Atmospheric Oxidation Capacity and Its Impact on the Secondary Inorganic Components of PM2.5 in Recent Years in Beijing: Enlightenment for PM2.5 Pollution Control in the Future
Abstract
:1. Introduction
2. Experiment
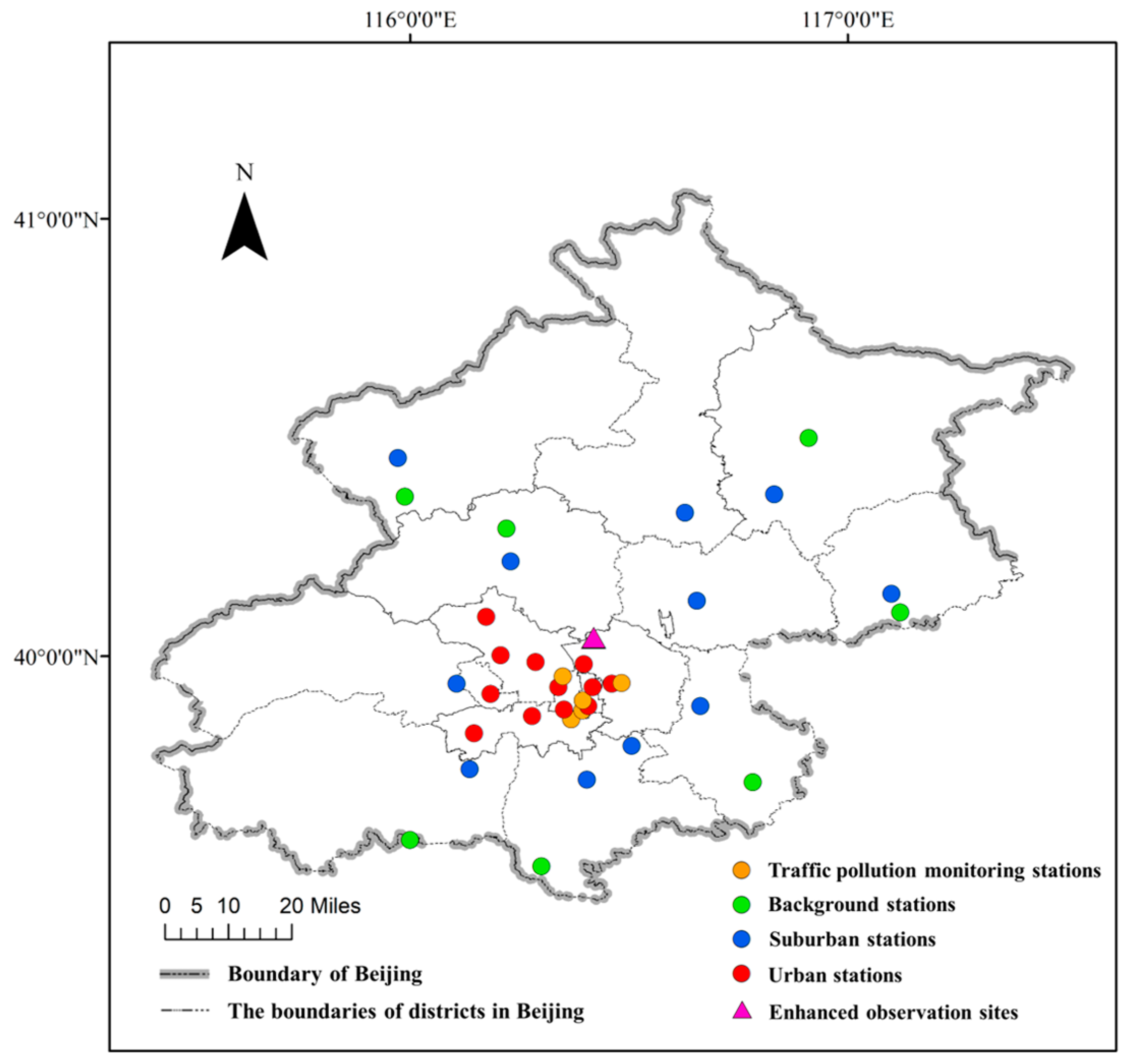
2.1. Observation Sites and Period
2.1.1. Data on Conventional Pollutants
2.1.2. Enhanced Field Observation
2.2. Observation Items and Analysis Methods
2.2.1. Data on Conventional Pollutants
2.2.2. PM2.5 Sampling and Inorganic Ion Component Analysis
2.3. Quality Control
2.3.1. Online Monitoring of Conventional Pollutants
2.3.2. Offline Sampling of PM2.5 and Ion Component Analysis
2.4. Data Analysis and Processing
2.4.1. Correlation Analysis
2.4.2. Estimation of Secondary Particle Generation
- (1)
- R0: MDA8 O3 ≤ 100 μg/m3;
- (2)
- R1: 100 < MDA8 O3 ≤ 160 μg/m3;
- (3)
- R2: 160 < MDA8 O3 ≤ 200 μg/m3;
- (4)
- R3: MDA8 O3 > 200 μg/m3.
