Indoor Air Quality in the Most Crowded Public Places of Tehran: An Inhalation Health Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
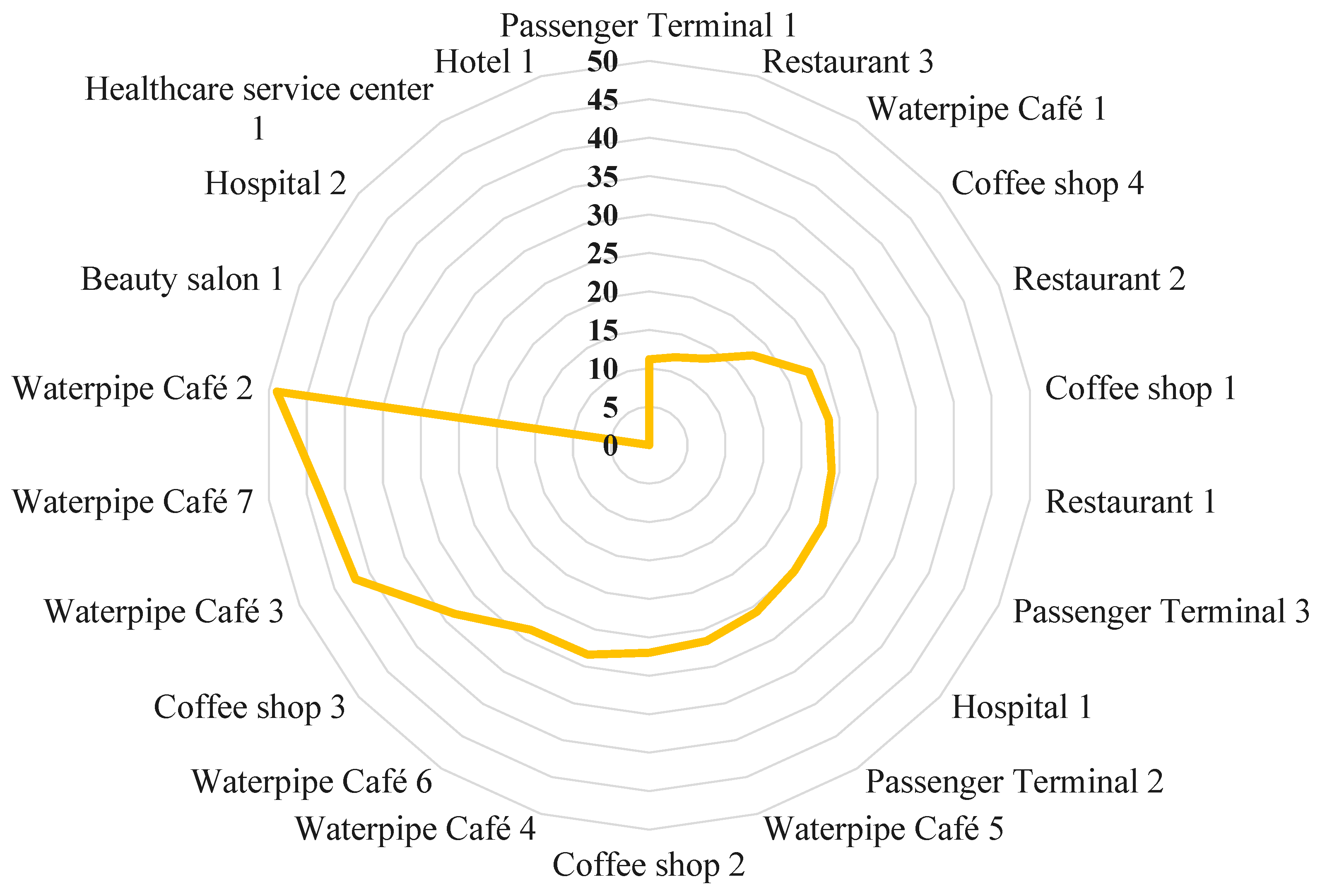
2.1. Study Area and Public Places
2.2. Indoor Air Sampling
2.2.1. Real-Time Sampling
2.2.2. Off-Site Analyzing
2.3. Sample Preparation and Analytical Procedures
2.4. Quality Control/Quality Assurance (QC/QA)
2.5. Outdoor Air Quality Data Gathering
2.6. Cancer and Non-Cancer Risk Assessment
2.7. Statistical Analysis
3. Results
3.1. Indoor Temperature, Humidity, and CO2
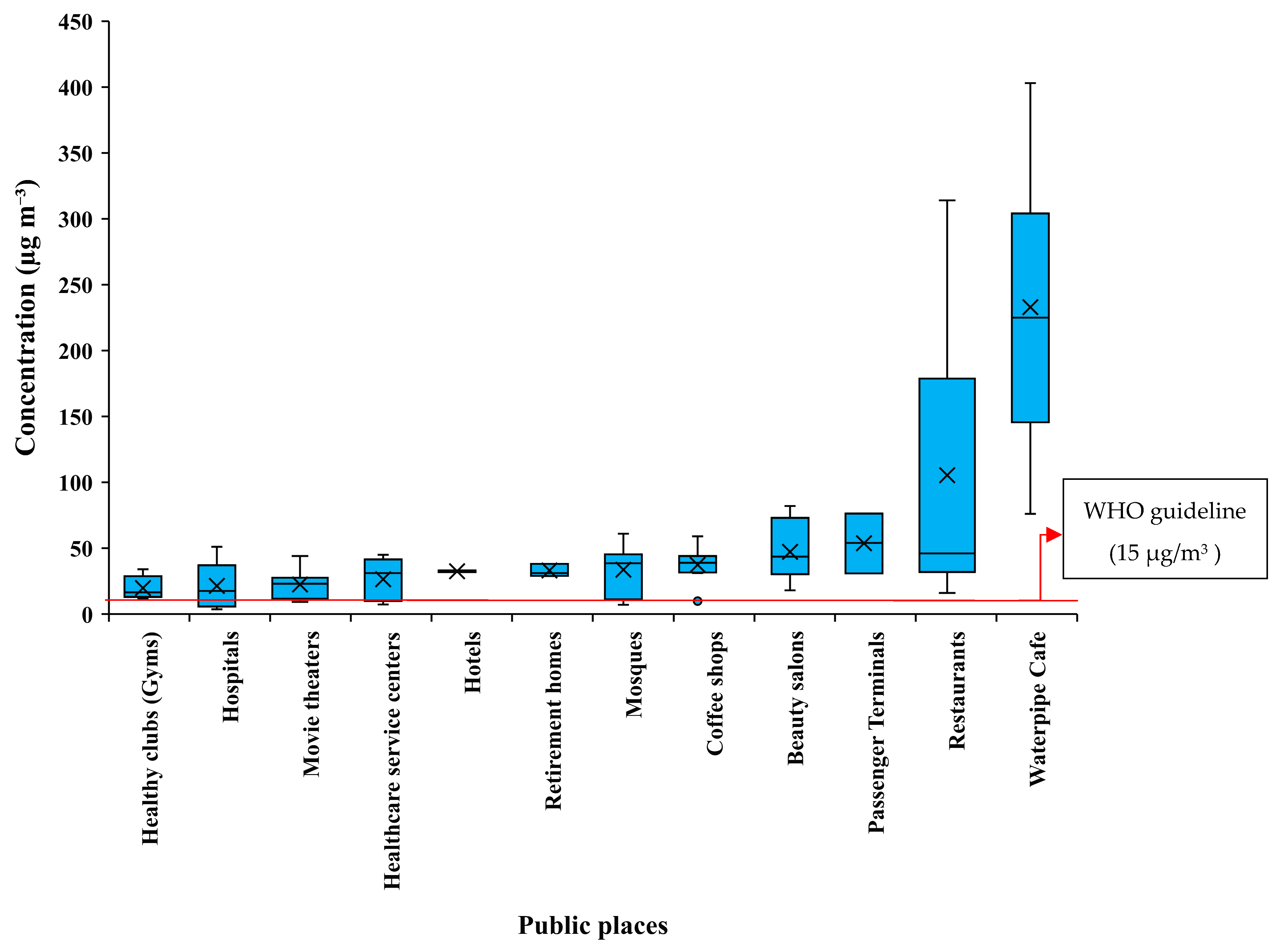
3.2. PM2.5 Mass Concentration
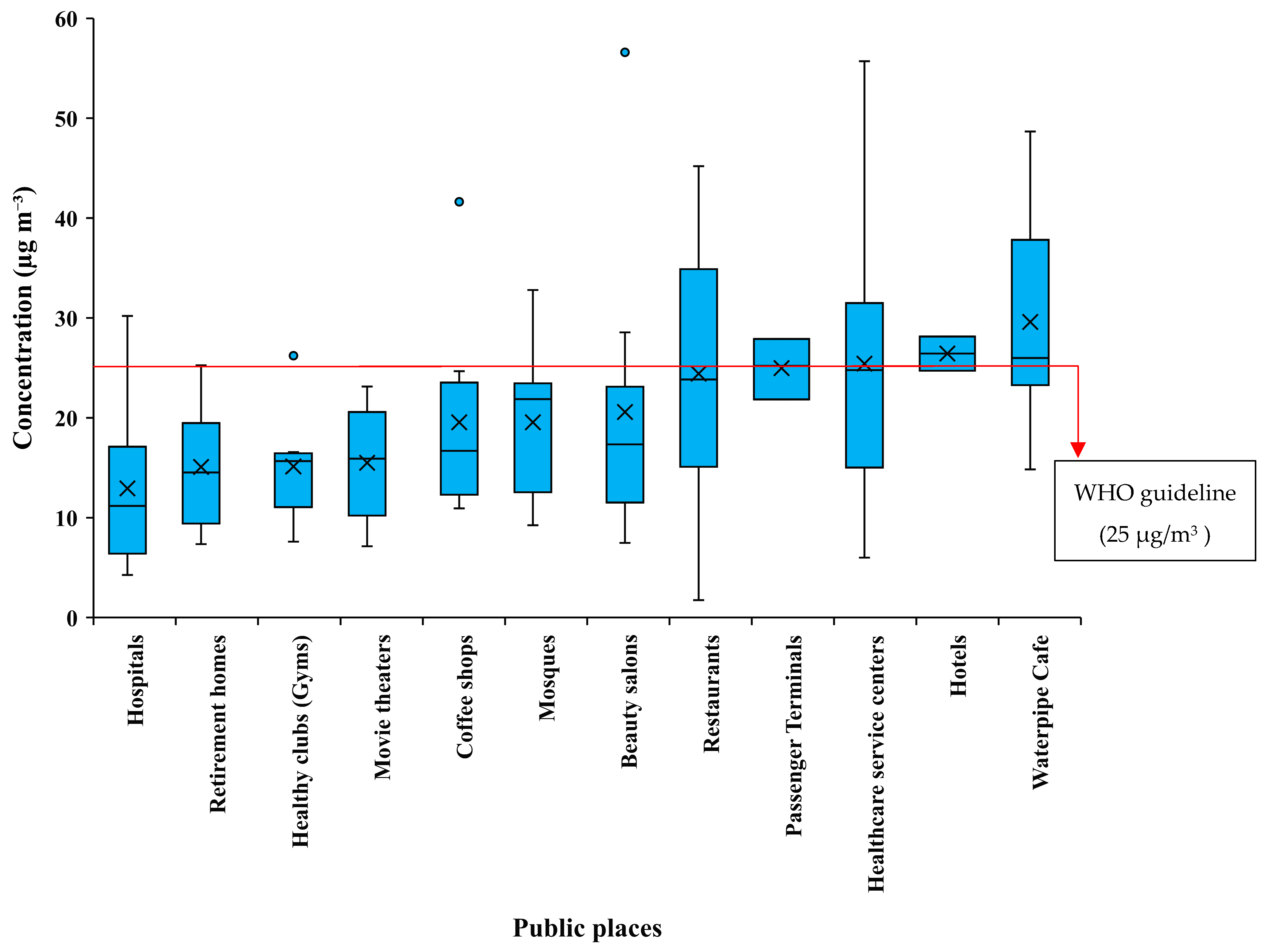
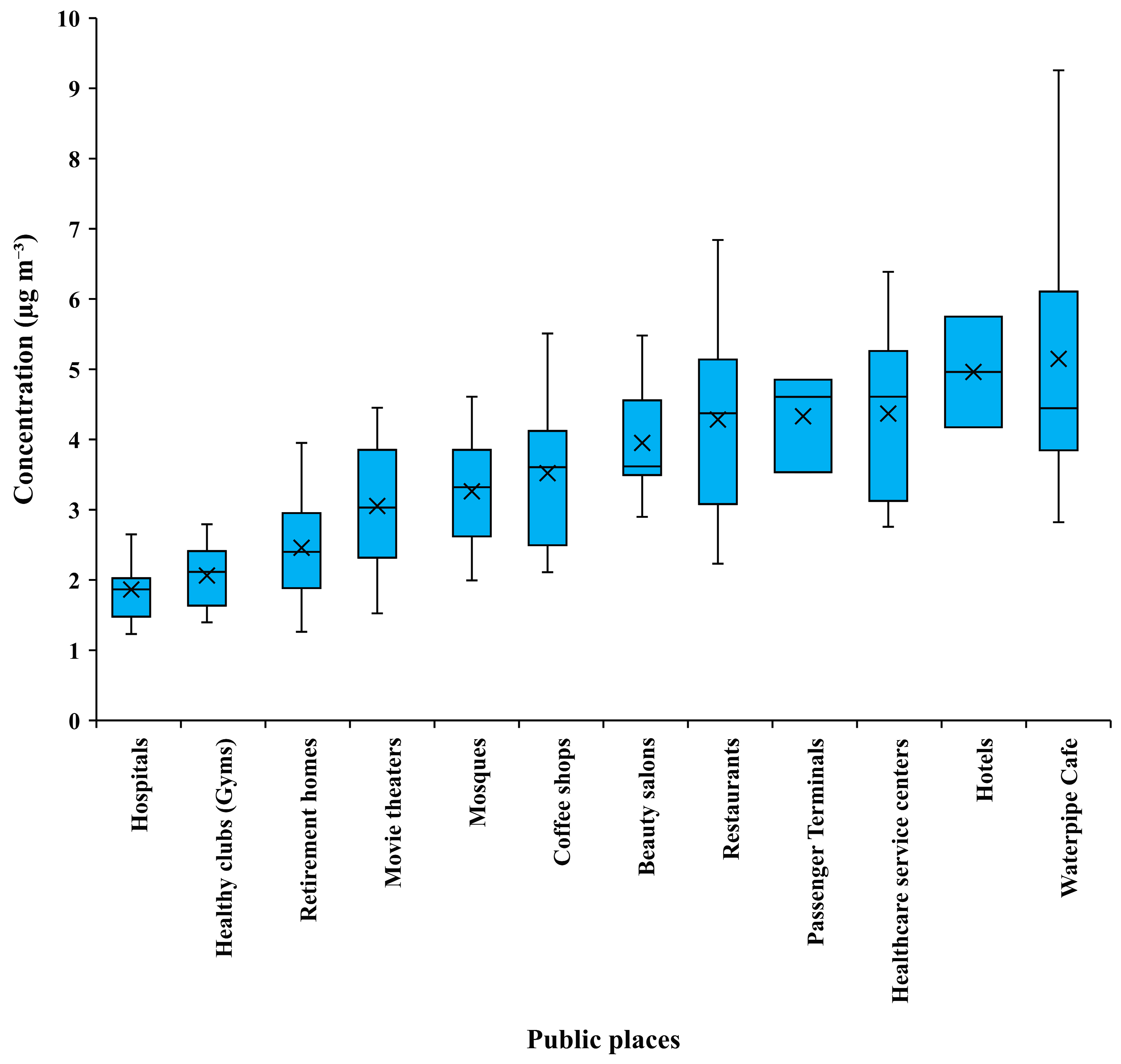
3.3. NO2 and SO2 Concentration
3.4. The Indoor-to-Outdoor Concentration Ratio
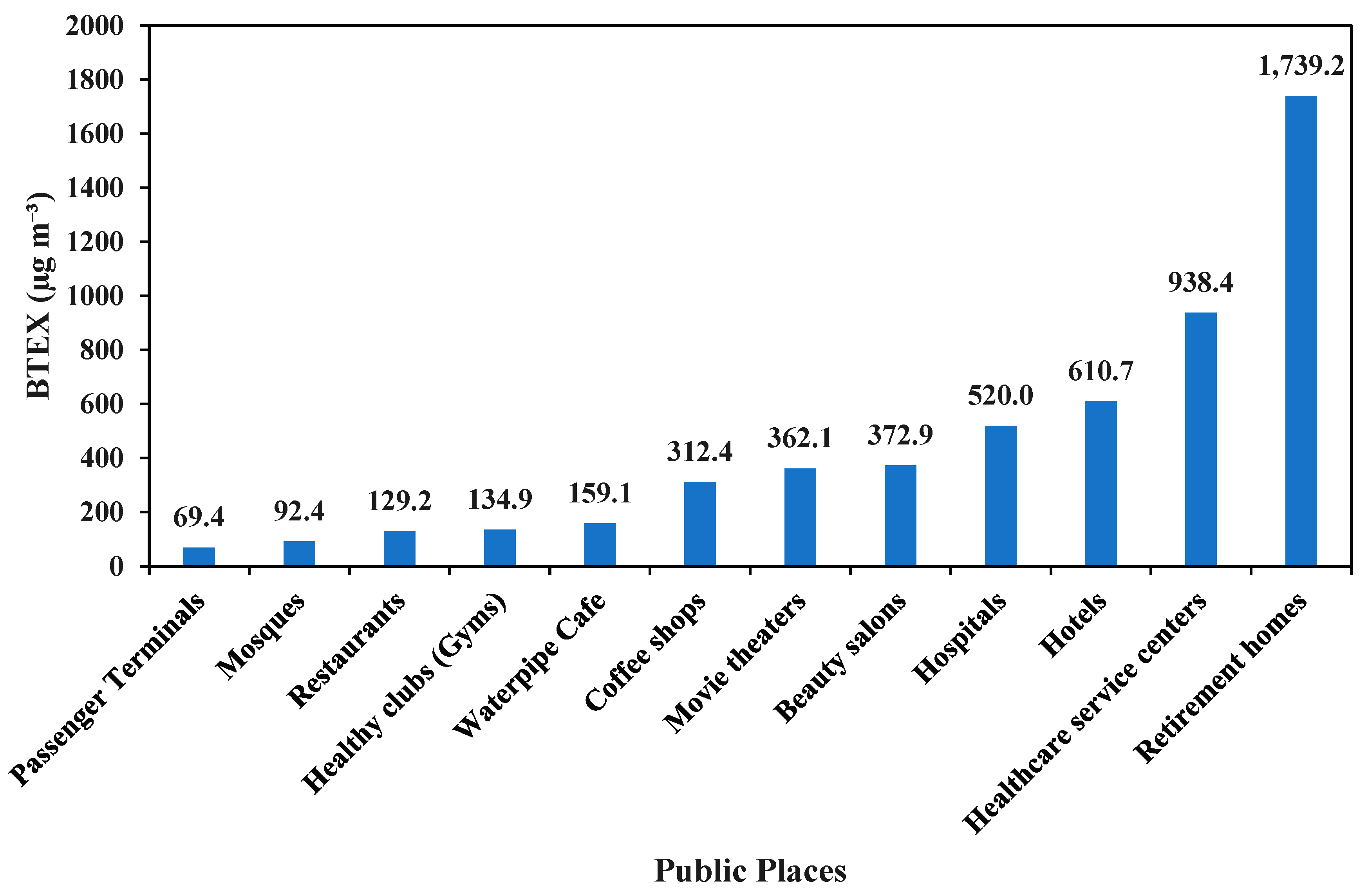
3.5. BTEX and Nicotine Concentration
3.6. BTEX’s Health Risk Assessment
3.6.1. Non-Carcinogenic Risk
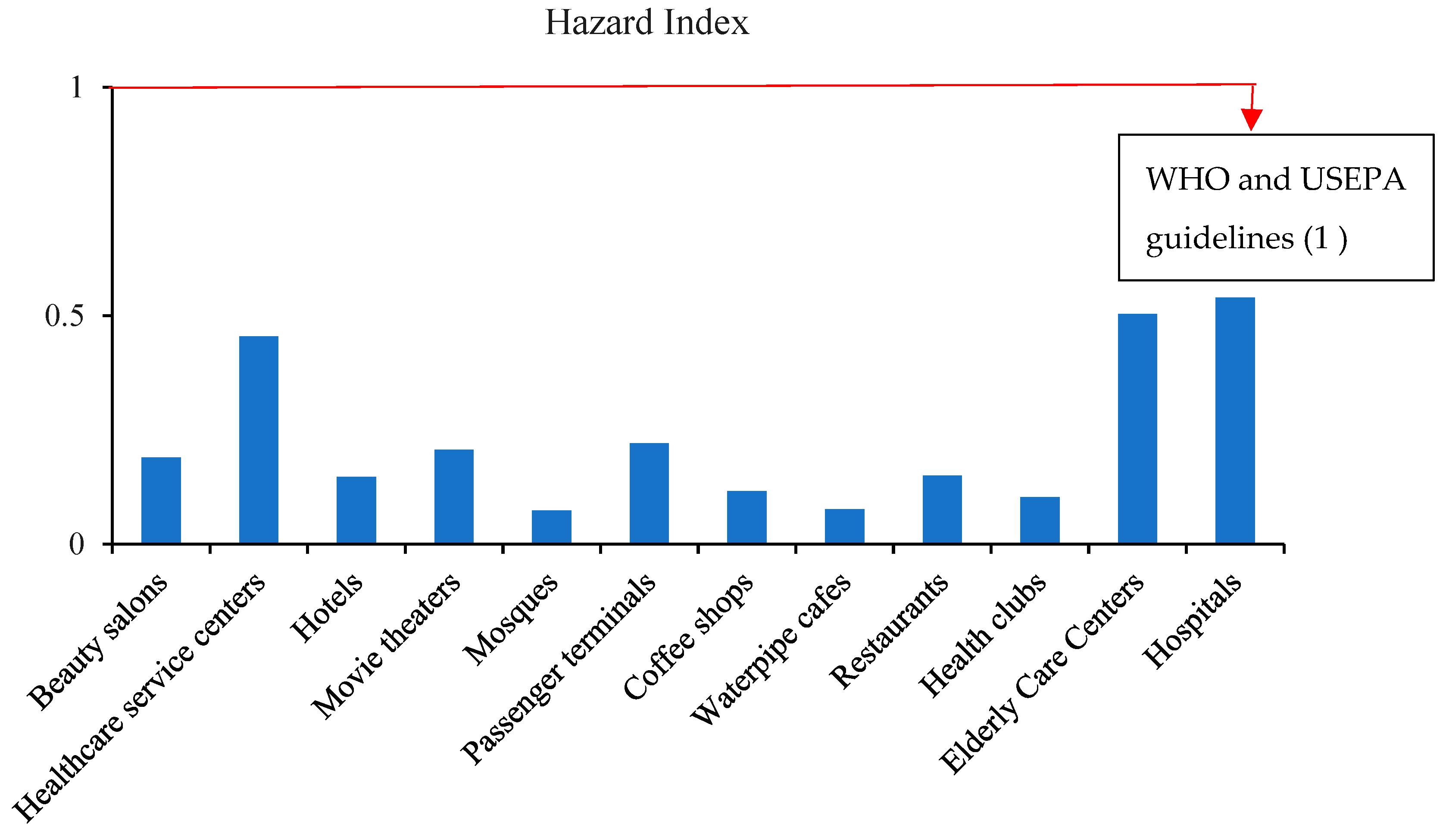
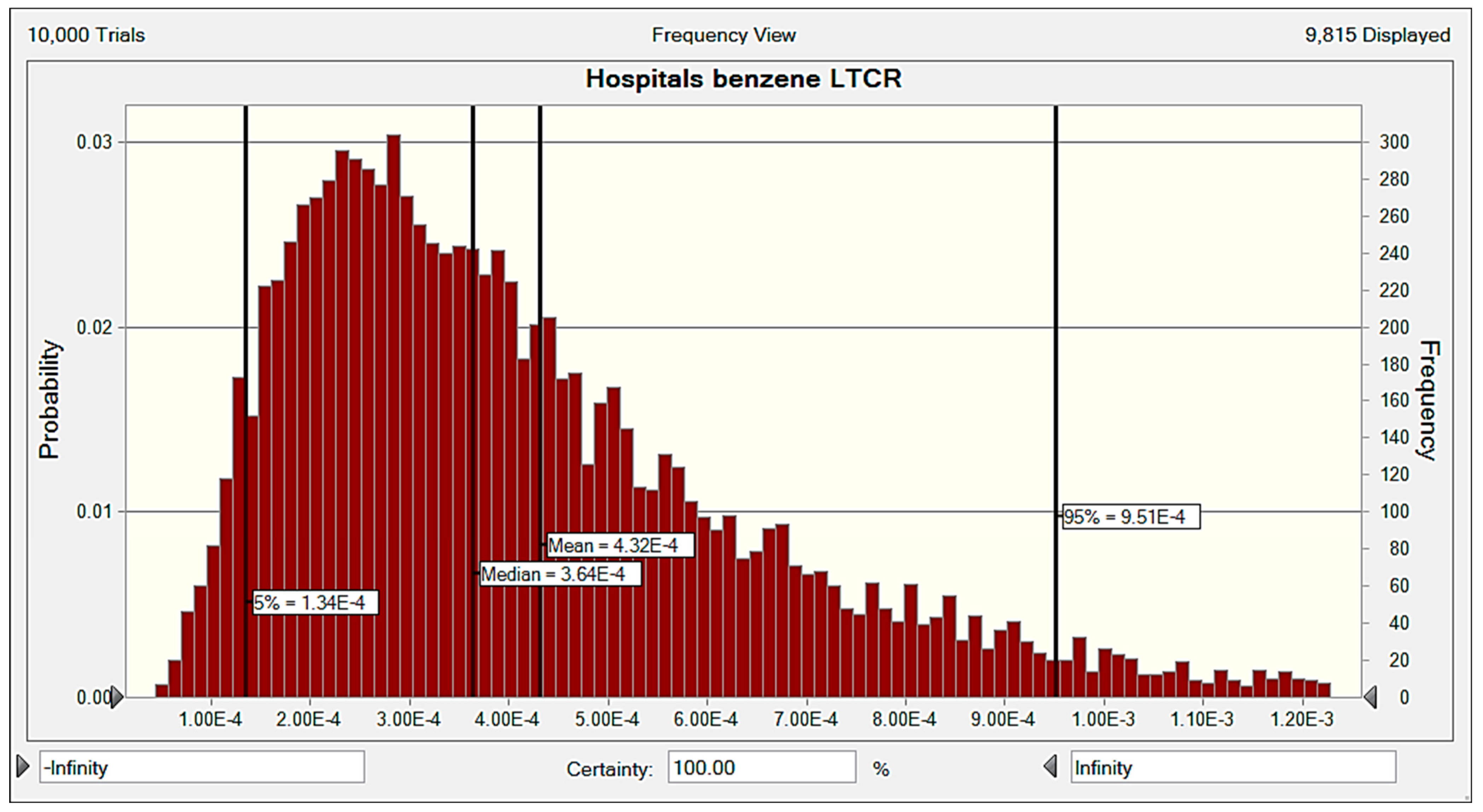
3.6.2. Carcinogenic Risk
