The Effect of Diluent on the Release of Benzene Series from Nitrocellulose-Lacquered MDF
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Instruments
- (1)
- Fifteen L small environmental chamber: It was independently developed by the laboratory of Northeast Forestry University, and it had good air-tightness. It has been verified to have a good correlation with the gas release measured of the 1 m3 climate chamber [10];
- (2)
- Intelligent vacuum pump, which was produced by Chengdu Xinweicheng Technology Co., Ltd. (Chengdu, China), was used for collecting cabin gas;
- (3)
- Tenax tube, which was produced by Markes Company (Llantrisant, UK), was used for adsorbing gas;
- (4)
- Thermal analysis of the aging instrument, which was produced by Beijing Beifen Temple Instrument Technology Co., Ltd. (Beijing, China), removed the adsorbent in the tube under a high temperature;
- (5)
- Gas analysis equipment: Thermal desorption autosampler, thermal desorption instrument and DSQII GC-MS. The thermal desorption autosampler was manufactured by Markes (Llantrisant, UK), and the model was Ultra100 bit autosampler. The thermal desorption instrument was produced by Markes Company in the UK, and the host system Unity was connected to the GC-MS equipment; the carrier gas was nitrogen [17]. The GC-MS equipment was manufactured by Thermo (Waltham, MA, USA).
2.3. Experimental Methods
- (1)
- Veneer-overlaid MDF: The veneer of Manchuria japonica was used. The hot pressing temperature of the MDF was within the range of 180~230 °C, and the hot pressing factor was 10 s/mm. The MDF was kept in the lab for 30 d after it had been taken off production line. The adhesive used in the hot pressing of the overlaid veneer was prepared independently. The modified urea–formaldehyde resin glue and emulsion glue were mixed at the ratio of 6:4, and 80 g/m2 amount of finish was used. After the secondary overlaid operation of hot pressing at the temperature of 165 °C for 60 s and the cutting the veneer overlaid MDF were performed, the boards with dimensions of (length × width × thickness) 150 mm × 75 mm × 8 mm and 150 mm × 75 mm × 18 mm were obtained [9].
- (2)
- Surface painting: After the veneer overlaid board was left to dry naturally at room temperature, the surface was polished using an electric sander, and the floating dust was cleaned using a brush. Then, the plate without bubbling and cracking was selected for painting. The main paint and diluent at a ratio of 2:1 were used for the bottom and surface paint. The bottom and surface paint were applied three times and twice, respectively. The sandpaper with the sand mesh of 400# was used to finely polish the surface before each painting stage, and the finish quality of the products meets the requirements of “GB/T 37005-2018 Painted Veneered Wood-based panel”. At the end of the painting process, the specimen sides were sealed with aluminum tape so that the Benzene Series could only be released from the top and bottom surface of the board. Finally, the painted specimens were placed in a naturally ventilated room with an ambient temperature of 23 °C for 28 d, then, the Benzene Series released from the painted MDF were collected and analyzed after reaching balance (see GB/T 29899-2013).
- (3)
- Collection of Benzene Series: In the experiment, the indoor environment was simulated with a 15 L, small environmental chamber [18]. The MDF and painted MDF were placed in the middle of the chamber for 4 h for balanced circulation, and then, they were sampled with a loading factor of 1.5 m2/m3 for both of them. The basic parameters of the environment chamber were set as follows: a gas exchange rate of 1 h−1, a room temperature of (23 ± 0.5) °C, and a relative humidity of (50 ± 3)%. The intelligent vacuum pump was used to collect gas for 12 min, and the gas flow rate was 250 mL/min. A total of 3 L of gas was collected. [9] The gas was collected 3~6 times in each coating condition. After the collection, brass caps were used to seal both ends of the adsorption tube for detection.
- (4)
- Gas analysis: The adsorption tube after the gas collection was heated and desorbed by a thermal desorption instrument, and the gas was pressurized and injected into the GC-MS. The carrier gas was high-purity helium; the chromatographic column type was DB-5MS; the resolution temperature was 300 °C; the pipeline temperature was 85 °C; the thermal resolution time was 10 min. According to China’s Indoor Air Quality Standard (GB/T18883-2002) [19], qualitative and quantitative analyses were conducted on all of the Benzene Series released from the board [8]. Based on their peak diagrams and total ion flow diagrams, the release of Benzene Series from different MDF was determined.
