Effects of Climate Change and Nitrogen Addition on Carbon Loss in Alpine Wetland of Qinghai–Tibet Plateau
Abstract
:1. Introduction
2. Materials and Methods
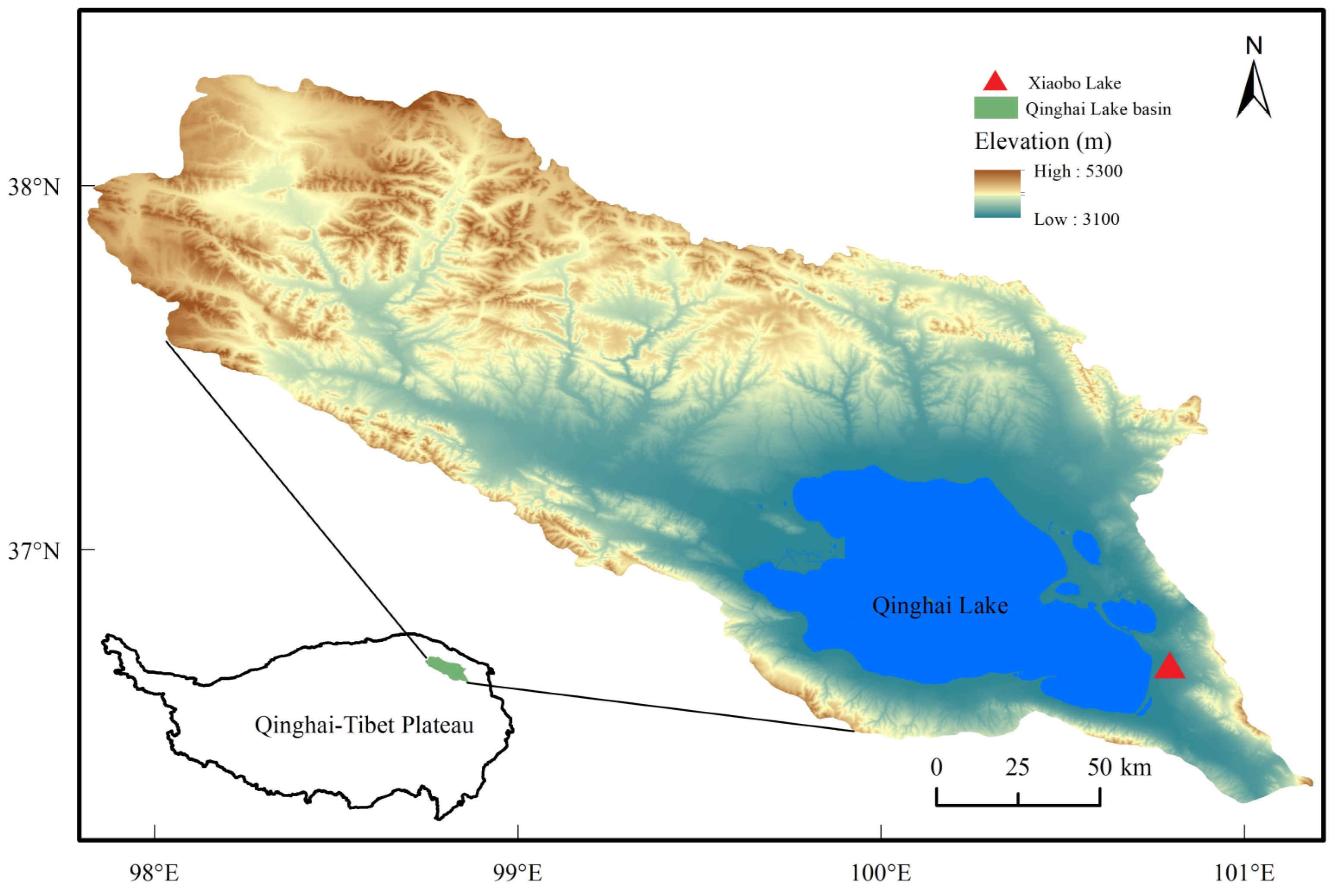
2.1. Study Area