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faridi, S.; Niazi, S.; Yousefian, F.; Azimi, F.; Pasalari, H.; Momeniha, F.; Mokammel, A.; Gholampour, A.; Hassanvand, M.S.; Naddafi, K. Spatial homogeneity and heterogeneity of ambient air pollutants in Tehran. Sci. Total Environ. 2019, 697, 134123. [Google Scholar] [CrossRef] [PubMed]
- Marlier, M.E.; Jina, A.S.; Kinney, P.L.; DeFries, R.S. Extreme air pollution in global megacities. Curr. Clim. Change Rep. 2016, 2, 15–27. [Google Scholar]
- Stranger, M.; Potgieter-Vermaak, S.; Van, R.G. Characterization of indoor air quality in primary schools in Antwerp, Belgium. Indoor Air 2008, 18, 454–463. [Google Scholar] [CrossRef]
- Hamidin, N.; Yu, J.; Phung, D.T.; Connell, D.; Chu, C. Volatile aromatic hydrocarbons (VAHs) in residential indoor air in Brisbane, Australia. Chemosphere 2013, 92, 1430–1435. [Google Scholar]
- Delgado-Saborit, J.M.; Aquilina, N.J.; Meddings, C.; Baker, S.; Harrison, R.M. Relationship of personal exposure to volatile organic compounds to home, work and fixed site outdoor concentrations. Sci. Total Environ. 2011, 409, 478–488. [Google Scholar] [PubMed]
