Development of an Indexed Score to Identify the Most Suitable Sampling Method to Assess Occupational Exposure to Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Approach and Analyses Performed
2.2. Indexed Score Applied
2.3. Statistical Analyses Performed
3. Results
3.1. Score Criteria Results
3.1.1. Fungal Species Diversity
3.1.2. Complexity in Field Work
3.1.3. Cost
3.1.4. Complexity in Laboratory Work
3.1.5. Temporal Variation for Results
3.2. Statistical Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sustainable Development—United Nations. Available online: https://sdgs.un.org/goals (accessed on 8 July 2022).
- World Health Organization—Compendium of WHO and Other UN Guidance on Health and Environment. Available online: https://www.who.int/tools/compendium-on-health-and-environment/priority-settings-for-action (accessed on 8 July 2022).
- Rim, K.-T.; Lim, C.-H. Biologically hazardous agents at work and efforts to protect workers’ health: A review of recent reports. Saf. Health Work 2014, 5, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Viegas, C.; Gomes, B.; Dias, M.; Carolino, E.; Caetano, L.A. Occupational exposure to Aspergillus section Fumigati in firefighter headquarters. Microorganisms 2021, 9, 2112. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Caetano, L.A.; Viegas, S. Occupational exposure to Aspergillus section Fumigati: Tackling the knowledge gap in Portugal. Environ. Res. 2021, 194, 110674. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Frederiksen, M.W.; Jacobsen, M.H.; Tendal, K. Towards a risk evaluation of workers’ exposure to handborne and airborne microbial species as exemplified with waste collection workers. Environ. Res. 2020, 183, 109177. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Almeida, B.; Caetano, L.A.; Afanou, A.; Straumfors, A.; Veríssimo, C.; Gonçalves, P.; Sabino, R. Algorithm to assess the presence of Aspergillus fumigatus resistant strains: The case of Norwegian sawmills. Int. J. Environ. Health Res. 2020, 32, 963–971. [Google Scholar] [CrossRef]
- Dias, M.; Gomes, B.; Cervantes, R.; Pena, P.; Viegas, S.; Viegas, C. Microbial Occupational Exposure Assessments in Sawmills—A Review. Atmosphere 2022, 13, 266. [Google Scholar] [CrossRef]
- Ramos, C.A.; Viegas, C.; Verde, S.C.; Wolterbeek, H.T.; Almeida, S.M. Characterizing the fungal and bacterial microflora and concentrations in fitness centres. Indoor Built Environ. 2016, 25, 872–882. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Twarużek, M.; Kosicki, R.; Marchand, G.; Viegas, S. Bioburden in health care centers: Is the compliance with Portuguese legislation enough to prevent and control infection? Build. Environ. 2019, 160, 106226. [Google Scholar] [CrossRef]
- Sastry, A.S.; Bhat, S. Essentials of Medical Microbiology, 1st ed.; Janagond, A., Ed.; The Health Sciences Publisher: London, UK, 2016; Volume 37. [Google Scholar]
