The Responses of N2O, CO2 Emissions, and Bacterial Communities to Nitrogen Addition in Saline–Alkaline Wetlands of Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples and Experimental Design
2.2. Soil Gas Emissions and Physicochemical Characteristics Analysis
2.3. High-Throughput Sequencing Analysis
2.4. Statistical Analysis
3. Results
3.1. Greenhouse Gas Emissions and Soil Physicochemical Properties
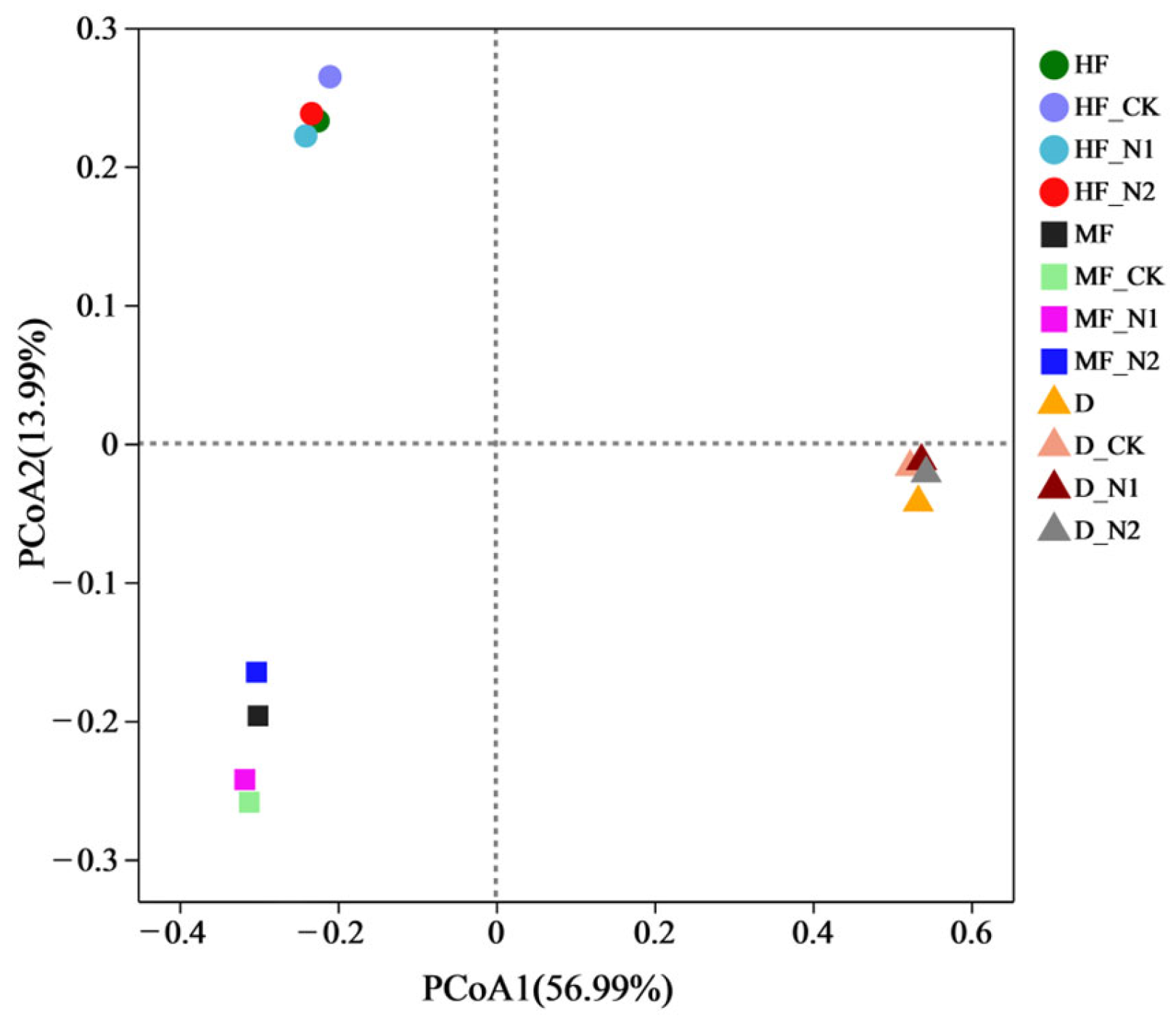
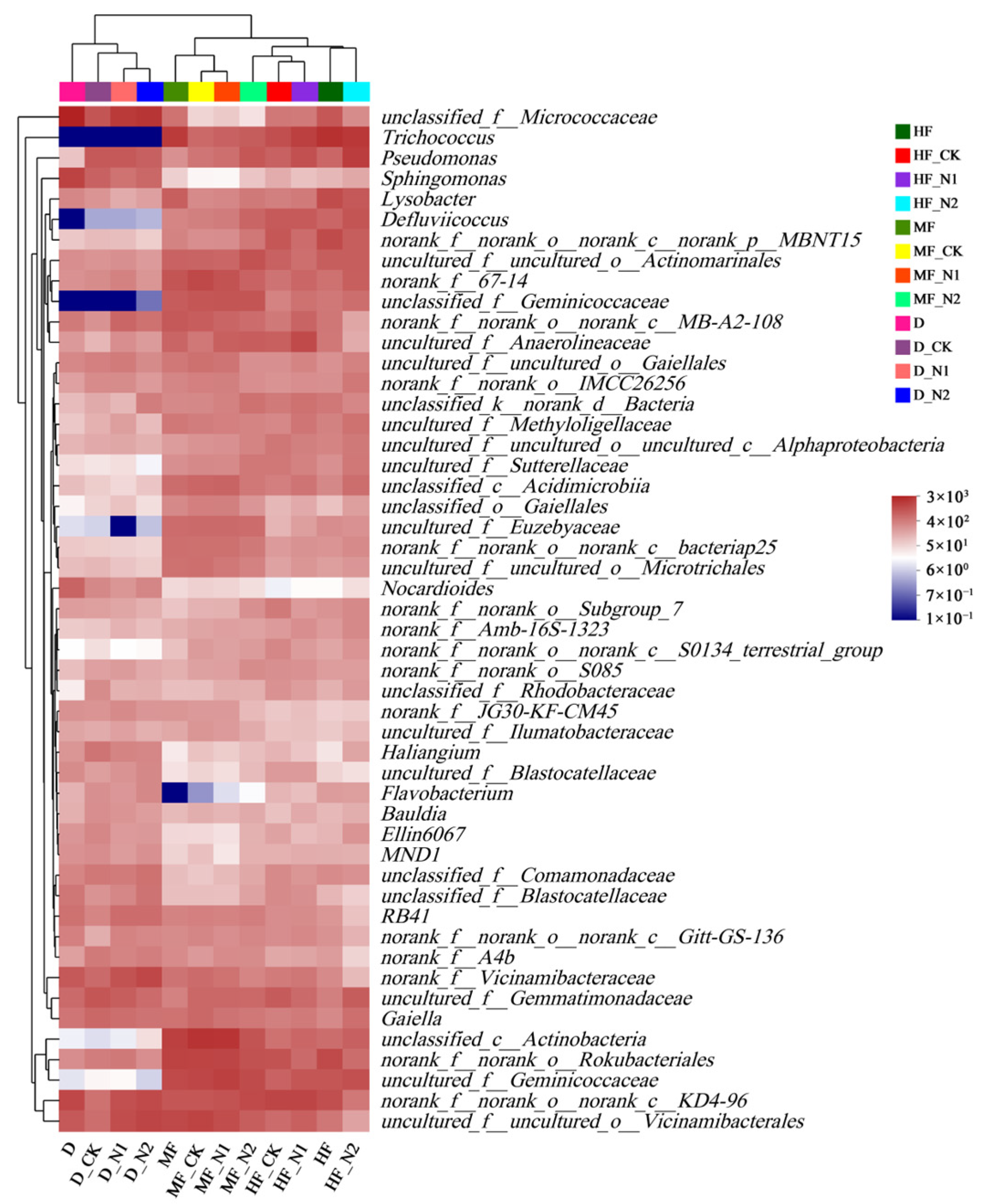
3.2. Bacterial Community Diversity and Composition
3.3. The Relationship between Bacterial and Soil Physicochemical Properties, Greenhouse Gas Emissions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanakidou, M.; Myriokefalitakis, S.; Daskalakis, N.; Fanourgakis, G.; Nenes, A.; Baker, A.; Tsigaridis, K.; Mihalopoulos, N. Past, present, and future atmospheric nitrogen deposition. J. Atmos. Sci. 2016, 73, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, D.; Millet, D.B.; Chen, X. Global estimates of inorganic nitrogen deposition across four decades. Glob. Biogeochem. Cycles 2019, 33, 100–107. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Aslam, R.W.; Shu, H.; Yaseen, A.; Sajjad, A.; Abidin, S.Z.U. Identification of time-varying wetlands neglected in Pakistan through remote sensing techniques. Environ. Sci. Pollut. Res. 2023, 30, 74031–74044. [Google Scholar] [CrossRef]
- Song, Q.; Zhao, R.; Fu, H.; Zhu, J.; Li, Y. Water Area Extraction and Water Level Prediction of Dongting Lake Based on Sentinel-1 Dual-Polarization Data Decomposition. Remote Sens. 2023, 15, 4655. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Liang, H.; Gao, D. Long-term harvesting of reeds affects greenhouse gas emissions and microbial functional genes in alkaline wetlands. Water Res. 2019, 164, 114936. [Google Scholar] [CrossRef]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Fiedler, D.J.; Clay, D.E.; Joshi, D.R.; Engel, A.; Marzano, S.Y.; Jakubowski, D.; Bhattarai, D.; Reese, C.L.; Bruggeman, S.A.; Clay, S.A. CO2 and N2O emissions and microbial community structure from fields that include salt-affected soils. Wiley Online Libr. 2021, 50, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, H.; Ning, Y.; Xu, C.; Zhang, H.; Lu, X.; Wang, J.; Xu, X.; Feng, Y.; Zhang, Y. Reduced nitrogen fertilization under flooded conditions cut down soil N2O and CO2 efflux: An incubation experiment. J. Environ. Manag. 2022, 324, 116335. [Google Scholar] [CrossRef]