3. Results and Discussion
3.1. Analysis of Benzene Series Released from MDF and Lacquered MDF
3.2. The Effect of Diluent on the Release of Benzene Series from Decorative NC-Lacquered MDF
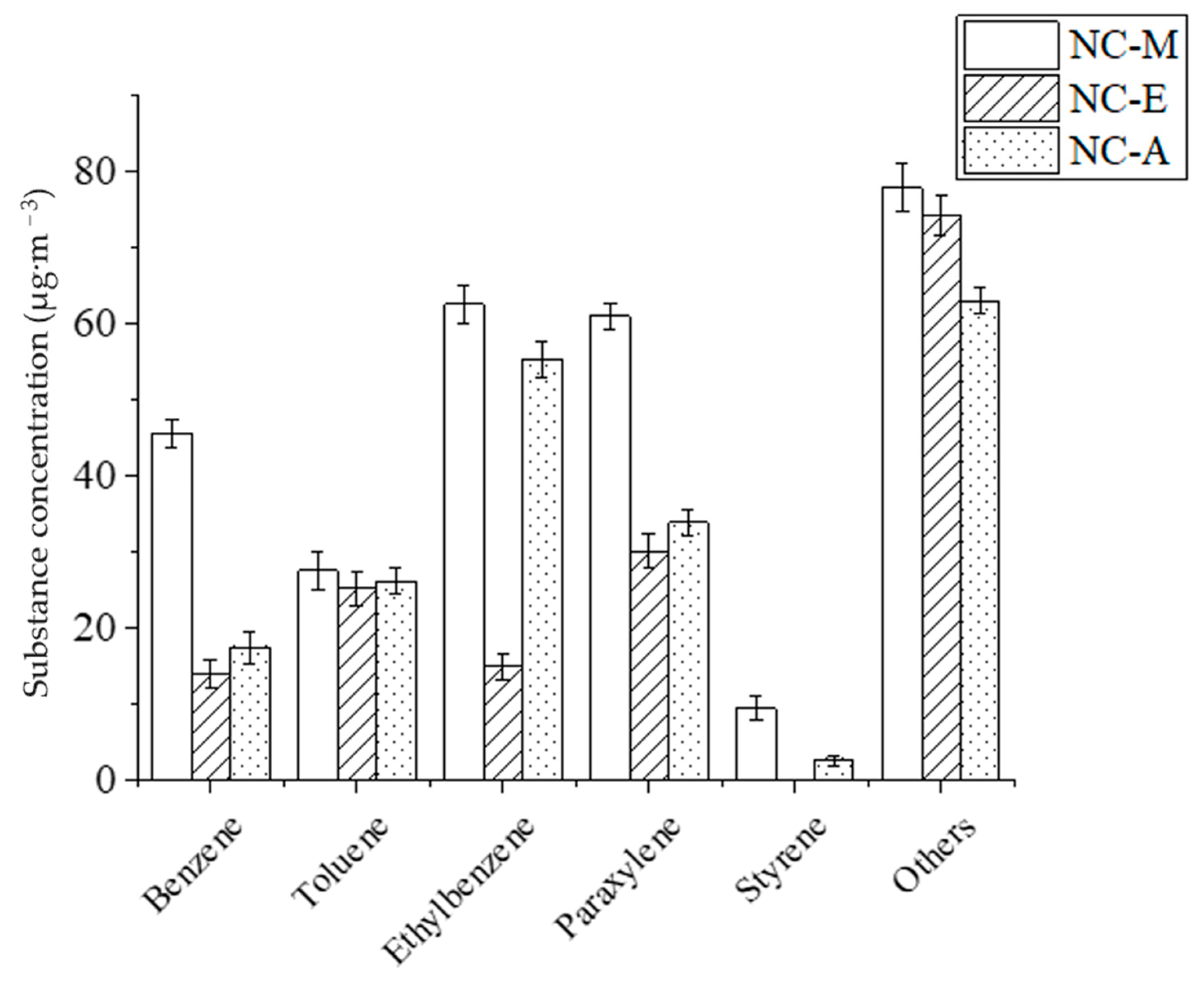
3.2.1. The Effect of Diluent on the Release Concentration of Benzene Series from NC-Painted MDF
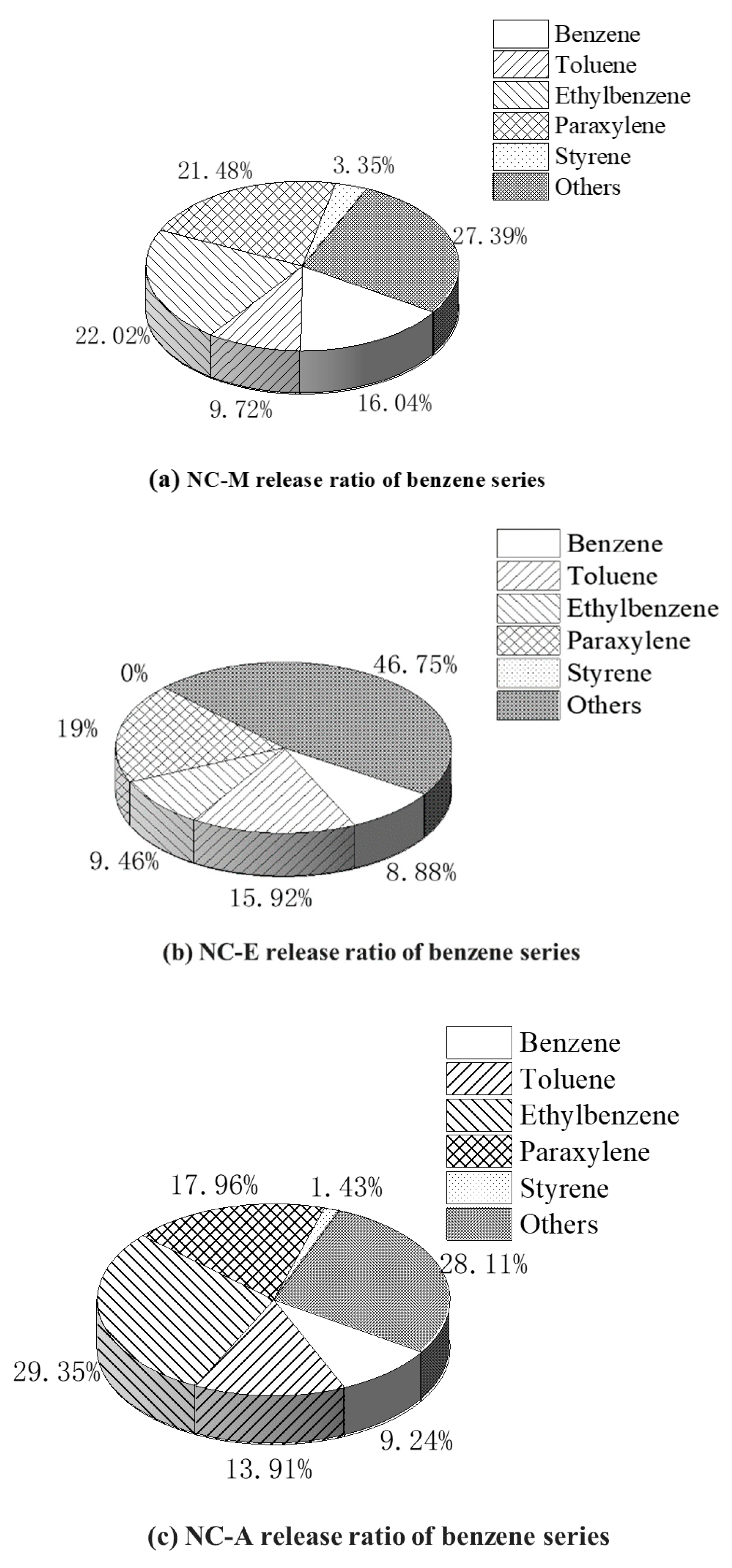
3.2.2. The Effect of Diluent on the Release Proportion of Benzene Series from NC-Painted MDF
3.2.3. The Effect of Alcohol Ester Ratio on the Release of Benzene Series from NC-Painted MDF
4. Conclusions
- (1)
- The concentration of Benzene Series released by MDF was 316.24 μg·m−3, and there are a total of 14 kinds of substances;
- (2)
- The concentrations of Benzene Series released by the NC-lacquered MDF with thicknesses of 8 mm and 18 mm were 281.06 μg·m−3 and 284.44 μg·m−3, respectively. The thicker MDF was, the higher the concentration of Benzene Series released was. However, the thickness of the MDF has no effect on the type of Benzene Series released. The NC-lacquered MDF with two thicknesses released 18 kinds of substances;
- (3)
- The release concentration order of Benzene Series in NC-lacquered MDF with different diluents on the 18 mm thickness panel is NC-M, NC-A, and NC-E from high to low;
- (4)
- The experimental data of the current study also adequately proves that single alcohols or ester solvents have an obvious effect on reducing the release of Benzene Series, and esters are one of the ideal choices for lacquer diluents, and where conditions permit, replacing mixed solvent containing Benzene Series with esters will reduce the release of Benzene Series from the source. To reduce the cost of the solvents, people can add a small amount of alcohol in the formula; we advise that they should try not to choose benzene as an auxiliary solvent;
- (5)