2.4.3. Calculation of Sulfur Oxidation Rate, Nitrogen Oxidation Rate, and Atmospheric Oxidant (OX) Concentrations
3. Results and Discussion
3.1. Changes in OX Concentration and Its Correlation with PM2.5 Concentration
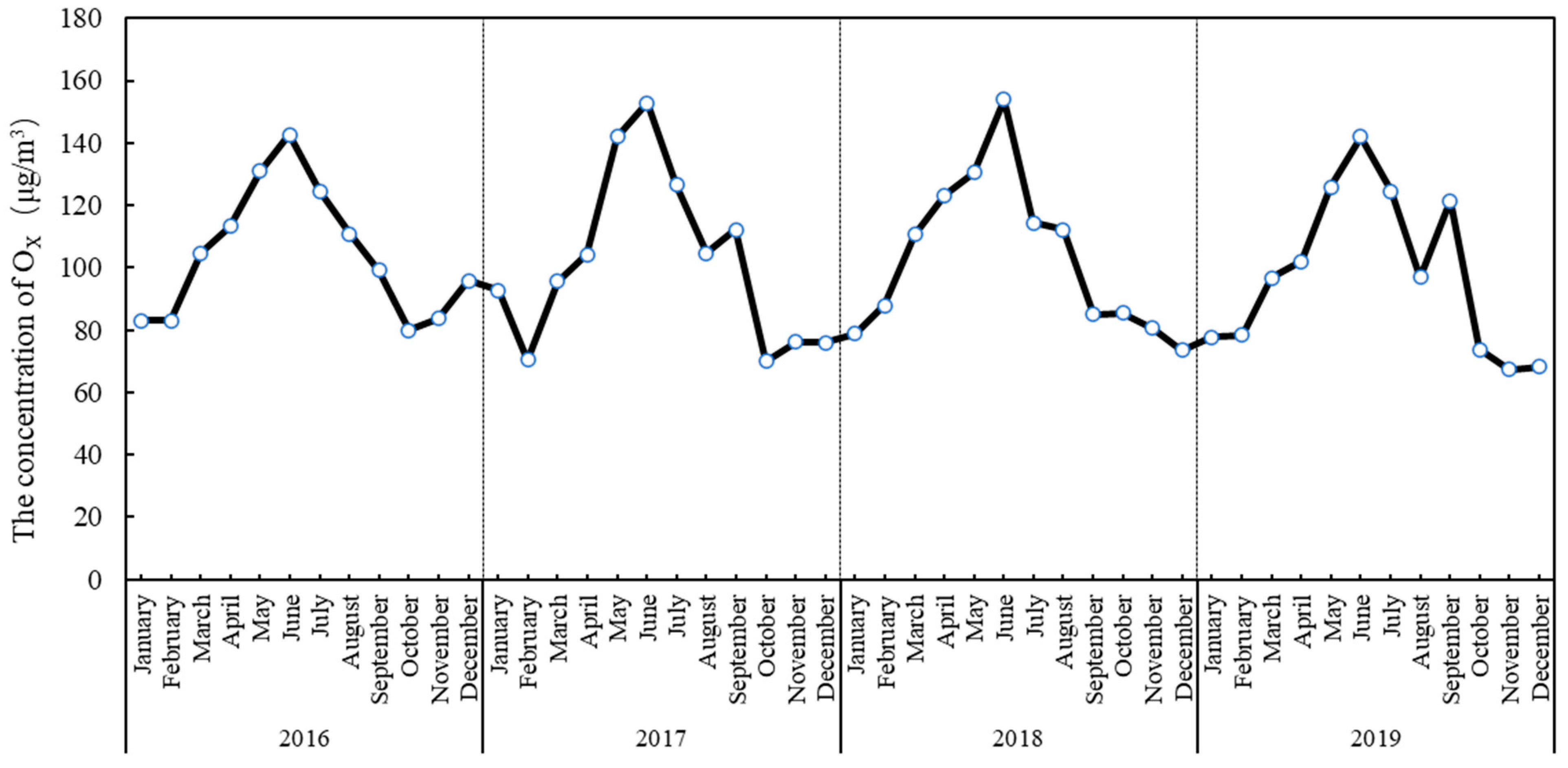
3.1.1. Interannual Change and Seasonal Change of OX Concentration