- Zhao, Z.; Dong, S.; Jiang, X.; Liu, S.; Ji, H.; Li, Y.; Han, Y.; Sha, W. Effects of warming and nitrogen deposition on CH4, CO2 and N2O emissions in alpine grassland ecosystems of the Qinghai-Tibetan Plateau. Sci. Total Environ. 2017, 592, 565–572. [Google Scholar] [CrossRef]
- Tahovská, K.; Kaňa, J.; Bárta, J.; Oulehle, F.; Richter, A.; Šantrůčková, H. Microbial N immobilization is of great importance in acidified mountain spruce forest soils. Soil Biol. Biochem. 2013, 59, 58–71. [Google Scholar] [CrossRef]
- Frey, B.; Moser, B.; Tytgat, B.; Zimmermann, S.; Alberti, J.; Biederman, L.A.; Borer, E.T.; Broadbent, A.A.; Caldeira, M.C.; Davies, K.F.; et al. Long-term N-addition alters the community structure of functionally important N-cycling soil microorganisms across global grasslands. Soil Biol. Biochem. 2023, 176, 108887. [Google Scholar] [CrossRef]
- Xu, X.; Shi, Z.; Li, D.; Rey, A.; Ruan, H.; Craine, J.M.; Liang, J.; Zhou, J.; Luo, Y. Soil properties control decomposition of soil organic carbon: Results from data-assimilation analysis. Geoderma 2016, 262, 235–242. [Google Scholar] [CrossRef]
- Lei, J.; Guo, X.; Zeng, Y.; Zhou, J.; Gao, Q.; Yang, Y. Temporal changes in global soil respiration since 1987. Nat. Commun. 2021, 12, 403. [Google Scholar] [CrossRef]
- Tian, J.; Dungait, J.A.; Lu, X.; Yang, Y.; Hartley, I.P.; Zhang, W.; Mo, J.; Yu, G.; Zhou, J.; Kuzyakov, Y. Long-term nitrogen addition modifies microbial composition and functions for slow carbon cycling and increased sequestration in tropical forest soil. Glob. Change Biol. 2019, 25, 3267–3281. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Z.; Barberán, A.; Yang, Y.; Hu, S.; Guo, H. Nitrogen-induced acidification plays a vital role driving ecosystem functions: Insights from a 6-year nitrogen enrichment experiment in a Tibetan alpine meadow. Soil Biol. Biochem. 2021, 153, 108107. [Google Scholar] [CrossRef]
- Zak, D.R.; Freedman, Z.B.; Upchurch, R.A.; Steffens, M.; Kögel-Knabner, I. Anthropogenic N deposition increases soil organic matter accumulation without altering its biochemical composition. Glob. Change Biol. 2017, 23, 933–944. [Google Scholar] [CrossRef]