- When MDF was painted with a mixed diluent prepared using different proportions of alcohol and ester, the Benzene Series released the lowest concentration the ratio of anhydrous ethanol to ethyl acetate of 1:3, and the Benzene Series released the lowest amount of components at the ratio of 1:2. Obviously the use of alcohol and ester thinner can reduce the release of Benzene Series, and the mixing ratio of 1:3 is recommended.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H. Overview of present situation and development trend of medium density MDF production in the world. Wood Ind. 1991, 5, 43–45. [Google Scholar]
- Lu, z.; Wang, Q.; Sun, G.; Wu, L. Research on the harmfulness of different veneer particleboards based on multiple VOC coexistence evaluation method. For. Eng. 2020, 36, 49–54+80. [Google Scholar]
- Huang, H. Determination and Pollution Control of Benzene Series Compounds in air. Master's Thesis, South-Central University for Nationalities, Wuhan, China, 2013. [Google Scholar]
- Zhong, Y. Determination of the Benzene Series in Solid Waste Leachate by Headspace- Gas chromatography / Mass spectrometry. Arid Environ. Monit. 2016, 30, 58–61. [Google Scholar]
- Standeker, S.; Novak, Z.; Knez, Z. Removal of BTEX vapours from waste gas streams using silica aerogels of different hydrophobicity. J. Hazard. Mater. 2009, 165, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Hamidin, N.; Yu, J.; Phung D., T.; Connell, D.; Chu, C. Volatile aromatic hydrocarbons (VAHs) in residential indoor air in Brisbane. Chemosphere 2013, 92, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Guang, Y. Study on the Harm of Formaldehyde and Benzene to Female Reproduction in Decoration Room. Master's Thesis, University of South China, Hengyang, China, 2011. [Google Scholar]
- Dong, H.; Jiang, L.; Shen, J.; Zhao, Z.; Wang, Q.; Shen, X. Identification and analysis of odor-active substances from PVC-overlaid MDF. Environ. Sci. Pollut. Res. 2019, 26, 20769–20779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, J.; Shao, Y.; Dong, H.; Li, Z.; Shen, X. Volatile organic compounds and odor emissions from veneered particleboards coated with water-based lacquer detected by gas chromatography-mass spectrometry/olfactometry. Eur. J. Wood Wood Prod. 2019, 77, 771–781. [Google Scholar]