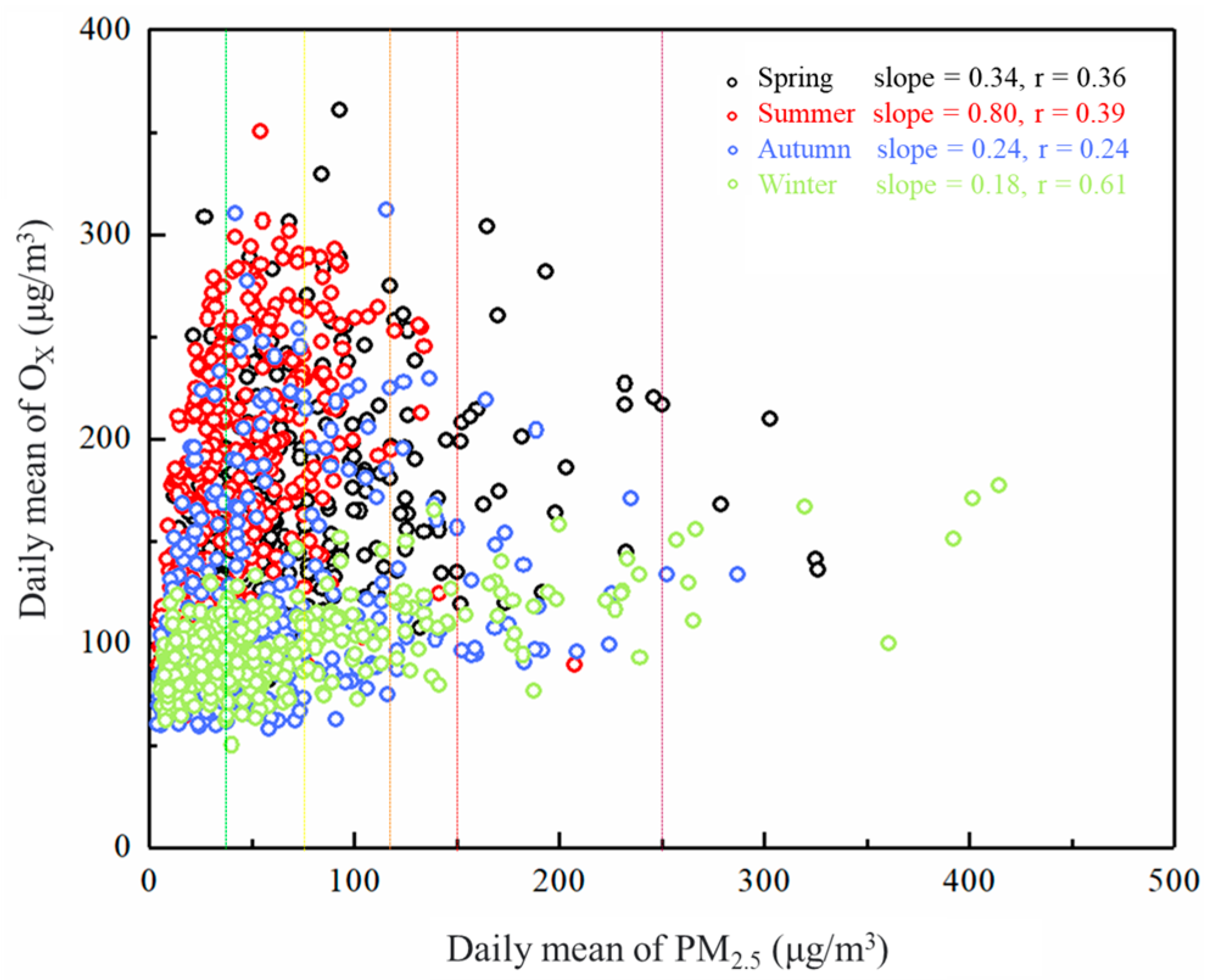
3.1.2. Correlation between OX and PM2.5 Concentrations
3.2. Evaluation of the Effect of Atmospheric Photochemical Reaction Activity on Atmospheric Secondary Particle Generation
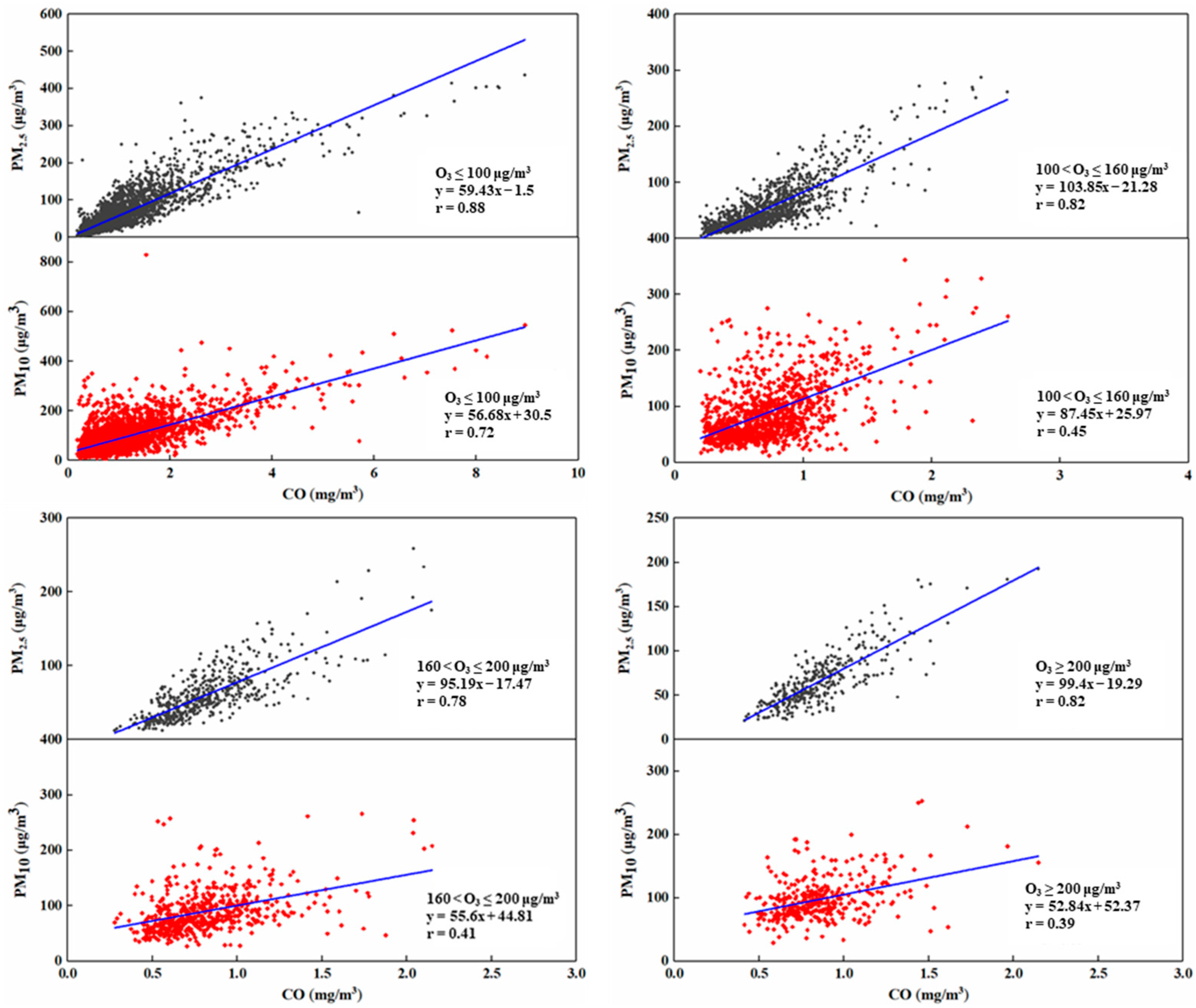
3.2.1. Classification of Photochemical Reaction Activity