- Liu, H.Y.; Huang, N.; Zhao, C.M.; Li, J.H. Responses of carbon cycling and soil organic carbon content to nitrogen addition in grasslands globally. Soil Biol. Biochem. 2023, 186, 109164. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.Y. Mapping global nitrogen deposition impacts on soil respiration. Sci. Total Environ. 2023, 871, 161986. [Google Scholar] [CrossRef]
- Stein, L.Y. The long-term relationship between microbial metabolism and greenhouse gases. Trends Microbiol. 2020, 28, 500–511. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Moser, G.; Gorenflo, A.; Brenzinger, K.; Keidel, L.; Braker, G.; Marhan, S.; Clough, T.J.; Müller, C. Explaining the doubling of N2O emissions under elevated CO2 in the Giessen FACE via in-field 15N tracing. Glob. Change Biol. 2018, 24, 3897–3910. [Google Scholar] [CrossRef] [PubMed]
- Berthrong, S.T.; Yeager, C.M.; Gallegos-Graves, L.; Steven, B.; Eichorst, S.A.; Jackson, R.B.; Kuske, C.R. Nitrogen fertilization has a stronger effect on soil nitrogen-fixing bacterial communities than elevated atmospheric CO2. Appl. Environ. Microbiol. 2014, 80, 3103–3112. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, H.; Li, F.; Su, H.; Li, H.; Gao, D. Water level of inland saline wetlands with implications for CO2 and CH4 fluxes during the autumn freeze–thaw period in Northeast China. Environ. Sci. Pollut. Res. 2023, 30, 50125–50133. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Wu, Y.; Peng, Q.A.; Lin, S.; Mo, Y.; Wu, L.; Hu, R.G.; Zhou, W. Effects of dicyandiamide and dolomite application on N2O emission from an acidic soil. Environ. Sci. Pollut. Res. 2016, 23, 6334–6342. [Google Scholar] [CrossRef] [PubMed]
- Pareja-Sánchez, E.; Cantero-Martínez, C.; Álvaro-Fuentes, J.; Plaza-Bonilla, D. Impact of tillage and N fertilization rate on soil N2O emissions in irrigated maize in a Mediterranean agroecosystem. Agric. Ecosyst. Environ. 2020, 287, 106687. [Google Scholar] [CrossRef]
- Deng, L.; Huang, C.; Kim, D.G.; Shangguan, Z.; Wang, K.; Song, X.; Peng, C. Soil GHG fluxes are altered by N deposition: New data indicate lower N stimulation of the N2O flux and greater stimulation of the calculated C pools. Glob. Change Biol. 2020, 26, 2613–2629. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Y.; Qu, Z.; Zhu, T.; Yu, L. Responses of Soil N2O Emission and CH4 Uptake to N Input in Chinese Forests across Climatic Zones: A Meta-Study. Atmosphere 2022, 13, 1145. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Jia, J.; Bai, J.; Wang, W.; Yin, S.; Zhang, G.; Zhao, Q.; Wang, X.; Liu, X.; Cui, B. Salt stress alters the short-term responses of nitrous oxide emissions to the nitrogen addition in salt-affected coastal soils. Sci. Total Environ. 2020, 742, 140124. [Google Scholar] [CrossRef]