- Wang, Q.; Shen, J.; Du, J.; Cao, T.; Xiwei, S. Characterization of odorants in particleboard coated with nitrocellulose lacquer under different environment conditions. For. Prod. J. 2018, 68, 272–280. [Google Scholar]
- Wang, L.; Li, R.; Zhao, Z.; Liu, Z.; Bai, Y. The effect of environmental parameters on Benzene Series emission for nitrocellulose varnish. Environ. Chem. 2014, 33, 584–590. [Google Scholar]
- Wang, L.; Liu, Z.; Bai, Y.; Zhang, Y. Effect of paint film thickness on VOCs emission from nitrocellulose varnish. China Environ. Sci. 2009, 29, 790–794. [Google Scholar]
- Han, S. Study on application and detection technology of coating nitrocellulose. Master's Thesis, Northwest University, Xi’an, China, 2018. [Google Scholar]
- Yang, Y.; Chen, D. Research of 35%, 45% Environmental Protection Type Nitric Decorative Varnish. Guangzhou Chem. Ind. 2005, 33, 47–49+73. [Google Scholar]
- Qi, Y.; Li, X.; Huang, Q. VOC Emission Control in Wood Furniture Nitro Paint Finishing Workshop. For. Sci. Technol. Dev. 2015, 29, 11–16. [Google Scholar]
- Wang, Q.; Shen, J.; Zeng, B.; Wang, H. Identification and analysis of odor-active compounds from Choerospondias axillaris (Roxb.) Burtt et Hill with different moisture content levels and lacquer treatments. Sci. Rep. 2020, 10, 14856. [Google Scholar] [CrossRef]
- Shao, Y.; Shen, J.; Shen, X.; Qin, J. Effect of panel area-volume ratio on TVOC released from decorative particleboards. Woods Fiber Sci. 2018, 50, 1–11. [Google Scholar]
- Wang, Q.; Shen, J.; Cao, T.; Du, J.; Dong, H.; Shen, X. Emission characteristics and health risks of volatile organic compounds and odor from PVC-overlaid particleboard. BioResources 2019, 14, 4385–4402. [Google Scholar]
- GB/T 18883-2002; Indoor Air Quality Standard (S). Standards Press of China: Beijing, China, 2002.