- Azuma, K.; Uchiyama, I.; Uchiyama, S.; Kunugita, N. Assessment of inhalation exposure to indoor air pollutants: Screening for health risks of multiple pollutants in Japanese dwellings. Environ. Res. 2016, 145, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Martellini, T.; Berlangieri, C.; Dei, L.; Carretti, E.; Santini, S.; Barone, A.; Cincinelli, A. Indoor levels of volatile organic compounds at Florentine museum environments in Italy. Indoor Air 2020, 30, 900–913. [Google Scholar] [CrossRef]
- Nakhjirgan, P.; Kashani, H.; Naddafi, K.; Nabizadeh, R.; Amini, H.; Yunesian, M. Maternal exposure to air pollutants and birth weight in Tehran, Iran. J. Environ. Health Sci. Eng. 2019, 17, 711–717. [Google Scholar] [CrossRef]
- Baurès, E.; Blanchard, O.; Mercier, F.; Surget, E.; Le Cann, P.; Rivier, A.; Gangneux, J.-P.; Florentin, A. Indoor air quality in two French hospitals: Measurement of chemical and microbiological contaminants. Sci. Total Environ. 2018, 642, 168–179. [Google Scholar] [CrossRef] [PubMed]
- WHO. Household Air Pollution and Health. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health (accessed on 28 November 2022).