- Croston, T.L.; Nayak, A.P.; Lemons, A.R.; Goldsmith, W.T.; Gu, J.K.; Germolec, D.R.; Beezhold, D.H.; Green, B.J. Influence of Aspergillus fumigatus conidia viability on murine pulmonary microRNA and mRNA expression following subchronic inhalation exposure. Clin. Exp. Allergy 2016, 46, 1315–1327. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.; Viegas, C. Fungal Prevalence on Waste Industry—Literature Review. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Jeanvoine, A.; Rocchi, S.; Reboux, G.; Crini, N.; Crini, G.; Millon, L. Azole-resistant Aspergillus fumigatus in sawmills of Eastern France. J. Appl. Microbiol. 2017, 123, 172–184. [Google Scholar] [CrossRef]
- Leppänen, H.K.; Täubel, M.; Jayaprakash, B.; Vepsäläinen, A.; Pasanen, P.; Hyvärinen, A. Quantitative assessment of microbes from samples of indoor air and dust. J. Expo. Sci. Environ. Epidemiol. 2017, 28, 231–241. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Paciência, I.; Rufo, J.C.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Marchand, G.; et al. Settled dust assessment in clinical environment: Useful for the evaluation of a wider bioburden spectrum. Int. J. Environ. Health Res. 2019, 31, 160–178. [Google Scholar] [CrossRef]
- Viegas, C.; Dias, M.; Almeida, B.; Vicente, E.; Caetano, L.A.; Carolino, E.; Alves, C. Settleable Dust and Bioburden in Portuguese Dwellings. Microorganisms 2020, 8, 1799. [Google Scholar] [CrossRef]
- Liebers, V.; Van Kampen, V.; Bünger, J.; Düser, M.; Stubel, H.; Brüning, T.; Raulf-Heimsoth, M. Assessment of airborne exposure to endotoxin and pyrogenic active dust using Electrostatic Dustfall Collectors (EDCs). J. Toxicol. Environ. Health Part A 2012, 75, 501–507. [Google Scholar] [CrossRef]
- Meadow, J.F.; Altrichter, A.; Kembel, S.; Kline, J.; Mhuireach, G.; Moriyama, M.; Northcutt, D.; O’Connor, T.K.; Womack, A.M.; Brown, G.Z.; et al. Indoor airborne bacterial communities are influenced by ventilation, occupancy, and outdoor air source. Indoor Air 2013, 24, 41–48. [Google Scholar] [CrossRef]
- Viegas, C. Sampling methods for an accurate mycobiota occupational exposure assessment—Overview of several ongoing projects. In Occupational Safety and Hygiene VI; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Ribeiro, E.; Faria, I. Analyses Approaches for Bacteria. In Exposure to Microbiological Agents in Indoor and Occupational Environments, 1st ed.; Viegas, C., Viegas, S., Gomes, A., Täubel, M., Sabino, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 97–108. [Google Scholar] [CrossRef]
- Mao, J.; Tang, Y.; Wang, Y.; Huang, J.; Dong, X.; Chen, Z.; Lai, Y. Particulate Matter Capturing via Naturally Dried ZIF-8/Graphene Aerogels under Harsh Conditions. iScience 2019, 16, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, A.; Svingby, G. The use of scoring rubrics: Reliability, validity and educational consequences. Educ. Res. Rev. 2007, 2, 130–144. [Google Scholar] [CrossRef]