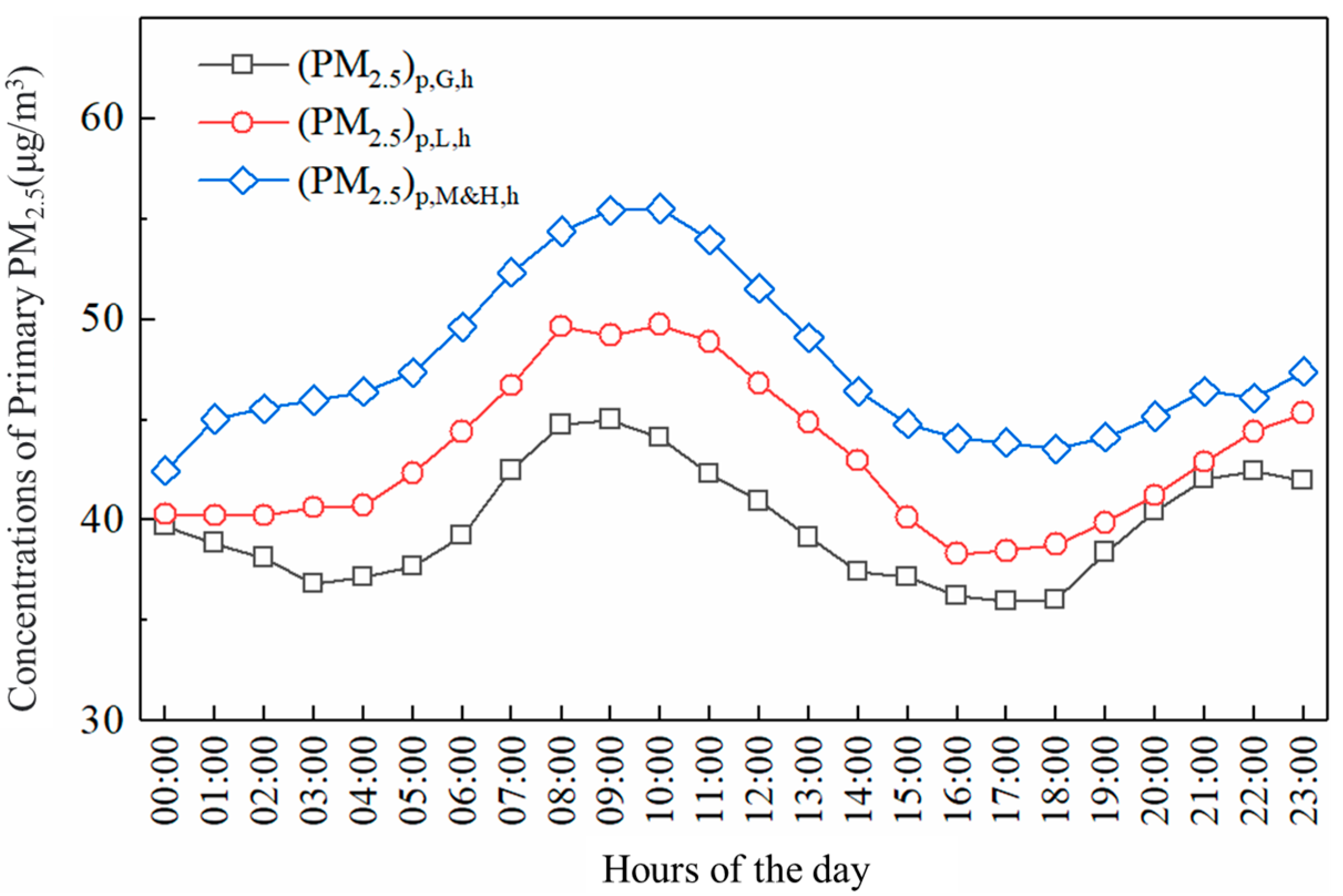
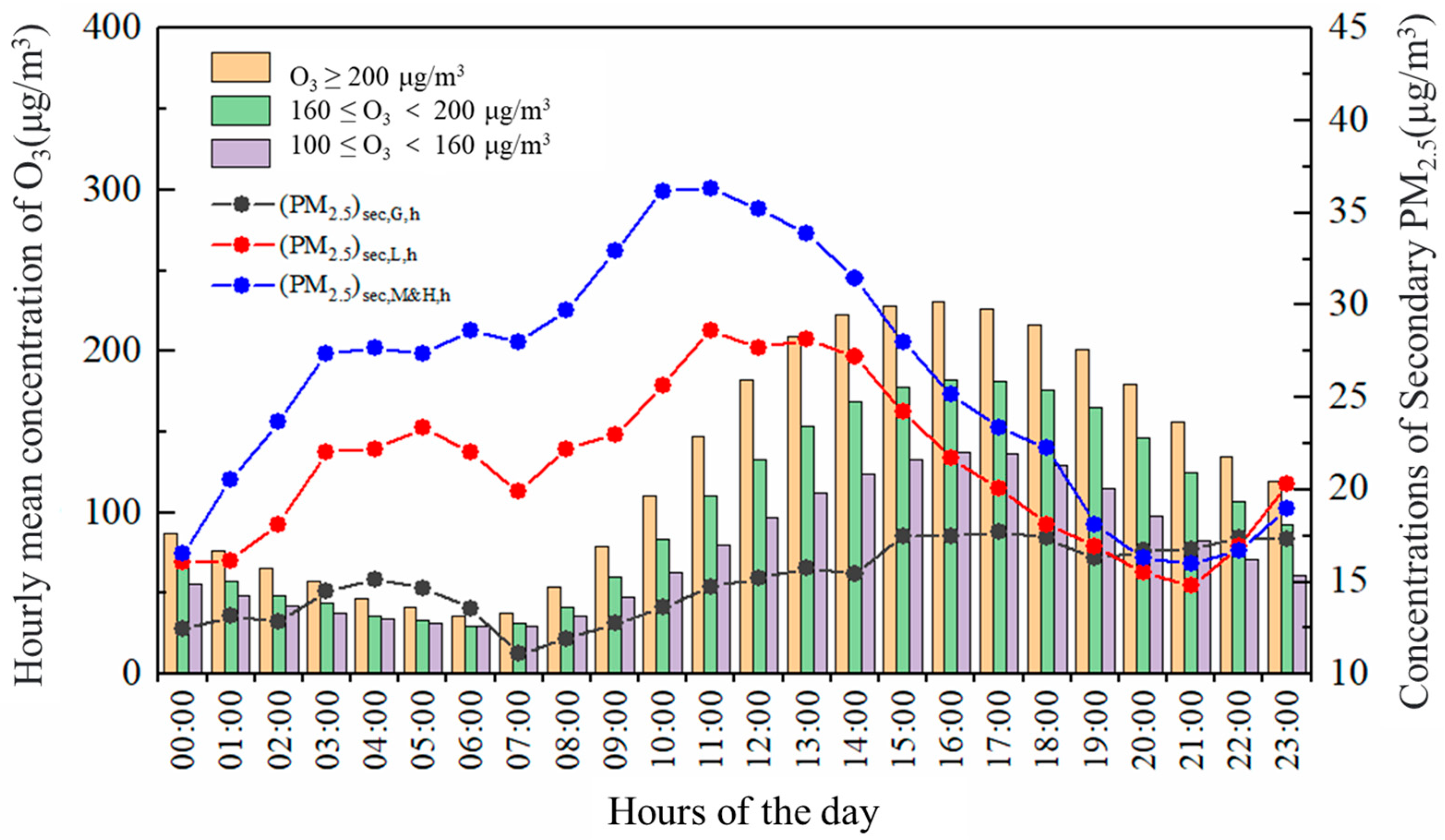
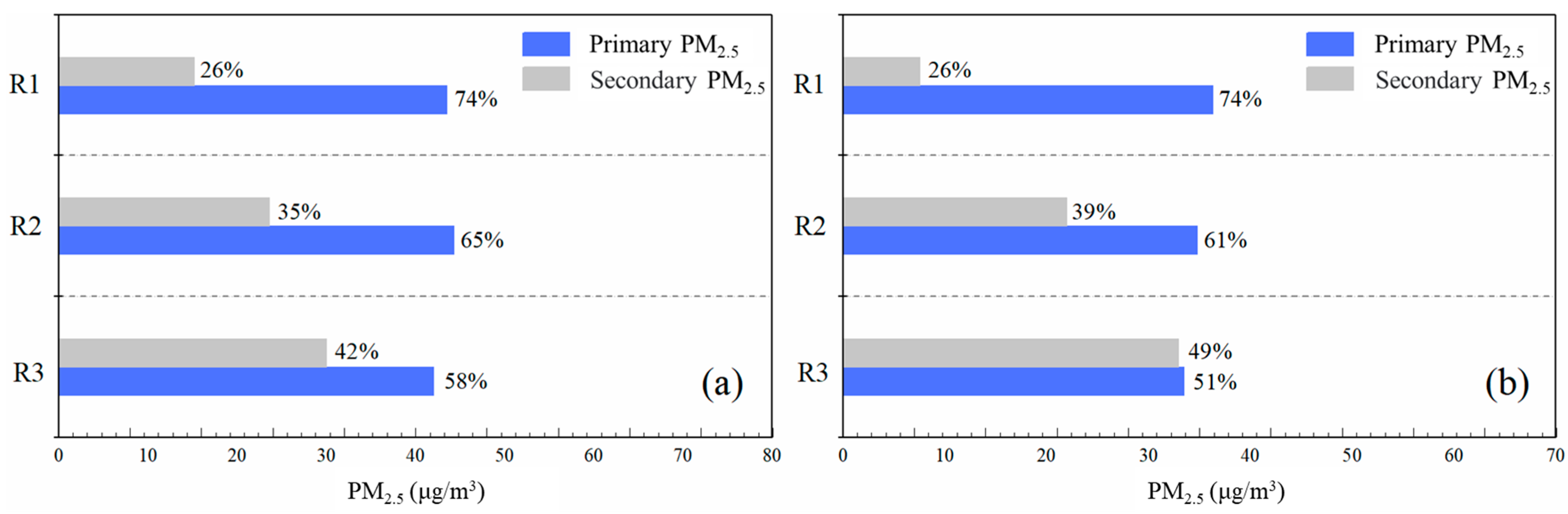
3.2.2. Effect of Different Photochemical Reaction Activities on the Generation of Atmospheric Secondary Particles
3.3. Effect of AOC on the Formation of Secondary Inorganic Components in Atmospheric Particulate Matter