- Śmiełowska, M.; Marć, M.; Zabiegała, B. Indoor air quality in public utility environments—A review. Environ. Sci. Pollut. Res. 2017, 24, 11166–11176. [Google Scholar]
- Ramos, C.; Wolterbeek, H.; Almeida, S. Exposure to indoor air pollutants during physical activity in fitness centers. Build. Environ. 2014, 82, 349–360. [Google Scholar]
- Romagnoli, P.; Balducci, C.; Perilli, M.; Vichi, F.; Imperiali, A.; Cecinato, A. Indoor air quality at life and work environments in Rome, Italy. Environ. Sci. Pollut. Res. 2016, 23, 3503–3516. [Google Scholar]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lee, S.; Li, W.; Cao, J. Source characterization of BTEX in indoor microenvironments in Hong Kong. Atmos. Environ. 2003, 37, 73–82. [Google Scholar] [CrossRef]
- Shallis, R.M.; Weiss, J.J.; Deziel, N.C.; Gore, S.D. A clandestine culprit with critical consequences: Benzene and acute myeloid leukemia. Blood Rev. 2021, 47, 100736. [Google Scholar]
- Ghaffari, H.R.; Kamari, Z.; Hassanvand, M.S.; Fazlzadeh, M.; Heidari, M. Level of air BTEX in urban, rural and industrial regions of Bandar Abbas, Iran; indoor-outdoor relationships and probabilistic health risk assessment. Environ. Res. 2021, 200, 111745. [Google Scholar] [CrossRef]
- Golkhorshidi, F.; Sorooshian, A.; Jafari, A.J.; Baghani, A.N.; Kermani, M.; Kalantary, R.R.; Ashournejad, Q.; Delikhoon, M. On the nature and health impacts of BTEX in a populated middle eastern city: Tehran, Iran. Atmos. Pollut. Res. 2019, 10, 921–930. [Google Scholar]
- Brauer, M.; Lencar, C.; Tamburic, L.; Koehoorn, M.; Demers, P.; Karr, C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ. Health Perspect. 2008, 116, 680–686. [Google Scholar] [CrossRef] [Green Version]
- Gauderman, W.J.; Avol, E.; Gilliland, F.; Vora, H.; Thomas, D.; Berhane, K.; McConnell, R.; Kuenzli, N.; Lurmann, F.; Rappaport, E. The effect of air pollution on lung development from 10 to 18 years of age. N. Engl. J. Med. 2004, 351, 1057–1067. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. Available online: https://apps.who.int/iris/handle/10665/345329 (accessed on 7 September 2021).