- Viegas, C.; Gomes, B.; Pimenta, R.; Dias, M.; Cervantes, R.; Caetano, L.A.; Carolino, E.; Twarużek, M.; Soszczyńska, E.; Kosicki, R.; et al. Microbial contamination in firefighter Headquarters’: A neglected occupational exposure scenario. Build. Environ. 2022, 213, 108862. [Google Scholar] [CrossRef]
- Hoog, D.; Guarro, J.; Gene, G.; Figueras, M. Atlas of Clinical Fungi—The Ultimate Benchtool for Diagnosis; Version 4.1.4; Utr. Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2016. [Google Scholar]
- Reponen, T. Sampling for Microbial Determinations. In Exposure to Microbiological Agents in Indoor and Occupational Environments; Viegas, C., Viegas, S., Gomes, A., Täubel, M., Sabino, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 85–96. [Google Scholar]
- Flannigan, B. Air sampling for fungi in indoor environments. J. Aerosol Sci. 1997, 28, 381–392. [Google Scholar] [CrossRef]
- Viegas, C.; Sousa, P.; Dias, M.; Caetano, L.A.; Ribeiro, E.; Carolino, E.; Twarużek, M.; Kosicki, R.; Viegas, S. Bioburden contamination and Staphylococcus aureus colonization associated with firefighter’s ambulances. Environ. Res. 2021, 197, 111125. [Google Scholar] [CrossRef]
- Bouillard, L.; Michel, O.; Dramaix, M.; Devleeschouwer, M. Bacterial contamination of indoor air, surfaces, and settled dust, and related dust endotoxin concentrations in healthy office buildings. Ann. Agric. Environ. Med. 2005, 12, 187–192. [Google Scholar] [PubMed]
- Macher, J.M. Review of Methods to Collect Settled Dust and Isolate Culturable Microorganisms. Indoor Air 2001, 11, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. 2017, 67, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.; Viegas, C. Fungal Prevalence on Waste Industry—Literature Review. In Encyclopedia of Mycology; Zaragoza, Ó., Casadevall, A., Eds.; Elsevier: Oxford, UK, 2021; Volume 2, pp. 99–106. [Google Scholar]
- Timm, M.; Madsen, A.M.; Hansen, J.V.; Moesby, L.; Hansen, E.W. Assessment of the Total Inflammatory Potential of Bioaerosols by Using a Granulocyte Assay. Appl. Environ. Microbiol. 2009, 75, 7655–7662. [Google Scholar] [CrossRef] [Green Version]
- Dannemiller, K.; Gent, J.F.; Leaderer, B.P.; Peccia, J. Influence of housing characteristics on bacterial and fungal communities in homes of asthmatic children. Indoor Air 2015, 26, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Jürgensen, C.W.; Madsen, A.M. Influence of everyday activities and presence of people in common indoor environments on exposure to airborne fungi. AIMS Environ. Sci. 2016, 3, 77–95. [Google Scholar] [CrossRef]