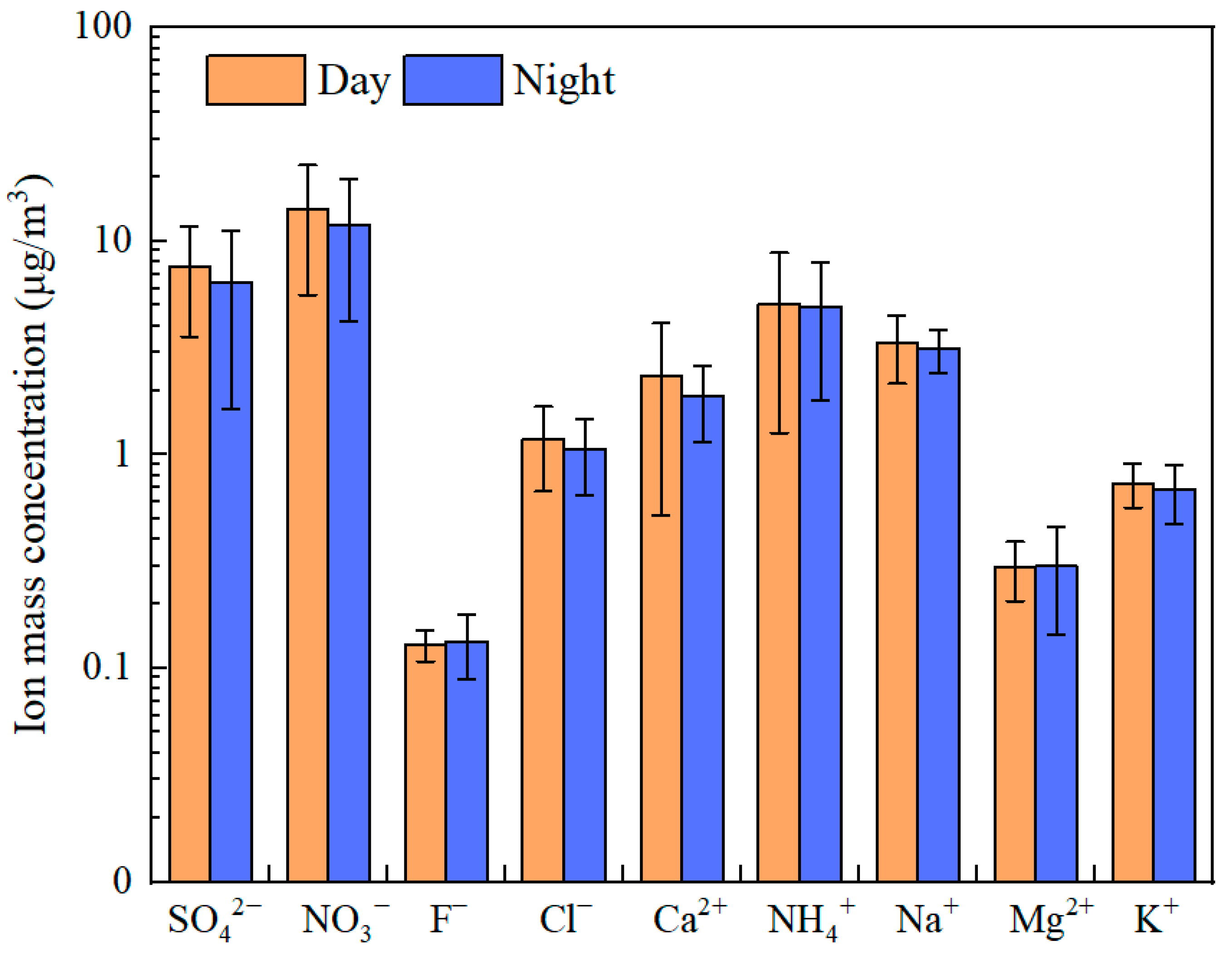
3.3.1. Composition Characteristics of Water-Soluble Inorganic Ions in PM2.5
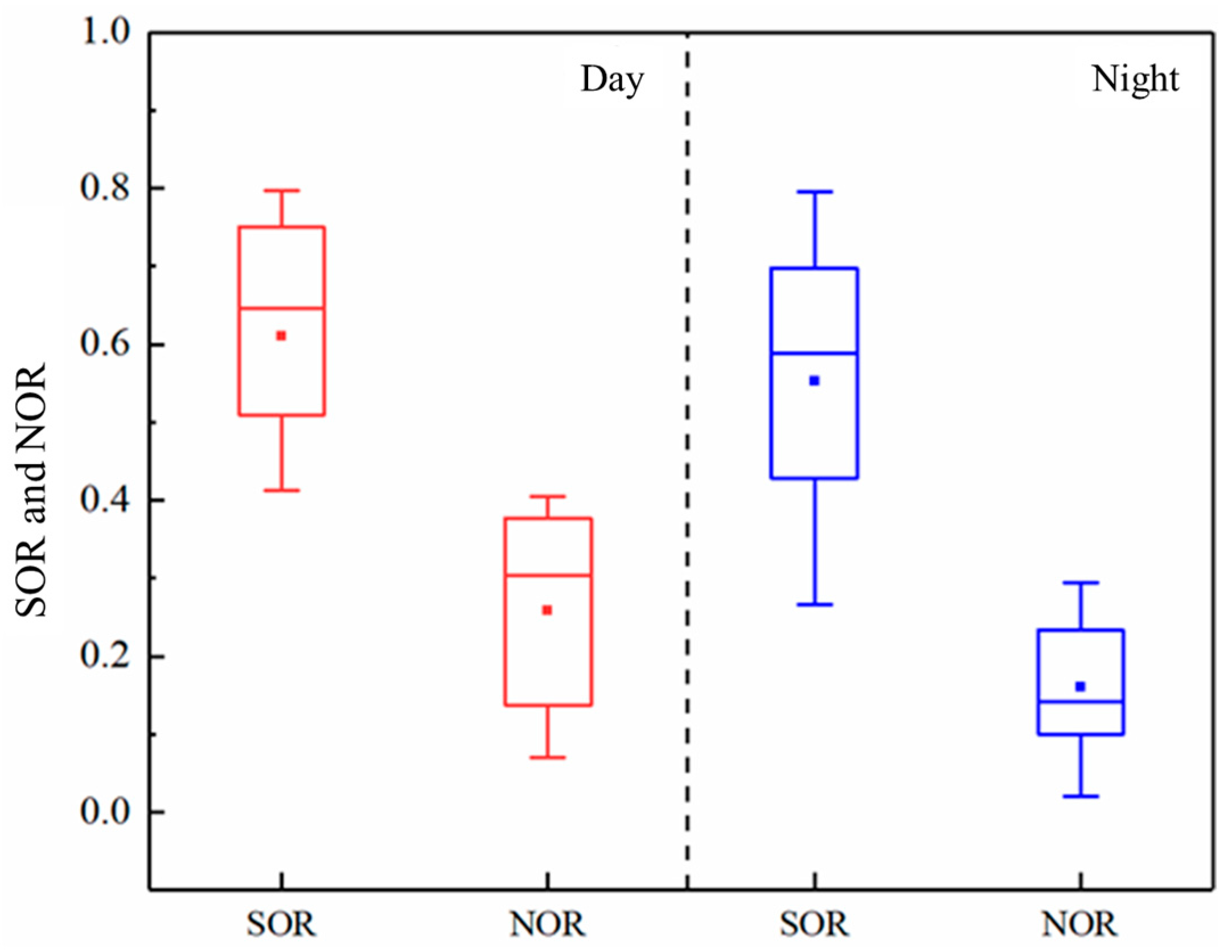
3.3.2. Sulfur and Nitrogen Conversion Rates of SO2 and NO2 in the Atmosphere
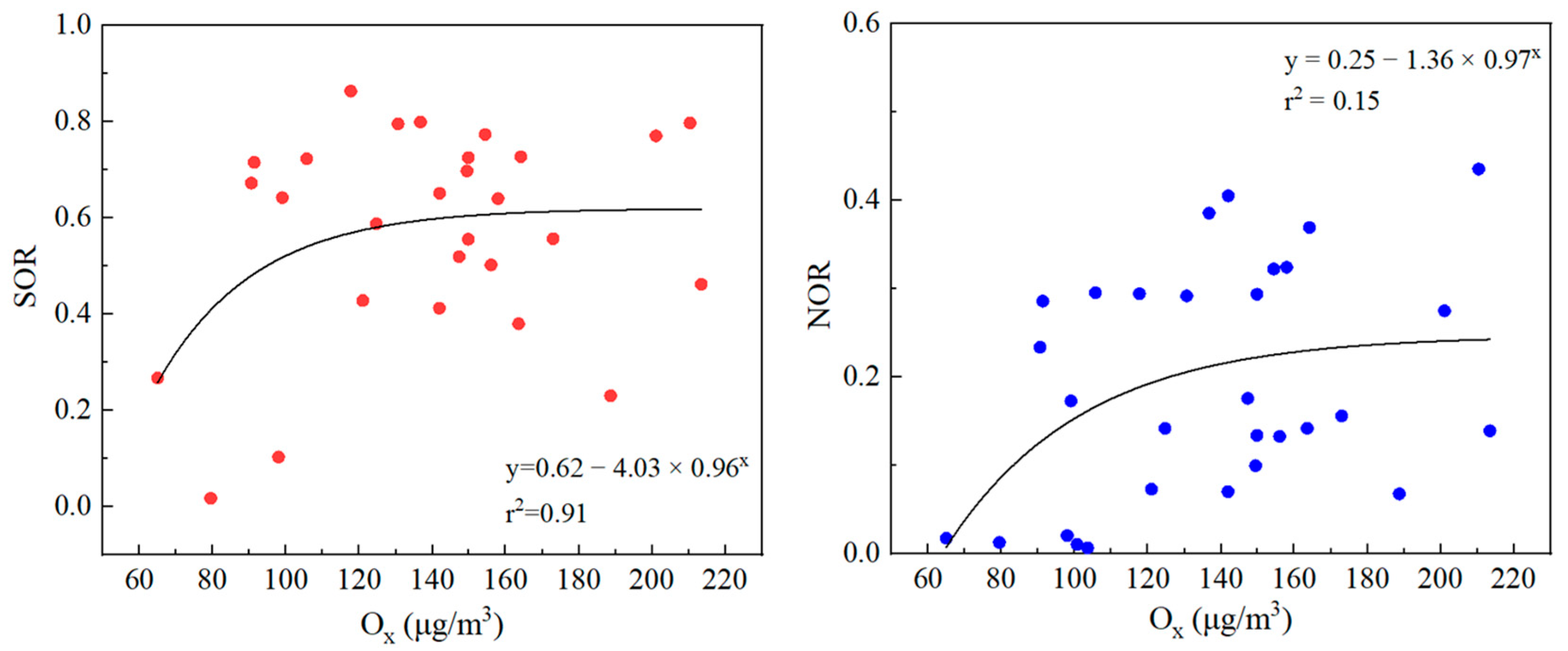
3.3.3. Correlation between OX and Secondary Inorganic Salts, SOR, and NOR in PM2.5
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beijing Municipal Ecology and Environment Bureau. Beijing Ecology and Environment Statement 2019; Beijing Municipal Ecology and Environment Bureau: Beijing, China, 2019.