- Li, W.-M.; Lee, S.C.; Chan, L.Y. Indoor air quality at nine shopping malls in Hong Kong. Sci. Total Environ. 2001, 273, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Chen, W.; Xue, Y.; Liu, W. A comprehensive evaluation method for indoor air quality of buildings based on rough sets and a wavelet neural network. Build. Environ. 2019, 162, 106296. [Google Scholar] [CrossRef]
- Mokammel, A.; Rostami, R.; Niazi, S.; Asgari, A.; Fazlzadeh, M. BTEX levels in rural households: Heating system, building characteristic impacts and lifetime excess cancer risk assessment. Environ. Pollut. 2022, 298, 118845. [Google Scholar] [CrossRef] [PubMed]
- Al Hanai, A.H.; Antkiewicz, D.S.; Hemming, J.D.; Shafer, M.M.; Lai, A.M.; Arhami, M.; Hosseini, V.; Schauer, J.J. Seasonal variations in the oxidative stress and inflammatory potential of PM2.5 in Tehran using an alveolar macrophage model; The role of chemical composition and sources. Environ. Int. 2019, 123, 417–427. [Google Scholar]
- Statical_Center_of_Iran. Population-and-Housing-Censuses. 2016. Available online: https://www.amar.org.ir/english/Population-and-Housing-Censuses (accessed on 13 June 2023).
- Faridi, S.; Yousefian, F.; Niazi, S.; Ghalhari, M.R.; Hassanvand, M.S.; Naddafi, K. Impact of SARS-CoV-2 on ambient air particulate matter in Tehran. Aerosol Air Qual. Res. 2020, 20, 1805–1811. [Google Scholar] [CrossRef]
- Bayat, R.; Ashrafi, K.; Motlagh, M.S.; Hassanvand, M.S.; Daroudi, R.; Fink, G.; Künzli, N. Health impact and related cost of ambient air pollution in Tehran. Environ. Res. 2019, 176, 108547. [Google Scholar]
- Amini, H.; Schindler, C.; Hosseini, V.; Yunesian, M.; Künzli, N. Land use regression models for Alkylbenzenes in a middle eastern megacity: Tehran study of exposure prediction for environmental Health Research (Tehran SEPEHR). Environ. Sci. Technol. 2017, 51, 8481–8490. [Google Scholar] [CrossRef] [Green Version]
- Rohani-Rasaf, M.; Rohani-Rasaf, M.R.; Asadi-Lari, M.; Hashemi Nazari, S.S. Regional differences in cancer incidence trend in tehran, Iran: A contextual study on the effect of socioeconomic status at regional level. Int. J. Cancer Manag. 2018, 11. [Google Scholar] [CrossRef]
- KIMO. Kimo HD110 Thermo-Hygrometer. Available online: https://www.onetemp.com.au/hd110 (accessed on 13 June 2023).
- Dehghani, M.H.; Baghani, A.N.; Fazlzadeh, M.; Ghaffari, H.R. Exposure and risk assessment of BTEX in indoor air of gyms in Tehran, Iran. Microchem. J. 2019, 150, 104135. [Google Scholar] [CrossRef]
- NIOSH. Indoor Environmental Quality: HVAC Management. 2021. Available online: https://www.cdc.gov/niosh/topics/indoorenv/hvac.html (accessed on 25 February 2022).
- WHO. Indoor Air—WHO/Europe. 2021. Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/156146/WA754ES.pdf (accessed on 20 June 2023).
- Vehviläinen, T.; Lindholm, H.; Rintamäki, H.; Pääkkönen, R.; Hirvonen, A.; Niemi, O.; Vinha, J. High indoor CO2 concentrations in an office environment increases the transcutaneous CO2 level and sleepiness during cognitive work. J. Occup. Environ. Hyg. 2016, 13, 19–29. [Google Scholar] [CrossRef]
- Mackinson, F. Occupational health guidelines for chemical hazards. NIOSH/OSHA 1981, 81–123. [Google Scholar]
- Standard 62.1-2019; Ventilation for Acceptable Indoor Air Quality. ASHRAE Standing Standard Project Committee 62.1. ASHRAE: Atlanta, GA, USA, 2019.
- Viveiros, F.; Gaspar, J.L.; Ferreira, T.; Silva, C. Hazardous indoor CO2 concentrations in volcanic environments. Environ. Pollut. 2016, 214, 776–786. [Google Scholar]
- Slezakova, K.; Peixoto, C.; do Carmo Pereira, M.; Morais, S. Indoor air quality in health clubs: Impact of occupancy and type of performed activities on exposure levels. J. Hazard. Mater. 2018, 359, 56–66. [Google Scholar]
- Morawska, L.; Tang, J.W.; Bahnfleth, W.; Bluyssen, P.M.; Boerstra, A.; Buonanno, G.; Cao, J.; Dancer, S.; Floto, A.; Franchimon, F. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020, 142, 105832. [Google Scholar] [CrossRef]
- Jung, C.-C.; Wu, P.-C.; Tseng, C.-H.; Su, H.-J. Indoor air quality varies with ventilation types and working areas in hospitals. Build. Environ. 2015, 85, 190–195. [Google Scholar]
- Loupa, G.; Zarogianni, A.-M.; Karali, D.; Kosmadakis, I.; Rapsomanikis, S. Indoor/outdoor PM2.5 elemental composition and organic fraction medications, in a Greek hospital. Sci. Total Environ. 2016, 550, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Naddafi, K.; Nabizadeh, R.; Rostami, R.; Ghaffari, H.R.; Fazlzadeh, M. Formaldehyde and acetaldehyde in the indoor air of waterpipe cafés: Measuring exposures and assessing health effects. Build. Environ. 2019, 165, 106392. [Google Scholar]