- Pongracic, J.A.; O’Connor, G.T.; Muilenberg, M.L.; Vaughn, B.; Gold, D.R.; Kattan, M.; Morgan, W.J.; Gruchalla, R.S.; Smartt, E.; Mitchell, H.E. Differential effects of outdoor versus indoor fungal spores on asthma morbidity in inner-city children. J. Allergy Clin. Immunol. 2010, 125, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Bundy, K.W.; Gent, J.F.; Beckett, W.; Bracken, M.B.; Belanger, K.; Triche, E.; Leaderer, B.P. Household airborne Penicillium associated with peak expiratory flow variability in asthmatic children. Ann. Allergy Asthma Immunol. 2009, 103, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Inal, A.; Karakoc, G.B.; Altintas, D.U.; Guvenmez, H.K.; Aka, Y.; Gelisken, R.; Yilmaz, M.; Kendirli, S.G.; Altıntaş, D.U.; Yılmaz, M. Effect of Indoor Mold Concentrations on Daily Symptom Severity of Children with Asthma and/or Rhinitis Monosensitized to Molds. J. Asthma 2007, 44, 543–546. [Google Scholar] [CrossRef]
- Turyk, M.; Curtis, L.; Scheff, P.; Contraras, A.; Coover, L.; Hernández, E.; Freels, S.; Persky, V. Environmental Allergens and Asthma Morbidity in Low-Income Children. J. Asthma 2006, 43, 453–457. [Google Scholar] [CrossRef]
- Beard, J.T.; Iachetta, F.A.; Lilleleht, L.U. APTI (Air Pollution Training Institute) Course 427: Combustion Evaluation, Student Manual; Associated Environmental Consultants: Charlottesville, VA, USA, 1980. [Google Scholar]
- Santos, J.; Ramos, C.; Vaz-Velho, M.; Vasconcelos Pinto, M. Occupational Exposure to Biological Agents. In Advances in Safety Management and Human Performance; Advances in Intelligent Systems and Computing; Arezes, P.M., Boring, R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 61–67. [Google Scholar]
- Nevalainen, A.; Pastuszka, J.; Liebhaber, F.; Willeke, K. Performance of bioaerosol samplers: Collection characteristics and sampler design considerations. Atmos. Environ. Part A Gen. Top. 1992, 26, 531–540. [Google Scholar] [CrossRef]
- Dias, M.; Sousa, P.; Viegas, C. Occupational Exposure to Bioburden in Portuguese Ambulances. In Occupational and Environmental Safety and Health III; Studies in Systems, Decision and Control; Arezes, P.M., Baptista, J.S., Carneiro, P., Castelo Branco, J., Costa, N., Duarte, J., Guedes, J.C., Melo, R.B., Miguel, A.S., Perestrelo, G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 167–173. [Google Scholar] [CrossRef]
- Badyda, A.; Gayer, A.; Czechowski, P.O.; Majewski, G.; Dąbrowiecki, P. Pulmonary Function and Incidence of Selected Respiratory Diseases Depending on the Exposure to Ambient PM10. Int. J. Mol. Sci. 2016, 17, 1954. [Google Scholar] [CrossRef] [Green Version]
- Viegas, C.; Monteiro, A.; Caetano, L.A.; Faria, T.; Carolino, E.; Viegas, S. Electrostatic Dust Cloth: A Passive Screening Method to Assess Occupational Exposure to Organic Dust in Bakeries. Atmosphere 2018, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.; Mbareche, H.; Lindsley, W.G.; Duchaine, C. Field sampling of indoor bioaerosols. Aerosol Sci. Technol. 2019, 54, 572–584. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Viegas, S. Aspergillus Spp. Prevalence in Different Portuguese Occupational Environments: What Is the Real Scenario in High Load Settings? J. Occup. Environ. Hyg. 2017, 14, 771–785. [Google Scholar] [CrossRef]
