar elimination in a chemical-looping reforming reactor operated with biomass producer gas. Ind. Eng. Chem. Res. 2012, 51, 16610–16616. [Google Scholar] [CrossRef]
- Lind, F.; Berguerand, N.; Seemann, M.; Thunman, H. Ilmenite and nickel as catalysts for upgrading of raw gas derived from biomass gasification. Energy Fuel 2013, 27, 997–1007. [Google Scholar] [CrossRef]
- Marinkovic, J.; Berguerand, N.; Lind, F.; Seemann, M.; Thunman, H. Using a manganese ore as catalyst for upgrading biomass derived gas. Biomass Convers. Biorefinery 2015, 5, 75–83. [Google Scholar] [CrossRef]
- Keller, M.; Leion, H.; Mattisson, T.; Thunman, H. Investigation of natural and synthetic bed materials for their utilization in chemical looping reforming for tar elimination in biomass-derived gasification gas. Energy Fuel 2014, 28, 3833–3840. [Google Scholar] [CrossRef]
- Keller, M.; Fung, J.; Leion, H.; Mattisson, T. Cu-impregnated alumina/silica bed materials for chemical looping reforming of biomass gasification gas. Fuel 2016, 180, 448–456. [Google Scholar] [CrossRef]
- Tian, X.; Niu, P.; Ma, Y.; Zhao, H. Chemical-looping gasification of biomass: Part II. Tar yields and distributions. Biomass Bioenergy 2018, 108, 178–189. [Google Scholar] [CrossRef]
- Ma, M.; Muller, M.; Richter, J.; Kriegel, R.; Bohning, D.; Beckmann, M.; Glusing, J.; Ruhe, N. Investigation of combined catalyst and oxygen carrier systems for the partial oxidation of naphthalene as model tar from biomass gasification. Biomass Bioenergy 2013, 53, 65–71. [Google Scholar] [CrossRef]
- Xu, T.; Xu, F.; Moyo, G.G.; Sun, Y.; Chen, Z.; Xiao, B.; Wang, X.; Hu, Z. Comparative study of MxOy (M = Cu, Fe and Ni) supported on dolomite for syngas production via chemical looping reforming with toluene. Energy Convers. Manag. 2019, 199, 111937. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.; Fan, L.-S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhu, M.; Tao, H.; Zhang, J.; Wu, J.; Tian, H.; Wu, J. Chemical looping reforming of toluene as a biomass tar model compound over two types of oxygen carriers: 2CuO-2NiO/Al2O3 and CaFe2O4. Fuel 2018, 222, 375–384. [Google Scholar] [CrossRef]
- Haryanto, A.; Fernando, S.D.; Pordesimo, L.O.; Adhikari, S. Upgrading of syngas derived from biomass gasification: A thermodynamic analysis. Biomass Bioenergy 2009, 33, 882–889. [Google Scholar] [CrossRef]
- Kale, G.R.; Kulkarni, B.D. Thermodynamic analysis of dry autothermal reforming of glycerol. Fuel Process. Technol. 2010, 91, 520–530. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Song, X.; Chen, J. Dry autothermal reforming of glycerol with in situ hydrogen separation via thermodynamic evaluation. Int. J. Hydrogen Energy 2017, 42, 838–847. [Google Scholar] [CrossRef]
- Iruretagoyena, D.; Hellgardt, K.; Chadwick, D. Towards autothermal hydrogen production by sorption-enhanced water gas shift and methanol reforming: A thermodynamic analysis. Int. J. Hydrogen Energy 2018, 43, 4211–4222. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Li, L.; Guo, Y.; Deng, T. Progresses on Thermodynamic Databases. IOP Conf. Ser. Mater. Sci. Eng. 2018, 382, 052018. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Sun, R.; Shen, L.; Bai, H.; Jiang, S.; Xiao, Y.; Song, T. Hydrogen-rich syngas production with tar elimination via biomass chemical looping gasification (BCLG) using BaFe2O4/Al2O3 as oxygen carrier. Chem. Eng. J. 2020, 387, 124107. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Zhao, Z.; He, F.; Huang, Z.; Wei, G. Identifying the roles of MFe2O4 (M=Cu, Ba, Ni, and Co) in the chemical looping reforming of char, pyrolysis gas and tar resulting from biomass pyrolysis. Int. J. Hydrogen Energy 2019, 44, 4674–4687. [Google Scholar] [CrossRef]
- Liu, S.; Xiang, D.; Xu, Y.; Sun, Z.; Cao, Y. Relationship between electronic properties of Fe3O4 substituted by Ca and Ba and their reactivity in chemical looping process: A first-principles study. Appl. Energy 2017, 202, 550–557. [Google Scholar] [CrossRef]