- Zhang, Y.; Xie, Z. Environmental biogeochemical processes of styrene and human health. 2012 Annual Conference Proceedings of Chinese Society of Environmental Sciences, Shanghai, China, 9–11 September 2012; Volume 4, pp. 791–794. [Google Scholar]
- Guo, Y. Related Regulations and Laws for Application of Plasticizer Phthalates in Food Packing Materials. Plast. Addit. 2012, 4, 18–20+39. [Google Scholar]
- Gao, Z. Source characteristics of VOC emissions from typical industrial painting sources. Master's Thesis, South China University of Technology, Guangzhou, China, 2015. [Google Scholar]

| Composition of Benzene Series | Molecular Formula | Structural Formula | Functional Group | Toxicity | Painted MDF (18 mm in Thickness) | Painted MDF (8 mm in Thickness) | MDF (18 mm in Thickness) |
|---|---|---|---|---|---|---|---|
| Benzene | C6H6 |  | Non | Low toxicity | 45.61 | 45.42 | 44.40 |
| Methylbenzene | C7H8 |  | Methyl | Low toxicity | 27.65 | 14.75 | 60.24 |
| Ethylbenzene | C8H10 |  | Ethyl | Low toxicity | 62.62 | 63.35 | 59.37 |
| P-xylene | C8H10 |  | Methyl | Slight toxicity | 61.10 | 72.11 | 43.17 |
| Benzaldehyde | C7H6O |  | Aldehyde group | Low toxicity | 3.09 | 3.82 | / |
| Styrene | C8H8 |  | Carbon–carbon double bond | Slight toxicity | 9.54 | 7.54 | / |
| 1-methylene 1 H-ninhydrin | C10H8 |  | Carbon–carbon double bond | Low toxicity | 2.57 | 2.45 | / |
| 2-vinylnaphthalene | C12H10 |  | Carbon–carbon double bond | Low toxicity | 5.22 | 3.61 | 4.98 |
| Biphenyl | C12H10 |  | / | Low toxicity | 1.96 | 1.51 | 10.47 |
| Acenaphthene | C12H10 |  | / | Low toxicity | 3.14 | 3.79 | 12.38 |
| Dimethyl phthalate | C10H10O4 |  | / | Low toxicity | 3.45 | 3.58 | 3.15 |
| Dibutyl phthalate | C6H22O4 |  | Ester group | Low toxicity | 28.57 | 27.65 | 21.49 |
| 1,2,3-trimethylbenzene | C9H12 |  | Methyl | Low toxicity | 1.59 | 1.21 | 5.31 |
| Naphthalene | C10H8 |  | / | Low toxicity | 4.09 | 4.20 | 21.20 |
| Acetophenone | C8H8O |  | Hydroxyl | Low toxicity | 1.49 | 2.42 | / |
| Diphenylene-oxide | C12H8O |  | Hydroxyl | Low toxicity | 6.91 | 6.14 | 1.95 |
| Fluorene | C13H10 |  | / | Low toxicity | 10.12 | 11.59 | 16.64 |
| 1-methyl naphthalene | C11H10 |  | Methyl | Low toxicity | / | / | 11.49 |
| 9-methylene-9H-fluorene | C14H10 |  | Carbon–carbon double bond | Low toxicity | 5.72 | 5.92 | / |
| Total | - | - | - | - | 284.44 | 281.06 | 316.24 |
| Composition of Benzene Series | Molecular Formula | Structural Formula | Functional Group | Toxicity | NC-M | NC-A | NC-E |
|---|---|---|---|---|---|---|---|
| Benzene | C6H6 |  | / | Low toxicity | 45.61 | 14.1 | 17.44 |
| Methylbenzene | C7H8 |  | Methyl | Low toxicity | 27.65 | 25.28 | 26.26 |
| Ethylbenzene | C8H10 |  | Ethyl | Low toxicity | 62.62 | 15.02 | 55.41 |
| P-xylene | C8H10 |  | Methyl | Slight toxicity | 61.10 | 30.16 | 33.90 |
| Benzaldehyde | C7H6O |  | Aldehyde group | Low toxicity | 3.09 | 5.32 | 4.51 |