- Cox, J.; Indugula, R.; Vesper, S.; Zhu, Z.; Jandarov, R.; Reponen, T. Comparison of indoor air sampling and dust collection methods for fungal exposure assessment using quantitative PCR. Environ. Sci. Process. Impacts 2017, 19, 1312–1319. [Google Scholar] [CrossRef]
- Szulc, J.; Okrasa, M.; Majchrzycka, K.; Sulyok, M.; Nowak, A.; Ruman, T.; Nizioł, J.; Szponar, B.; Gutarowska, B. Microbiological and Toxicological Hazards in Sewage Treatment Plant Bioaerosol and Dust. Toxins 2021, 13, 691. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; Meneses, M.; Carolino, E.; Viegas, S.; Gomes, A.; Sabino, R.F.P. Analysis of surfaces for characterization of fungal burden—Does it matter? Int. J. Occup. Med. Environ. Health 2016, 29, 623–632. [Google Scholar] [CrossRef] [Green Version]

| Sampling Methods | Description | |
|---|---|---|
| Andersen six-stage | This sampling method requires a small, yet specific, and technical, number of steps. All the equipment parts must be disinfected between sampling sites. After the disinfection, a plate from the chosen media needs to be placed in each of the six stages and the equipment must be properly closed to ensure a suitable sampling. The equipment has only a power switch, which means that the collection time must be controlled by the user depending on the number of litres needed, which is directly dependent on the expected contamination from the sampling site. |  |
| Millipore air sampler | Millipore air sampler requires a small number of steps. The top part of the equipment (where the air passes) must be disinfected between sampling sites. After the disinfection, a plate from the chosen media needs to be placed into the equipment which must be properly closed to ensure the sampling. The equipment allows the choice of the number of liters needed which is dependent on the estimated contamination from the sampling location. The device automatically controls the time of the sampling collection. |  |
| Surface swabs | Sampling with surface swabs requires the use of not only the swab, but also a square of known size, to limit a portion of the surface and make it possible to later calculate the densities per square meter. To facilitate the sampling collection, it is advisable to wet the swab in saline or distilled water. |  |
| EDC | EDC have a very short sampling protocol since it is only necessary to place them in the sampling site. However, the cloth to be used has to be sterilized under UV light. It is recommended to attach the plate with tape to the collection site to minimize disturbance during the sampling period. |  |
| Settled dust | This sampling method has an extraction protocol with a few steps, although it depends on the equipment used. The vacuuming filter needs to be properly attached to the equipment. The collection time must be controlled by the user, depending on the number of grams needed to perform the analyses. The filter and/or the dust (depending on the equipment used) must be stored in a sterile bag. |  |