- Li, Q.W.; Zhang, X.Y.; Gao, J.Q.; Song, M.H.; Liang, J.F.; Yue, Y. Effects of N addition frequency and quantity on Hydrocotyle vulgaris growth and greenhouse gas emissions from wetland microcosms. Sustainability 2019, 11, 1520. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, X.; Song, C.; Li, M.; Gao, S.; Liu, Z.; Gao, J.; Wang, X. Soil CO2 and N2O emissions and microbial abundances altered by temperature rise and nitrogen addition in active-layer soils of permafrost peatland. Front. Microbiol. 2022, 13, 1093487. [Google Scholar] [CrossRef]
- Zhang, P.; Wen, T.; Hu, Y.; Zhang, J.; Cai, Z. Can N Fertilizer Addition Affect N2O Isotopocule Signatures for Soil N2O Source Partitioning? Int. J. Environ. Res. Public Health 2021, 18, 5024. [Google Scholar] [CrossRef]
- Jia, D.; Qi, F.; Xu, X.; Feng, J.; Wu, H.; Guo, J.; Lu, W.; Peng, R.; Zhu, X.; Luo, Y.; et al. Co-regulations of Spartina alterniflora invasion and exogenous nitrogen loading on soil N2O efflux in subtropical mangrove mesocosms. PLoS ONE 2016, 11, e0146199. [Google Scholar] [CrossRef] [PubMed]
- Bakken, L.R.; Bergaust, L.; Liu, B.; Frostegård, Å. Regulation of denitrification at the cellular level: A clue to the understanding of N2O emissions from soils. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Liu, C.; Zhang, B.; Dong, J. Effects of inorganic and organic nitrogen additions on CO2 emissions in the coastal wetlands of the Yellow River Delta, China. Atmos. Environ. 2018, 185, 159–167. [Google Scholar] [CrossRef]
- Qu, S.; Ri, X.; Yu, J.; Li, F.; Wei, D.; Borjigidai, A. Nitrogen deposition accelerates greenhouse gas emissions at an alpine steppe site on the Tibetan Plateau. Sci. Total Environ. 2021, 765, 144277. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Cao, Y.; Zhou, B.; Xiao, W.; Tian, X.; Li, M. Combined application of biochar and N increased temperature sensitivity of soil respiration but still decreased the soil CO2 emissions in moso bamboo plantations. Sci. Total Environ. 2020, 730, 139003. [Google Scholar] [CrossRef]