| Styrene | C8H8 |  | Carbon–carbon double bond | Slight toxicity | 9.54 | / | 2.69 |
| 1-methylene 1 H-ninhydrin | C10H8 |  | Carbon–carbon double bond | Low toxicity | 2.57 | 2.61 | 2.28 |
| 2-vinylnaphthalene | C12H10 |  | Carbon–carbon double bond | Low toxicity | 5.22 | 3.34 | / |
| Biphenyl | C12H10 |  | / | Low toxicity | 1.96 | / | 1.71 |
| Acenaphthene | C12H10 |  | / | Low toxicity | 3.14 | / | 3.48 |
| Dimethyl phthalate | C10H10O4 |  | / | Low toxicity | 3.45 | 5.78 | 3.63 |
| Dibutyl phthalate | C6H22O4 |  | Ester group | Low toxicity | 28.57 | 26.52 | 18.22 |
| 1,2,3-trimethylbenzene | C9H12 |  | Methyl | Low toxicity | 1.59 | 3.36 | / |
| Naphthalene | C10H8 |  | / | Low toxicity | 4.09 | 5.29 | 3.99 |
| Acetophenone | C8H8O |  | / | Low toxicity | 1.49 | / | 3.63 |
| Dibenzofuran | C12H8O |  | Carboxyl | Low toxicity | 6.91 | 7.36 | 6.02 |
| Fluorene | C13H10 |  | / | Low toxicity | 10.12 | 9.44 | 9.01 |
| 9-methylene-9H-fluorene | C14H10 |  | Carbon–carbon double bond | Low toxicity | 5.72 | 5.24 | 6.58 |
| Total | - | - | - | - | 284.44 | 198.76 | 158.82 |
| Benzene Series | Alcohol: Ester = 2:1 | Alcohol: Ester = 1:1 | Alcohol: Ester = 1:2 | Alcohol: Ester = 1:3 |
|---|---|---|---|---|
| Benzene | 40.75 | 52.35 | 41.32 | 31.34 |
| Methylbenzene | 43.68 | 27.83 | 42.14 | 36.45 |
| Ethylbenzene | 14.05 | 14.64 | 13.61 | 11.51 |
| P-xylene | 34.05 | 36.23 | 30.52 | 28.04 |
| Benzaldehyde | 5.73 | 7.98 | 4.73 | 4.08 |
| 1,2,4-trimethyl-benzene | 3.90 | 3.83 | 3.46 | 3.67 |
| Naphthalene | 4.81 | 6.56 | 7.24 | / |
| 1-ethylene-1H-ninhydrin | 2.24 | 2.26 | 2.19 | 2.08 |
| Dibenzofuran | 5.75 | 5.83 | 5.27 | 5.35 |
| Diethyl phthalate | 3.35 | 4.44 | 4.29 | 3.15 |
| Fluorene | 10.65 | 10.32 | 11.74 | 10.11 |
| Phenanthrene | 7.95 | 8.42 | 12.50 | 5.52 |
| Dimethyl phthalate | 7.08 | 8.12 | 7.27 | 3.94 |
| Acenaphthene | 3.14 | / | 3.39 | 3.11 |
| 1-methylene-1H-ninhydrin | 9.43 | 5.67 | / | 3.99 |
| Dibutyl phthalate | 18.83 | 14.52 | / | 12.34 |
| Styrene | / | 1.77 | / | 1.58 |
| Total concentration | 215.39 | 209.77 | 189.67 | 163.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Ma, J.; Li, H. The Effect of Diluent on the Release of Benzene Series from Nitrocellulose-Lacquered MDF. Atmosphere 2023, 14, 21. https://doi.org/10.3390/atmos14010021
Cui X, Ma J, Li H. The Effect of Diluent on the Release of Benzene Series from Nitrocellulose-Lacquered MDF. Atmosphere. 2023; 14(1):21. https://doi.org/10.3390/atmos14010021
Chicago/Turabian StyleCui, Xiaolei, Junhong Ma, and Huifang Li. 2023. "The Effect of Diluent on the Release of Benzene Series from Nitrocellulose-Lacquered MDF" Atmosphere 14, no. 1: 21. https://doi.org/10.3390/atmos14010021
APA StyleCui, X., Ma, J., & Li, H. (2023). The Effect of Diluent on the Release of Benzene Series from Nitrocellulose-Lacquered MDF. Atmosphere, 14(1), 21. https://doi.org/10.3390/atmos14010021