- Beijing Municipal Ecology and Environment Bureau. Beijing Ecology and Environment Statement 2016; Beijing Municipal Ecology and Environment Bureau: Beijing, China, 2016.
- Zang, H.; Zhao, Y.; Huo, J.; Zhao, Q.; Fu, Q.; Duan, Y.; Shao, J.; Huang, C.; An, J.; Xue, L.; et al. High atmospheric oxidation capacity drives wintertime nitrate pollution in the eastern Yangtze River Delta of China. Atmos. Chem. Phys. 2022, 22, 4355–4374. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, H.; Lu, K.; Dong, H.; Liu, Y.; Zeng, L.; Hu, M.; Zhang, Y. An Observational Based Modeling of the Surface Layer Particulate Nitrate in the North China Plain during Summertime. JGR Atomspheres 2021, 126, e2021JD035623. [Google Scholar] [CrossRef]
- Liu, P.; Ye, C.; Xue, C.; Zhang, C.; Mu, Y.; Sun, X. Formation mechanisms of atmospheric nitrate and sulfate during the winter haze pollution periods in Beijing: Gas-phase, heterogeneous and aqueous-phase chemistry. Atmos. Chem. Phys. 2020, 20, 4153–4165. [Google Scholar] [CrossRef] [Green Version]
- Jia, M.; Zhao, T.; Cheng, X.; Gong, S.; Zhang, X.; Tang, L.; Liu, D.; Wu, X.; Wang, L.; Chen, Y. Inverse Relations of PM2.5 and O3 in Air Compound Pollution between Cold and Hot Seasons over an Urban Area of East China. Atmosphere 2017, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Chen, L.; Liao, H.; Dang, R. Correlations between PM2.5 and Ozone over China and Associated Underlying Reasons. Atmosphere 2019, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhu, S.; Zhang, M.; Shao, T. Atmospheric oxidation capacity and its contribution tosecondary pollutants formation. Chin. Sci. Bull. 2022, 67, 2069–2078. [Google Scholar] [CrossRef]
- Wen, L.; Xue, L.; Wang, X.; Xu, C.; Chen, T.; Yang, L.; Wang, T.; Zhang, Q.; Wang, W. Summertime fine particulate nitrate pollution in the North China Plain: Increasing trends, formation mechanisms and implications for control policy. Atmos. Chem. Phys. 2018, 18, 11261–11275. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Song, C.H.; Ghim, Y.S.; Won, J.G.; Yoon, S.C.; Carmichael, G.R.; Woo, J.H. An investigation on NH3 emissions and particulate NH4+–NO3− formation in East Asia. Atmos. Environ. 2006, 40, 2139–2150. [Google Scholar] [CrossRef]
- Feng, T.; Zhao, S.; Bei, N.; Liu, S.; Li, G. Increasing atmospheric oxidizing capacity weakens emission mitigation effort in Beijing during autumn haze events. Chemosphere 2021, 281, 130855. [Google Scholar] [CrossRef]
- Xie, X.; Hu, J.; Qin, M.; Guo, S.; Hu, M.; Wang, H.; Lou, S.; Li, J.; Sun, J.; Li, X.; et al. Modeling particulate nitrate in China: Current findings and future directions. Environ. Int. 2022, 166, 107369. [Google Scholar] [CrossRef]
- Peng, J.; Hu, M.; Shang, D.; Wu, Z.; Du, Z.; Tan, T.; Wang, Y.; Zhang, F.; Zhang, R. Explosive Secondary Aerosol Formation during Severe Haze in the North China Plain. Environ. Sci. Technol. 2021, 55, 2189–2207. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Automated Methods for Ambient Air Quality Monitoring; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2005.
- Ministry of Ecology and Environment of the People’s Republic of China. Specifications and Test Procedures for Ambient Air Quality Continuous Automated Monitoring System for PM10 and PM2.5 (HJ 653–2021); Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2021.
- Ministry of Ecology and Environment of the People’s Republic of China. Ambient Air Quality Standards (GB 3095-2012); Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2012.
- Ministry of Ecology and Environment of the People’s Republic of China. Technical Regulation for Ambient Air Quality Assessment (On Trial) (HJ 663-2013); Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2013.