- Meng, C.; Tian, D.S.; Zeng, H.; Li, Z.; Yi, C.; Niu, S. Global soil acidification impacts on belowground processes. Environ. Res. Lett. 2019, 14, 074003. [Google Scholar] [CrossRef]
- Tang, J.; Liang, S.; Li, Z.; Zhang, H.; Wang, S.; Zhang, N. Emission laws and influence factors of greenhouse gases in saline-alkali paddy fields. Sustainability 2016, 8, 163. [Google Scholar] [CrossRef]
- Gagnon, B.; Ziadi, N.; Rochette, P.; Chantigny, M.H.; Angers, D.A.; Bertrand, N.; Smith, W.N. Soil-surface carbon dioxide emission following nitrogen fertilization in corn. Can. J. Soil Sci. 2016, 96, 219–232. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, J.; Cai, W.; Li, G.; Mei, Y.; Yang, S. Different amounts of nitrogen fertilizer applications alter the bacterial diversity and community structure in the rhizosphere soil of sugarcane. Front. Microbiol. 2021, 12, 2490. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, W.; Wang, J.; Shi, X. Bacterial Diversity and Community in Response to Long-Term Nitrogen Fertilization Gradient in Citrus Orchard Soils. Diversity 2021, 13, 282. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Z.; Zhu, J. Effects of Climate Change and Nitrogen Addition on Carbon Loss in Alpine Wetland of Qinghai–Tibet Plateau. Atmosphere 2023, 14, 1342. [Google Scholar] [CrossRef]
- Sun, K.; Cai, J.; Liu, X.; Yang, L.; Li, H.; Wang, G.; Xu, X.; Yu, F. Effects of nitrogen and phosphorus supply levels and ratios on soil microbial diversity-ecosystem multifunctionality relationships in a coastal nontidal wetland. Sci. Total Environ. 2023, 874, 162472. [Google Scholar] [CrossRef]
- Ge, Y.; Sun, T. Soil Microbial Community Structure and Diversity of Potato in Rhizosphere and Non-rhizosphere Soil. Ecol. Environ. 2020, 29, 141. [Google Scholar] [CrossRef]
- Goordial, J.; Davila, A.; Greer, C.W.; Cannam, R.; DiRuggiero, J.; McKay, C.P.; Whyte, L.G. Comparative activity and functional ecology of permafrost soils and lithic niches in a hyper-arid polar desert. Environ. Microbiol. 2017, 19, 443–458. [Google Scholar] [CrossRef]
- Tian, X.Y.; Zhang, C.S. Illumina-based analysis of endophytic and rhizosphere bacterial diversity of the coastal halophyte Messerschmidia sibirica. Front. Microbiol. 2017, 8, 2288. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D.; Jiang, Z.; Sun, P.; Xiao, H.; Yu, X.W.; Chen, J. Changes in the soil microbial communities of alpine steppe at Qinghai-Tibetan Plateau under different degradation levels. Sci. Total Environ. 2019, 651, 2281–2291. [Google Scholar] [CrossRef]