| Sampling Methods | Criteria Application |
|---|---|
| Andersen six-stage | Considering the complexity of handling the equipment and the sampling, we determined that it requires training, and technical expertise, as it requires knowledge of the equipment’s operation and purpose. In terms of the complexity of lab work and protocols, as well as the overall complexity of all work (from the field to the lab) and time consumption, it is a relatively easy sampling approach because all that is required after sampling is to incubate the plates before densities calculation and identification. In terms of cost, this sample method requires little processing material (plates and media), but it is dependent on the acquisition of equipment. |
| Millipore | As per the previous sampling method, the device employment will require training and technical skill because it demands an understanding of the equipment’s operation and purpose. It is a remarkably straightforward sampling method in terms of lab work and protocols, as well as the overall complexity of all work (from the field to the lab) and time consumption, because all that is necessary after sampling is to incubate the plates before densities calculation and identification. Concerning costs, this sampling method is similar to the preceding method and only dependent on the equipment purchase, besides plates and media. |
| Surface swabs | The surface swabs technique is easy to perform. As in the other sampling methods, it demands knowledge of the sampling protocol. It is considered an accessible sampling approach since, while it does require an extraction protocol, it only has a few steps that take little time. In terms of cost, this sample approach involves the use of additional materials, but it is not dependent on equipment, and only needs some lab consumables and the extraction solution, besides plates and media. |
| EDC | This also needs comprehension of the sampling protocol and the specifics, and requires specific training. Since the EDC must stay in the sampling location for 15 to 30 days, the extraction protocol comprises several steps that take time and, considering the complexity of all the work (from the field to the lab), it was rated as a difficult sample method. This sampling method involves the use of additional lab materials (besides plates and media) and consumables (extraction solution), but it is not reliant on equipment. |
| Settled dust | This sampling method also requires training. Regarding the field and lab work after collection and the time required, it was considered more difficult than the other passive sampling methods employed. Concerning costs, this sampling method needs less specific equipment which is less expensive than the equipment used in active sampling, besides lab materials and consumables (extraction solution). |