- Zhang, J.; He, T.; Wang, Z.; Zhu, M.; Zhang, K.; Li, B.; Wu, J. The search of proper oxygen carriers for chemical looping partial oxidation of carbon. Appl. Energy 2017, 190, 1119–1125. [Google Scholar] [CrossRef]
- Fan, L.-S. Chemical Looping Systems for Fossil Energy Conversions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Kale, G.R.; Kulkarni, B.D.; Bharadwaj, K.V. Chemical looping reforming of ethanol for syngas generation: A theoretical investigation. Int. J. Energy Res. 2013, 37, 645–656. [Google Scholar] [CrossRef]
- Bendoni, R.; Miccio, F.; Medri, V.; Benito, P.; Vaccari, A.; Landi, E. Geopolymer composites for the catalytic cleaning of tar in biomass-derived gas. Renew. Energy 2019, 131, 1107–1116. [Google Scholar] [CrossRef]
- Zeng, J.; Xiao, R.; Zhang, H.; Wang, Y.; Zeng, D.; Ma, Z. Chemical looping pyrolysis-gasification of biomass for high H2/CO syngas production. Fuel Process. Technol. 2017, 168, 116–122. [Google Scholar] [CrossRef]
- Wnukowski, M.; Jamrózb, P. Microwave plasma treatment of simulated biomass syngas: Interactions between the permanent syngas compounds and their influence on the model tar compound conversion. Fuel Process. Technol. 2018, 173, 229–242. [Google Scholar] [CrossRef]
- Wei, G.-Q.; Feng, J.; Hou, Y.-L.; Li, F.-Z.; Li, W.-Y.; Huang, Z.; Zheng, A.-Q.; Li, H.-B. Ca-enhanced hematite oxygen carriers for chemical looping reforming of biomass pyrolyzed gas coupled with CO2 splitting. Fuel 2021, 285, 119125. [Google Scholar] [CrossRef]
- Wang, Z.; He, T.; Qin, J.; Wu, J.; Li, J.; Zi, Z.; Liu, G.; Wu, J.; Sun, L. Gasification of biomass with oxygen-enriched air in a pilot scale two-stage gasifier. Fuel 2015, 150, 386–393. [Google Scholar] [CrossRef]





| Oxygen Carrier | Reducible Oxygen Amount, mol/mol | Reduced Products Considered |
|---|---|---|
| CuO | 1 | Cu, Cu2O |
| NiO | 1 | Ni |
| Fe2O3 | 3 | Fe, FeO |
| Mn2O3 | 3 | Mn, MnO |
| CaFe2O4 | 3 | CaO, Fe, FeO, CaCO3 |
| BaFe2O4 | 3 | BaO, Fe, FeO, BaCO3 |
| Reaction Description | Reaction Equation | ||
|---|---|---|---|
| Oxidation: | C7H8(g) + 18MeO = 7CO2 + 4H2O + 8Me | ΔH298K < 0 * | (1) |
| Partial oxidation | C7H8(g) + 7MeO = 7CO + 4H2 + 18Me | ΔH298K < 0 | (2) |
| Decomposition | C7H8(g) = 7C + 4H2 | ΔH298K = −50.0 kJ/mol | (3) |
| Methanation | 2CO + 2H2 = CH4 + CO2 CO + 3H2 = CH4 + H2O(g) CO2 + 4H2 = CH4 + 2H2O(g) | ΔH298K = −247.1 kJ/mol ΔH298K = −206.0 kJ/mol ΔH298K = −164.8 kJ/mol | (4) (5) (6) |
| Steam/CO2 reforming | C7H8(g) + 7H2O(g)= 7CO + 11H2 C7H8(g) + 7CO2 = 14CO + 4H2 | ΔH298K = 869.4 kJ/mol ΔH298K = 1157.5 kJ/mol | (7) (8) |
| Water gas shift | CO + H2O(g) = H2 + CO2 | ΔH298K = −41.2 kJ/mol | (9) |
| Carbon formation | 2CO = CO2 + C CO + H2 = C + H2O(g) CO2+ 2H2 = C + 2H2O(g) CH4 = 2H2 + C | ΔH298K = −131.3 kJ/mol ΔH298K = −90.2 kJ/mol ΔH298K = −172.5 kJ/mol ΔH298K = 74.6 kJ/mol | (10) (11) (12) (13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, J.; Wu, J.; He, T.; Wu, J. Evaluation of Different Oxygen Carriers for Chemical Looping Reforming of Toluene as Tar Model Compound in Biomass Gasification Gas: A Thermodynamic Analysis. Atmosphere 2022, 13, 887. https://doi.org/10.3390/atmos13060887
Wang Z, Zhang J, Wu J, He T, Wu J. Evaluation of Different Oxygen Carriers for Chemical Looping Reforming of Toluene as Tar Model Compound in Biomass Gasification Gas: A Thermodynamic Analysis. Atmosphere. 2022; 13(6):887. https://doi.org/10.3390/atmos13060887
Chicago/Turabian StyleWang, Zhiqi, Jinzhi Zhang, Jingli Wu, Tao He, and Jinhu Wu. 2022. "Evaluation of Different Oxygen Carriers for Chemical Looping Reforming of Toluene as Tar Model Compound in Biomass Gasification Gas: A Thermodynamic Analysis" Atmosphere 13, no. 6: 887. https://doi.org/10.3390/atmos13060887
APA StyleWang, Z., Zhang, J., Wu, J., He, T., & Wu, J. (2022). Evaluation of Different Oxygen Carriers for Chemical Looping Reforming of Toluene as Tar Model Compound in Biomass Gasification Gas: A Thermodynamic Analysis. Atmosphere, 13(6), 887. https://doi.org/10.3390/atmos13060887