- Hanada, S. Filamentous anoxygenic phototrophs in hot springs. Microbes Environ. 2003, 18, 51–61. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Lücker, S.; Vejmelkova, D.; Kostrikina, N.A.; Kleerebezem, R.; Rijpstra, W.I.C.; Damsté, J.S.S.; Le, P.D.; Muyzer, G.; Wagner, M.; et al. Nitrification expanded: Discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME J. 2012, 6, 2245–2256. [Google Scholar] [CrossRef]
- Li, Y.T.; He, J.S.; Wang, H.; Zhou, J.Z.; Yang, Y.F.; Chu, H.Y. Lowered water table causes species substitution while nitrogen amendment causes species loss in alpine wetland microbial communities. Pedosphere 2021, 31, 912–922. [Google Scholar] [CrossRef]
- Wang, G.H.; Liu, J.J.; Yu, Z.H.; Wang, X.Z.; Jin, J.; Liu, X.B. Research progress of Acidobacteria ecology in soils. Biotechnol. Bull. 2016, 32, 14. [Google Scholar] [CrossRef]
- Begmatov, S.; Beletsky, A.V.; Dedysh, S.N.; Mardanov, A.V.; Ravin, N.V. Genome analysis of the candidate phylum MBNT15 bacterium from a boreal peatland predicted its respiratory versatility and dissimilatory iron metabolism. Front. Microbiol. 2022, 13, 951761. [Google Scholar] [CrossRef]
- Hu, D.; Zang, Y.; Mao, Y.; Gao, B. Identification of molecular markers that are specific to the class thermoleophilia. Front. Microbiol. 2019, 10, 1185. [Google Scholar] [CrossRef]
- Al, D.A.; Alkufeidy, R.M.; Alkubaisi, N.A.; Alshammari, M.K. Polynuclear aromatic anthracene biodegradation by psychrophilic Sphingomonas sp., cultivated with tween-80. Chemosphere 2021, 263, 128115. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, S.; Guo, J.; Ge, S. Light irradiation enables rapid start-up of nitridation through suppressing nxrB gene expression and stimulating ammonia-oxidizing bacteria. Environ. Sci. Technol. 2021, 55, 13297–13305. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Zhao, Y.; Yu, X.; Guo, W.; Qiao, Z.; Ismail, S.; Ni, S.Q. Feasibility of Partial Nitrification Combined with Nitrite-Denitrification Phosphorus Removal and Simultaneous Nitrification–Endogenous Denitrification for Synchronous Chemical Oxygen Demand, Nitrogen, and Phosphorus Removal. ACS ES&T Water 2022, 2, 1119–1131. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Xue, T.; Li, B.; Dai, X.; Peng, Y. Treating low carbon/nitrogen (C/N) wastewater in simultaneous nitrification-endogenous denitrification and phosphorous removal (SNDPR) systems by strengthening anaerobic intracellular carbon storage. Water Res. 2015, 77, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, J.J.; Banerjee, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Tian, C.Y. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef] [PubMed]
- Strepis, N.; Naranjo, H.D.; Meier-Kolthoff, J.; Göker, M.; Shapiro, N.; Kyrpides, N.; Klenk, H.P.; Schaap, P.J.; Stams, A.J.; Sousa, D.Z. Genome-guided analysis allows the identification of novel physiological traits in Trichococcus species. BMC Genom. 2020, 21, 24. [Google Scholar] [CrossRef]
- Tian, L.; Yan, B.; Ou, Y.; Liu, H.; Cheng, L.; Jiao, P. Effectiveness of exogenous Fe2+ on nutrient removal in gravel-based constructed wetlands. Int. J. Environ. Res. Public Health 2022, 19, 1475. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, J.; Zhang, L.; Yin, H.; Fan, C.; Wang, Z.; Zhao, M.; Ge, C.; Song, H. Enhanced nitrogen and phosphorus removal by natural pyrite–based constructed wetland with intermittent aeration. Environ. Sci. Pollut. Res. 2021, 28, 69012–69028. [Google Scholar] [CrossRef] [PubMed]





| Sampling Site | pH | Salt (%) | NH4+ (mg N kg−1) | NO3− (mg N kg−1) | TOC (g kg−1) | DOC (mg kg−1) | TC (g kg−1) | TN (g kg−1) |
|---|---|---|---|---|---|---|---|---|
| HF | 9.14 ± 0.09a | 0.10 ± 0.02a | 5.90 ± 0.19a | 9.57 ± 0.72a | 21.22 ± 0.18a | 176.05 ± 2.15a | 22.98 ± 0.82a | 1.05 ± 0.08a |
| MF | 8.95 ± 0.10a | 0.05 ± 0.01b | 9.00 ± 0.17b | 8.32 ± 0.16b | 25.94 ± 0.17b | 136.95 ± 1.34b | 31.50 ± 0.34b | 1.37 ± 0.11b |
| D | 9.08 ± 0.05a | 0.04 ± 0.01c | 7.84 ± 0.03c | 9.59 ± 0.18a | 10.42 ± 0.21c | 48.40 ± 0.68c | 12.69 ± 0.56c | 0.41 ± 0.02c |
| Cumulative N2O Emissions | Cumulative CO2 Emissions | |
|---|---|---|
| N addition level | *** | *** |
| Sampling site | *** | *** |
| N addition level × sampling site | *** | *** |
| Point | N Addition Level (mg N kg−1) | Linear Regression Equation | R2 | p Value |
|---|---|---|---|---|
| HF | 0–100 | y = 0.019x + 0.101 | 0.95 | p < 0.01 |
| MF | 30–100 | y = 0.002x − 0.037 | 0.99 | p < 0.05 |