- Chang, S.-C.; Lee, C.-T. Secondary aerosol formation through photochemical reactions estimated by using air quality monitoring data in Taipei City from 1994 to 2003. Atmos. Environ. 2007, 41, 4002–4017. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Y.; Bai, H.; Wang, X.; Cheng, S.; Wang, L. Insights into atmospheric oxidation capacity and its impact on PM2.5 in megacity Beijing via volatile organic compounds measurements. Atmos. Res. 2021, 258, 105632. [Google Scholar] [CrossRef]
- Lu, K.; Fuchs, H.; Hofzumahaus, A.; Tan, Z.; Wang, H.; Zhang, L.; Schmitt, S.H.; Rohrer, F.; Bohn, B.; Broch, S.; et al. Fast Photochemistry in Wintertime Haze: Consequences for Pollution Mitigation Strategies. Environ. Sci. Technol. 2019, 53, 10676–10684. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wang, M.; Yu, G. Spatial and Temporal Distribution and Potential Source of Atmospheric Pollution in Jiaozuo City. Res. Environ. Sci. 2020, 33, 820–830. [Google Scholar] [CrossRef]
- Chen, C.; Wang, T.; Li, Y.; Ma, H.; Chen, P.; Wang, D.; Zhang, Y.; Qiao, Q.; Li, G.; Wang, W. Pollution Characteristics and Source Apportionment of Fine Particulate Matter in Autumn and Winter in Puyang, China. Environ. Sci. 2019, 40, 3421–3430. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, C.; Wang, H.; Huang, C.; Su, L.; Chen, Y.; Li, L.; Qiao, Y.; Chen, M.; Huang, H.; et al. Chemical characteristics of particulate matters during air pollution episodes in autumn of Shanghai, China. Acta Sci. Circumstantiae 2012, 32, 81–92. [Google Scholar] [CrossRef]
- Shen, Z.; Huo, Z.; Han, Y.; Cao, J.; Zhao, J.; Zhang, T. Chemical Composition of Water-Soluble Ions in Aerosols over Xi’an in Heating and Non-Heating Seasons. Plateau Meteorol. 2009, 28, 151–158. [Google Scholar]
- Guo, W.; Wang, K.; Guo, X.; Yan, Y. Characteristics of sulfur and nitrogen conversion in the aerosol, Taiyuan. Environ. Chem. 2016, 35, 11–17. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Chen, T.; Zhang, D.; Sun, F.; Wang, X.; Huan, N.; Pan, L. Analysis on diurnal variation characteristics of ozone and correlations with its precursors in urban atmosphere of Beijing. China Environ. Sci. 2014, 34, 3001–3008. [Google Scholar]
- Huang, X.-F.; Cao, L.-M.; Tian, X.-D.; Zhu, Q.; Saikawa, E.; Lin, L.-L.; Cheng, Y.; He, L.-Y.; Hu, M.; Zhang, Y.-H.; et al. Critical Role of Simultaneous Reduction of Atmospheric Odd Oxygen for Winter Haze Mitigation. Environ. Sci. Technol. 2021, 55, 11557–11567. [Google Scholar] [CrossRef]
- Xue, J.; Yuan, Z.; Griffith, S.M.; Yu, X.; Lau, A.K.H.; Yu, J.Z. Sulfate Formation Enhanced by a Cocktail of High NOx, SO2, Particulate Matter, and Droplet pH during Haze-Fog Events in Megacities in China: An Observation-Based Modeling Investigation. Environ. Sci. Technol. 2016, 50, 7325–7334. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Liao, H.; Zhu, J.; Shah, V.; Shen, L.; Bates, K.H.; Zhang, Q.; Zhai, S. A two-pollutant strategy for improving ozone and particulate air quality in China. Nat. Geosci. 2019, 12, 906–910. [Google Scholar] [CrossRef]

| Seasons | OX Concentration (μg/m3) | O3/OX | NO2/OX | r(NO2) | r(O3) |
|---|---|---|---|---|---|
| Spring | 116 | 0.6 | 0.4 | 0.41 | 0.75 |
| Summer | 128 | 0.74 | 0.26 | 0.29 | 0.96 |
| Autumn | 88 | 0.57 | 0.43 | 0.27 | 0.73 |
| Winter | 82 | 0.64 | 0.36 | 0.81 | 0.35 |
| Cities | Periods | SOR | NOR | Sources |
|---|---|---|---|---|
| Beijing | July–September 2019 | 0.58 | 0.20 | This paper |
| Jiaozuo | January 2018 | 0.43 | 0.35 | Wang Liumin et al. [21] |
| Puyang | October 2017– January 2018 | 0.22 | 0.22 | Chen Chu et al. [22] |
| Shanghai | October–November 2010 | 0.24 | 0.15 | Zhou Min et al. [23] |
| Xi’an | June–August 2006 | 0.44 | 0.32 | Shen Zhenxing et al. [24] |
| Taiyuan | May–June 2013 | 0.13 | 0.08 | Guo Wendi et al. [25] |
| PM2.5 | OX | SO42− | NO3− | NH4+ | SOR | NOR | |
|---|---|---|---|---|---|---|---|
| PM2.5 | 1 | 0.46 | 0.71 | 0.9 | 0.86 | 0.79 | 0.85 |
| OX | 1 | 0.35 | 0.41 | 0.32 | 0.25 | 0.36 | |
| SO42− | 1 | 0.75 | 0.78 | 0.82 | 0.8 | ||
| NO3− | 1 | 0.94 | 0.8 | 0.91 | |||
| Ca2+ | 0.45 | 0.43 | 0.56 | ||||
| NH4+ | 1 | 0.83 | 0.89 | ||||
| SOR | 1 | 0.83 | |||||
| NOR | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, W.; Li, L.; Li, H.; Zhang, Y.; Chen, Y.; Zhi, G.; Yang, X.; Ji, Y.; Chai, F. Atmospheric Oxidation Capacity and Its Impact on the Secondary Inorganic Components of PM2.5 in Recent Years in Beijing: Enlightenment for PM2.5 Pollution Control in the Future. Atmosphere 2023, 14, 1252. https://doi.org/10.3390/atmos14081252
Chu W, Li L, Li H, Zhang Y, Chen Y, Zhi G, Yang X, Ji Y, Chai F. Atmospheric Oxidation Capacity and Its Impact on the Secondary Inorganic Components of PM2.5 in Recent Years in Beijing: Enlightenment for PM2.5 Pollution Control in the Future. Atmosphere. 2023; 14(8):1252. https://doi.org/10.3390/atmos14081252
Chicago/Turabian StyleChu, Wanghui, Ling Li, Hong Li, Yuzhe Zhang, Yizhen Chen, Guorui Zhi, Xin Yang, Yuanyuan Ji, and Fahe Chai. 2023. "Atmospheric Oxidation Capacity and Its Impact on the Secondary Inorganic Components of PM2.5 in Recent Years in Beijing: Enlightenment for PM2.5 Pollution Control in the Future" Atmosphere 14, no. 8: 1252. https://doi.org/10.3390/atmos14081252
APA StyleChu, W., Li, L., Li, H., Zhang, Y., Chen, Y., Zhi, G., Yang, X., Ji, Y., & Chai, F. (2023). Atmospheric Oxidation Capacity and Its Impact on the Secondary Inorganic Components of PM2.5 in Recent Years in Beijing: Enlightenment for PM2.5 Pollution Control in the Future. Atmosphere, 14(8), 1252. https://doi.org/10.3390/atmos14081252