- Fromme, H.; Dietrich, S.; Heitmann, D.; Dressel, H.; Diemer, J.; Schulz, T.; Jörres, R.A.; Berlin, K.; Völkel, W. Indoor air contamination during a waterpipe (narghile) smoking session. Food Chem. Toxicol. 2009, 47, 1636–1641. [Google Scholar] [CrossRef]
- Maziak, W.; Ali, R.A.; Fouad, M.; Rastam, S.; Wipfli, H.; Travers, M.; Ward, K.; Eissenberg, T. Exposure to secondhand smoke at home and in public places in Syria: A developing country’s perspective. Inhal. Toxicol. 2008, 20, 17–24. [Google Scholar] [CrossRef]
- Fiala, S.C.; Morris, D.S.; Pawlak, R.L. Measuring indoor air quality of hookah lounges. Am. J. Public Health 2012, 102, 2043–2045. [Google Scholar] [CrossRef] [PubMed]
- Travers, M.J.; Kulak, J.A.; Vogl, L. Waterpipe cafés are hazardous to your health: Determination of a waterpipe specific calibration factor. Int. J. Hyg. Environ. Health 2018, 221, 48–53. [Google Scholar] [CrossRef]
- Zhou, S.; Weitzman, M.; Vilcassim, R.; Wilson, J.; Legrand, N.; Saunders, E.; Travers, M.; Chen, L.-C.; Peltier, R.; Gordon, T. Air quality in New York City hookah bars. Tob. Control 2015, 24, e193–e198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naddafi, K.; Nabizadeh, R.; Rostamy, R.; Kalan, M.E.; Hassanvand, M.S.; Fazlzadeh, M. Indoor air quality in waterpipe cafés: Exposure level to particulate matter. Environ. Sci. Pollut. Res. 2019, 26, 26605–26616. [Google Scholar] [CrossRef] [PubMed]
- Mohammadyan, M.; Shabankhani, B. Indoor PM1, PM2.5, PM10 and outdoor PM2.5 concentrations in primary schools in Sari, Iran. Arh. Za Hig. Rada I Toksikol. 2013, 64, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.H.; Park, W.M. Indoor air concentrations of carbon dioxide (CO2), nitrogen dioxide (NO2), and ozone (O3) in multiple healthcare facilities. Environ. Geochem. Health 2020, 42, 1487–1496. [Google Scholar] [CrossRef]
- Agbo, K.E.; Walgraeve, C.; Eze, J.I.; Ugwoke, P.E.; Ukoha, P.O.; Van Langenhove, H. Household indoor concentration levels of NO2, SO2 and O3 in Nsukka, Nigeria. Atmos. Environ. 2021, 244, 117978. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, B. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmos. Environ. 2011, 45, 275–288. [Google Scholar] [CrossRef]
- Salonen, H.; Salthammer, T.; Morawska, L. Human exposure to NO2 in school and office indoor environments. Environ. Int. 2019, 130, 104887. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, B. Relationship between indoor and outdoor NO2: A review. Build. Environ. 2020, 180, 106909. [Google Scholar]
- Ebrahimi, M.; Qaderi, F. Determination of the most effective control methods of SO2 pollution in Tehran based on adaptive neuro-fuzzy inference system. Chemosphere 2021, 263, 128002. [Google Scholar] [CrossRef]
- Department of Environment, Iran. The Clean Air Act. 2017. Available online: https://www.doe.ir/ (accessed on 9 August 2017).
- Masih, A.; Dviwedi, S.; Lal, J. Source characterization and health risks of BTEX in indoor/outdoor air during winters at a terai precinct of North India. Environ. Geochem. Health 2021, 43, 2985–3003. [Google Scholar] [CrossRef]
- Halliday-Bell, J.A.; Gissler, M.; Jaakkola, J.J. Work as a hairdresser and cosmetologist and adverse pregnancy outcomes. Occup. Med. 2009, 59, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsigonia, A.; Lagoudi, A.; Chandrinou, S.; Linos, A.; Evlogias, N.; Alexopoulos, E.C. Indoor air in beauty salons and occupational health exposure of cosmetologists to chemical substances. Int. J. Environ. Res. Public Health 2010, 7, 314–324. [Google Scholar] [CrossRef]
- Kheirmand, M.; Barkhordari, A.; Mosaddegh, M.H.; Farajzadegan, Z. Determination of benzene, toluene, ethyl benzene and xylene in administration room’s air of hospitals using solid phase micro extraction/gas chromatography/flame ionization detector. Int. J. Environ. Health Eng. 2014, 3, 27. [Google Scholar]
- Baghani, A.N.; Rostami, R.; Arfaeinia, H.; Hazrati, S.; Fazlzadeh, M.; Delikhoon, M. BTEX in indoor air of beauty salons: Risk assessment, levels and factors influencing their concentrations. Ecotoxicol. Environ. Saf. 2018, 159, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, S.; Rostami, R.; Fazlzadeh, M. BTEX in indoor air of waterpipe cafés: Levels and factors influencing their concentrations. Sci. Total Environ. 2015, 524, 347–353. [Google Scholar] [CrossRef]
- Markowicz, P.; Löndahl, J.; Wierzbicka, A.; Suleiman, R.; Shihadeh, A.; Larsson, L. A study on particles and some microbial markers in waterpipe tobacco smoke. Sci. Total Environ. 2014, 499, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USEPA. Risk Assessment Guidance for Superfund: Volume III Part A, Process for Conducting Probabilistic Risk Assessment; US Environmental Protection Agency: Washington, DC, USA, 2001. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/rags3adt_complete.pdf (accessed on 20 June 2023).