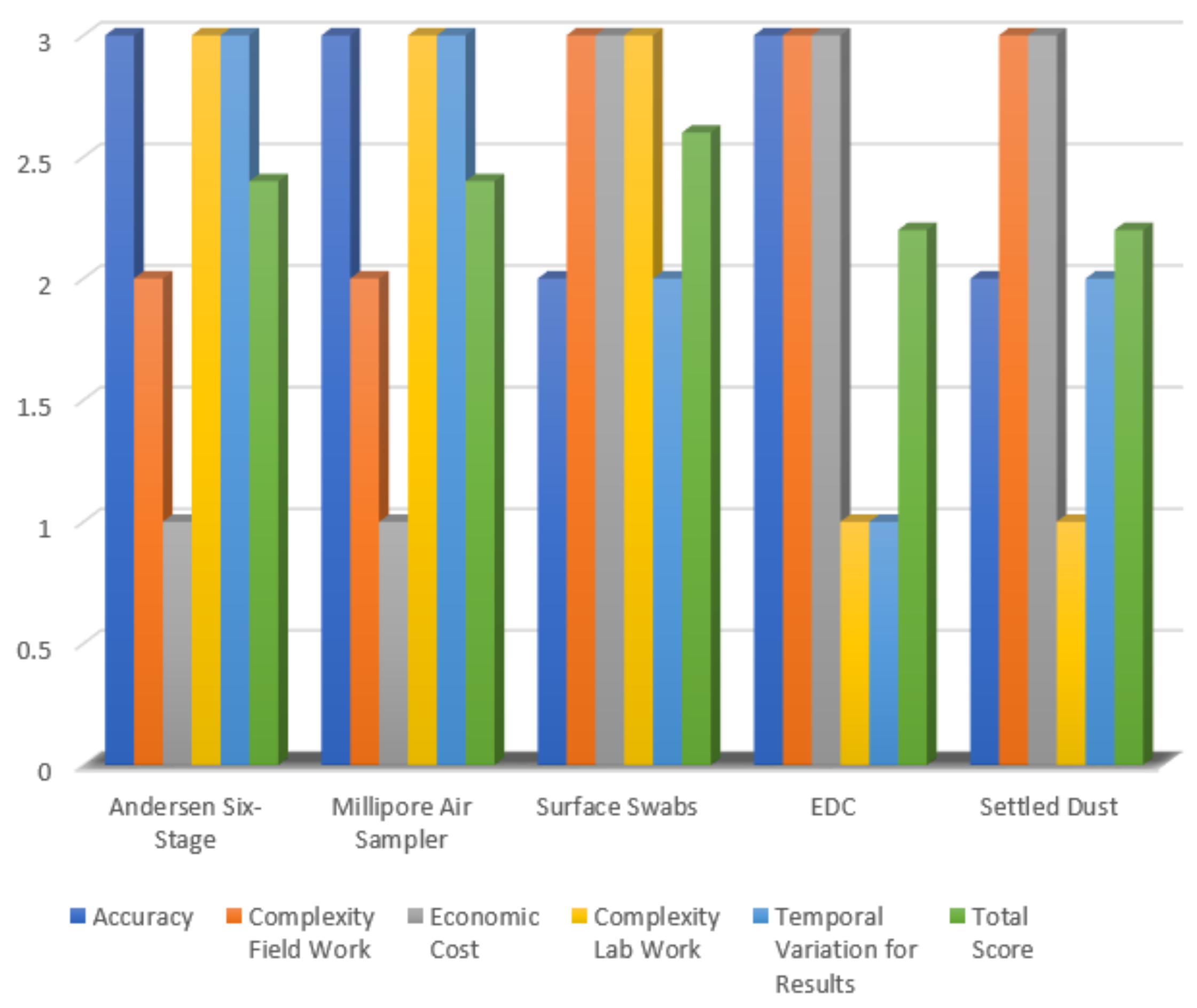
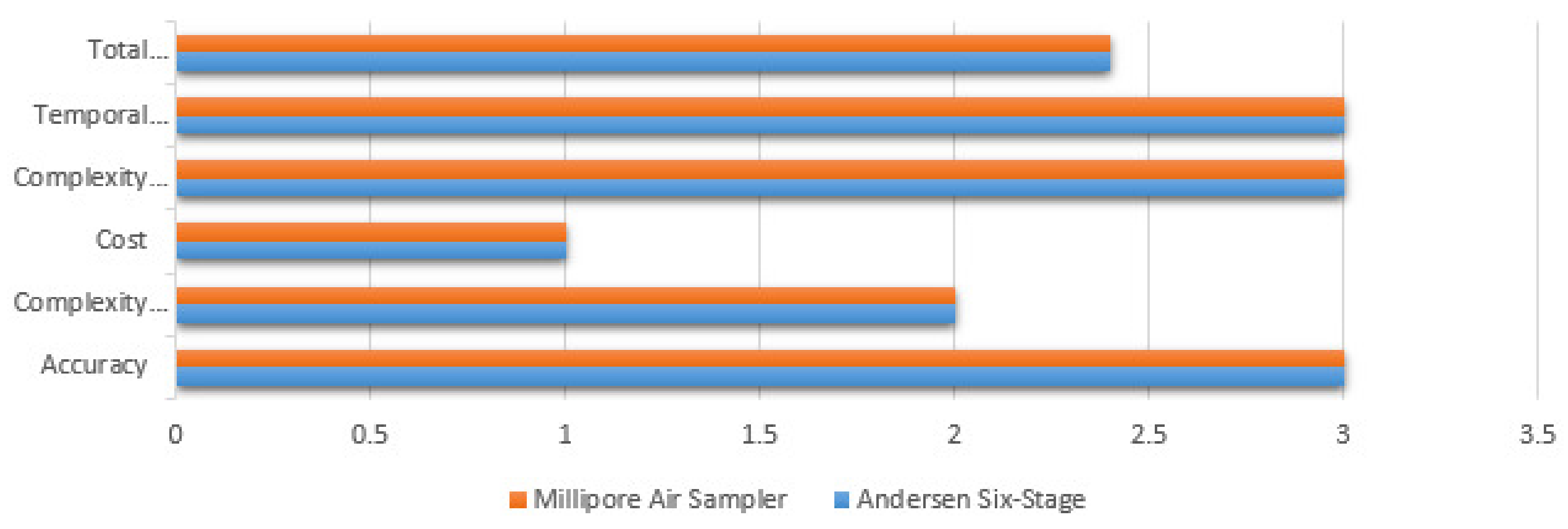
| Independent Variables | Andersen Six-Stage | Millipore Air Sampler | Surface Swabs | EDC | Settled Dust |
|---|---|---|---|---|---|
| Accuracy | 3 | 3 | 2 | 3 | 2 |
| Field Work Complexity | 2 | 2 | 3 | 3 | 3 |
| Economic Cost | 1 | 1 | 3 | 3 | 3 |
| Lab Work Complexity | 3 | 3 | 3 | 1 | 1 |
| Temporal Variation for Results | 3 | 3 | 2 | 1 | 2 |
| Total Score | 2.4 | 2.4 | 2.6 | 2.2 | 2.2 |
| Model | Independent Variables | |Devience/ (n-k-1)| | AIC | Omnibus Test | Model Effect | ||||
|---|---|---|---|---|---|---|---|---|---|
| X2 | Degrees of Freedom | Sig. | X2 | Degrees of Freedom | Sig. | ||||
| Model 1 | Sampling Site | 1.102 | 473.654 | 7.053 | 8 | 0.531 | 7.153 | 8 | 0.52 |
| Model 2 | Culture Media | 1.092 | 440.014 | 0.194 | 1 | 0.659 | 0.194 | 1 | 0.659 |
| Model 3 | Sampling Method | 0.56 | 409.997 | 48.711 | 4 | <0.001 | 46.717 | 4 | <0.001 |
| Model 4 | Sampling Method + Sampling Site | 0.527 | 446.942 | 55.764 | 12 | <0.001 | 46.717 | 4 | <0.001 |
| 7.153 | 8 | 0.52 | |||||||
| Model 5 | Sampling Method + Sampling Site + Culture Media | 0.531 | 452.248 | 55.958 | 13 | <0.001 | 46.717 | 4 | <0.001 |
| 7.153 | 8 | 0.52 | |||||||
| 0.194 | 1 | 0.659 | |||||||
| Parameter | B | Std. Error | 95% Wald Confidence Interval | Hypothesis Testing | Exp(B) | 95% Wald Confidence Interval for Exp(B) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Wald Chi-Square | df | Sig. | Lower | Upper | ||||
| (Intercept) | 2.140 | 0.0808 | 1.982 | 2.299 | 700.722 | 1 | 0.000 | 8.500 | 7.254 | 9.959 |
| Andersen six-stage | 0.129 | 0.1108 | −0.089 | 0.346 | 1.347 | 1 | 0.246 | 1.137 | 0.915 | 1.413 |
| Millipore air sampler | −0.294 | 0.1237 | −0.537 | −0.052 | 5.656 | 1 | 0.017 | 0.745 | 0.585 | 0.950 |
| EDC | −0.376 | 0.1267 | −0.625 | −0.128 | 8.825 | 1 | 0.003 | 0.686 | 0.535 | 0.880 |
| Surface swabs | −0.687 | 0.1397 | −0.960 | −0.413 | 24.149 | 1 | 0.000 | 0.503 | 0.383 | 0.662 |
| Settled dust | 0 a | 1 | ||||||||
| (Scale) | 1 b | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes, R.; Dias, M.; Gomes, B.; Carolino, E.; Viegas, C. Development of an Indexed Score to Identify the Most Suitable Sampling Method to Assess Occupational Exposure to Fungi. Atmosphere 2022, 13, 1123. https://doi.org/10.3390/atmos13071123
Cervantes R, Dias M, Gomes B, Carolino E, Viegas C. Development of an Indexed Score to Identify the Most Suitable Sampling Method to Assess Occupational Exposure to Fungi. Atmosphere. 2022; 13(7):1123. https://doi.org/10.3390/atmos13071123
Chicago/Turabian StyleCervantes, Renata, Marta Dias, Bianca Gomes, Elisabete Carolino, and Carla Viegas. 2022. "Development of an Indexed Score to Identify the Most Suitable Sampling Method to Assess Occupational Exposure to Fungi" Atmosphere 13, no. 7: 1123. https://doi.org/10.3390/atmos13071123