| D | 0–30 | y = 0.002x + 0.087 | 0.93 | p < 0.05 |
| 30–100 | y = −3.484x + 0.161 | 0.93 | p < 0.01 |
| N2O | CO2 | pH | TOC | EC | NH4+ | NO3− | |
|---|---|---|---|---|---|---|---|
| N2O | 1 | 0.22 | 0.15 | 0.34 | 0.26 | 0.73 * | 0.43 |
| CO2 | 1 | 0.71 * | 0.80 * | 0.53 | 0.52 | −0.30 | |
| pH | 1 | 0.30 | 0.09 | 0.50 | −0.60 | ||
| TOC | 1 | 0.44 | 0.43 | −0.08 | |||
| EC | 1 | 0.25 | 0.10 | ||||
| NH4+ | 1 | 0.56 | |||||
| NO3− | 1 |
| Point | N Addition Level (mg N kg−1) | Linear Regression Equation | R2 | p Value |
|---|---|---|---|---|
| HF | 0–100 | y = −0.456x + 165.923 | 0.86 | p < 0.05 |
| MF | 0–30 | y = 0.765x + 125.601 | 0.99 | p < 0.05 |
| 30–100 | y = −0.376x + 154.953 | 0.81 | p < 0.01 | |
| D | 0–30 | y = 0.760x + 53.514 | 0.99 | p < 0.05 |
| 30–100 | y = −0.105x + 77.733 | 0.79 | p < 0.01 |
| Treatments | Chao1 | Shannon | Treatments | Chao1 | Shannon | Treatments | Chao1 | Shannon |
|---|---|---|---|---|---|---|---|---|
| HF | 1831.60 | 6.27 | MF | 1549.96 | 6.07 | D | 2415.50 | 6.84 |
| HF_CK | 1898.85 | 6.61 | MF_CK | 1623.22 | 6.18 | D_CK | 2537.34 | 7.21 |
| HF_N1 | 2224.12 | 6.71 | MF_N1 | 1725.65 | 6.25 | D_N1 | 3215.64 | 7.31 |
| HF_N2 | 1705.27 | 6.25 | MF_N2 | 2065.51 | 6.50 | D_N2 | 6298.18 | 7.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Liang, H.; Li, F.; Xu, A.; Li, H.; Du, W.; Gao, Z. The Responses of N2O, CO2 Emissions, and Bacterial Communities to Nitrogen Addition in Saline–Alkaline Wetlands of Northeast China. Atmosphere 2023, 14, 1675. https://doi.org/10.3390/atmos14111675
Su H, Liang H, Li F, Xu A, Li H, Du W, Gao Z. The Responses of N2O, CO2 Emissions, and Bacterial Communities to Nitrogen Addition in Saline–Alkaline Wetlands of Northeast China. Atmosphere. 2023; 14(11):1675. https://doi.org/10.3390/atmos14111675
Chicago/Turabian StyleSu, Huihui, Hong Liang, Feng Li, Ao Xu, Huiju Li, Wei Du, and Zhongyan Gao. 2023. "The Responses of N2O, CO2 Emissions, and Bacterial Communities to Nitrogen Addition in Saline–Alkaline Wetlands of Northeast China" Atmosphere 14, no. 11: 1675. https://doi.org/10.3390/atmos14111675
APA StyleSu, H., Liang, H., Li, F., Xu, A., Li, H., Du, W., & Gao, Z. (2023). The Responses of N2O, CO2 Emissions, and Bacterial Communities to Nitrogen Addition in Saline–Alkaline Wetlands of Northeast China. Atmosphere, 14(11), 1675. https://doi.org/10.3390/atmos14111675