- Tunsaringkarn, T.; Prueksasit, T.; Morknoy, D.; Sawatsing, R.; Chinveschakitvanich, V.; Rungsiyothin, A.; Zapaung, K. Indoor air assessment, health risks, and their relationship among elderly residents in urban warrens of Bangkok, Thailand. Air Qual. Atmos. Health 2015, 8, 603–615. [Google Scholar] [CrossRef]
| Parameter | Value | Pollution | Rfc (µg/m3) | IUR (µg/m3)−1 |
|---|---|---|---|---|
| ET (h/day) | 8 | Benzene | 30 | 7.8 × 10−6 |
| EF (day/year) | 300 | Toluene | 5000 | |
| ED (year) | 30 | Ethylbenzene | 1000 | 2.5 × 10−6 |
| AT (h) | 262,800 | Xylenes | 100 | |
| IR (m3/day) | 18.7 | |||
| BW (kg) | 70 |
| Public Places | Tem. (°C) Mean (Min-Max) | RH (%) Mean (Min-Max) | CO2 (ppm) Mean (Min-Max) |
|---|---|---|---|
| Hospitals | 21 (19–24) | 24 (20–27) | 613 (512–842) |
| Elderly care centers | 24 (23–26) | 26 (26–29) | 734 (582–893) |
| Health clubs | 21 (19–24) | 29 (22–33) | 850 (637–1041) |
| Restaurants | 21 (18–25) | 26 (21–35) | 768 (518–1018) |
| Waterpipe cafés | 20 (18–23) | 30 (19–44) | 1150 (1064–1272) |
| Coffee shops | 22 (20–27) | 26 (21–30) | 628 (557–793) |
| Passenger terminals | 21 (19–23) | 29 (25–35) | 561 (477–644) |
| Mosques | 19 (16–22) | 26 (18–33) | 430 (409–520) |
| Movie theaters | 24 (22–26) | 26 (19–36) | 540 (410–730) |
| Hotels | 19 (17–21) | 26 (16–35) | 675 (490–883) |
| Beauty salons | 22 (20–25) | 32 (21–51) | 945 (736–1032) |
| Healthcare service centers | 19 (16–23) | 25 (18–29) | 470 (430–580) |
| Public Places | Indoor to Outdoor (I/O) Ratio | ||
|---|---|---|---|
| PM2.5 | NO2 | SO2 | |
| Hospitals | 0.67 | 0.31 | 0.38 |
| Elderly care centers | 1.22 | 0.33 | 0.42 |
| Health clubs | 0.80 | 0.31 | 0.43 |
| Restaurants | 4.41 | 0.49 | 0.78 |
| Waterpipe cafés | 11.09 | 0.50 | 0.84 |
| Coffee shops | 2.00 | 0.40 | 0.64 |
| Passenger terminals | 1.82 | 0.45 | 0.69 |
| Mosques | 0.86 | 0.39 | 0.57 |
| Movie theaters | 0.92 | 0.29 | 0.63 |
| Hotels | 1.23 | 0.53 | 0.74 |
| Beauty salons | 1.29 | 0.44 | 0.59 |
| Healthcare service centers | 1.04 | 0.63 | 0.72 |
| Public Places | Benzene | Toluene | Ethylbenzene | Xylene |
|---|---|---|---|---|
| Hospitals | 203.8 | 435.2 | 53.4 | 43.3 |
| Elderly care centers | 187.0 | 1563.2 | 22.8 | 31.9 |
| Health clubs | 36.0 | 92.6 | 18.5 | 17.1 |
| Restaurants | 57.7 | 93.4 | 13.0 | 9.5 |
| Waterpipe cafés | 25.4 | 158.4 | 11.7 | 15.8 |
| Coffee shops | 37.8 | 297.6 | 27.9 | 23.6 |
| Passenger terminals | 90.1 | 30.7 | 8.6 | - |
| Mosques | 28.2 | 78.5 | 8.4 | 3.6 |
| Movie theaters | 69.7 | 271.8 | 23.0 | 42.3 |
| Hotels | 46.3 | 529.0 | 24.9 | 33.5 |
| Healthcare service centers | 170.5 | 843.4 | 30.8 | 32.4 |
| Beauty salons | 69.8 | 305.8 | 14.1 | 18.3 |
| Public Places | Benzene | Toluene | Ethylbenzene | Xylenes |
|---|---|---|---|---|
| Hospitals | 0.4972 | 0.0064 | 0.0039 | 0.0317 |
| Elderly care centers | 0.4562 | 0.0229 | 0.0017 | 0.0233 |
| Health clubs | 0.0878 | 0.0014 | 0.0014 | 0.0125 |
| Restaurants | 0.1408 | 0.0014 | 0.0010 | 0.0070 |
| Waterpipe cafés | 0.0620 | 0.0023 | 0.0009 | 0.0116 |
| Coffee shops | 0.0922 | 0.0044 | 0.0020 | 0.0173 |
| Passenger terminals | 0.2198 | 0.0004 | 0.0006 | - |
| Mosques | 0.0688 | 0.0011 | 0.0006 | 0.0026 |
| Movie theaters | 0.1700 | 0.0040 | 0.0017 | 0.0310 |
| Hotels | 0.1130 | 0.0077 | 0.0018 | 0.0245 |
| Healthcare service centers | 0.4160 | 0.0123 | 0.0023 | 0.0237 |
| Beauty salons | 0.1703 | 0.0045 | 0.0010 | 0.0134 |
| Public Places | Benzene | Toluene | Ethylbenzene | Xylenes |
|---|---|---|---|---|
| Hospitals | 4.36 × 10−4 | - | 3.65 × 10−5 | - |
| Elderly care centers | 4.00 × 10−4 | - | 1.56 × 10−5 | - |
| Health clubs | 7.69 × 10−5 | - | 1.26 × 10−5 | - |
| Restaurants | 1.23 × 10−4 | - | 8.90 × 10−6 | - |
| Waterpipe cafés | 5.43 × 10−5 | - | 8.01 × 10−6 | - |
| Coffee shops | 8.08 × 10−5 | - | 1.91 × 10−5 | - |
| Passenger terminals | 1.93 × 10−4 | - | 5.89 × 10−6 | - |
| Mosques | 6.03 × 10−5 | - | 5.75 × 10−6 | - |
| Movie theaters | 1.49 × 10−4 | - | 1.57 × 10−5 | - |
| Hotels | 9.89 × 10−5 | - | 1.70 × 10−5 | - |
| Healthcare service centers | 3.64 × 10−4 | - | 2.10 × 10−5 | - |
| Beauty salons | 1.49 × 10−4 | - | 9.65 × 10−6 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derikvand, A.; Taherkhani, A.; Hassanvand, M.S.; Naddafi, K.; Nabizadeh, R.; Shamsipour, M.; Niazi, S.; Heidari, M.; Mokammel, A.; Faridi, S. Indoor Air Quality in the Most Crowded Public Places of Tehran: An Inhalation Health Risk Assessment. Atmosphere 2023, 14, 1080. https://doi.org/10.3390/atmos14071080
Derikvand A, Taherkhani A, Hassanvand MS, Naddafi K, Nabizadeh R, Shamsipour M, Niazi S, Heidari M, Mokammel A, Faridi S. Indoor Air Quality in the Most Crowded Public Places of Tehran: An Inhalation Health Risk Assessment. Atmosphere. 2023; 14(7):1080. https://doi.org/10.3390/atmos14071080
Chicago/Turabian StyleDerikvand, Ahmad, Ali Taherkhani, Mohammad Sadegh Hassanvand, Kazem Naddafi, Ramin Nabizadeh, Mansour Shamsipour, Sadegh Niazi, Mohsen Heidari, Adel Mokammel, and Sasan Faridi. 2023. "Indoor Air Quality in the Most Crowded Public Places of Tehran: An Inhalation Health Risk Assessment" Atmosphere 14, no. 7: 1080. https://doi.org/10.3390/atmos14071080