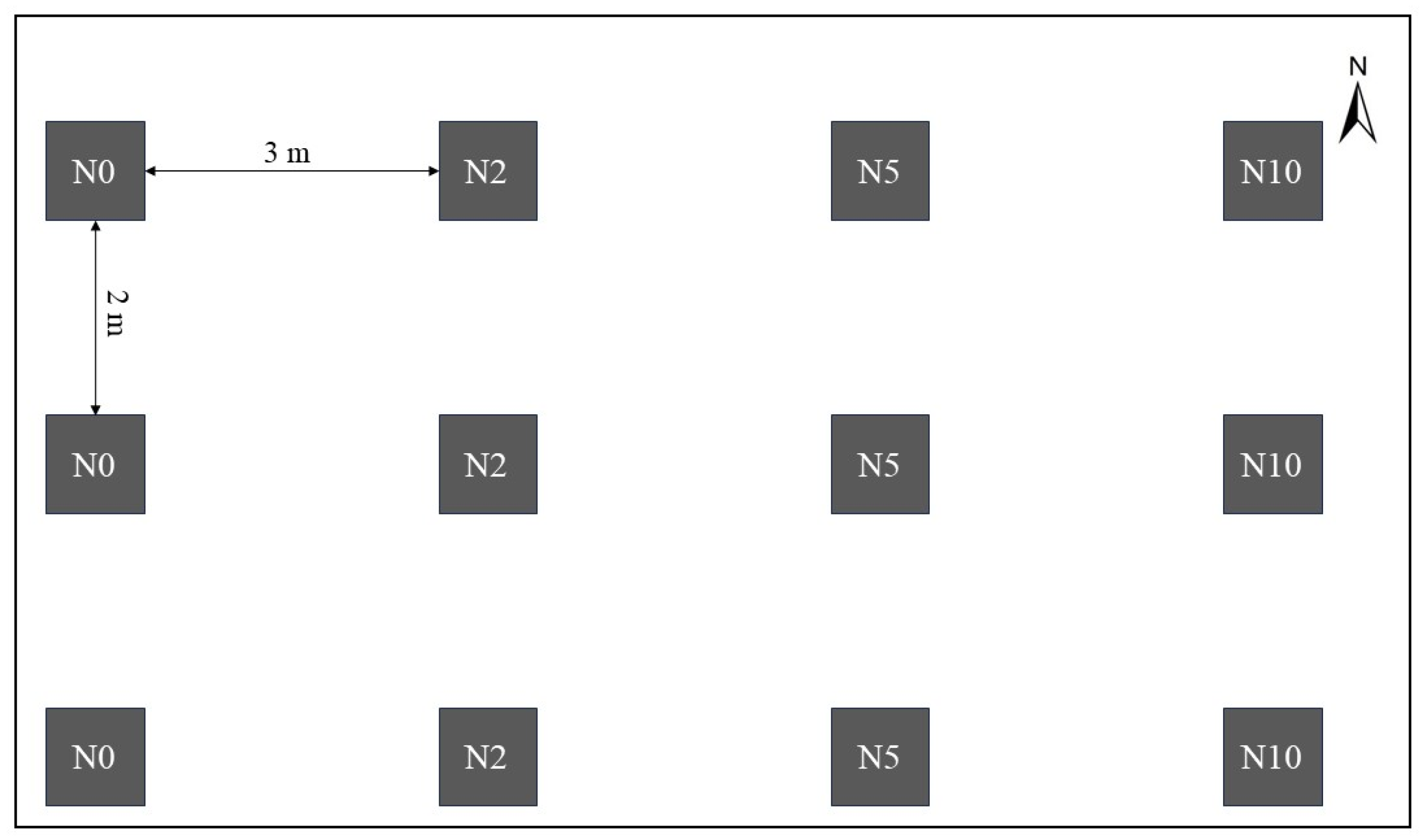
2.2. Experimental Design
2.3. Sample Processing and Determination
2.3.1. Determination of Physical and Chemical Properties of Soils
2.3.2. Soil Microbiology Determination
2.3.3. Climate Data
3. Results
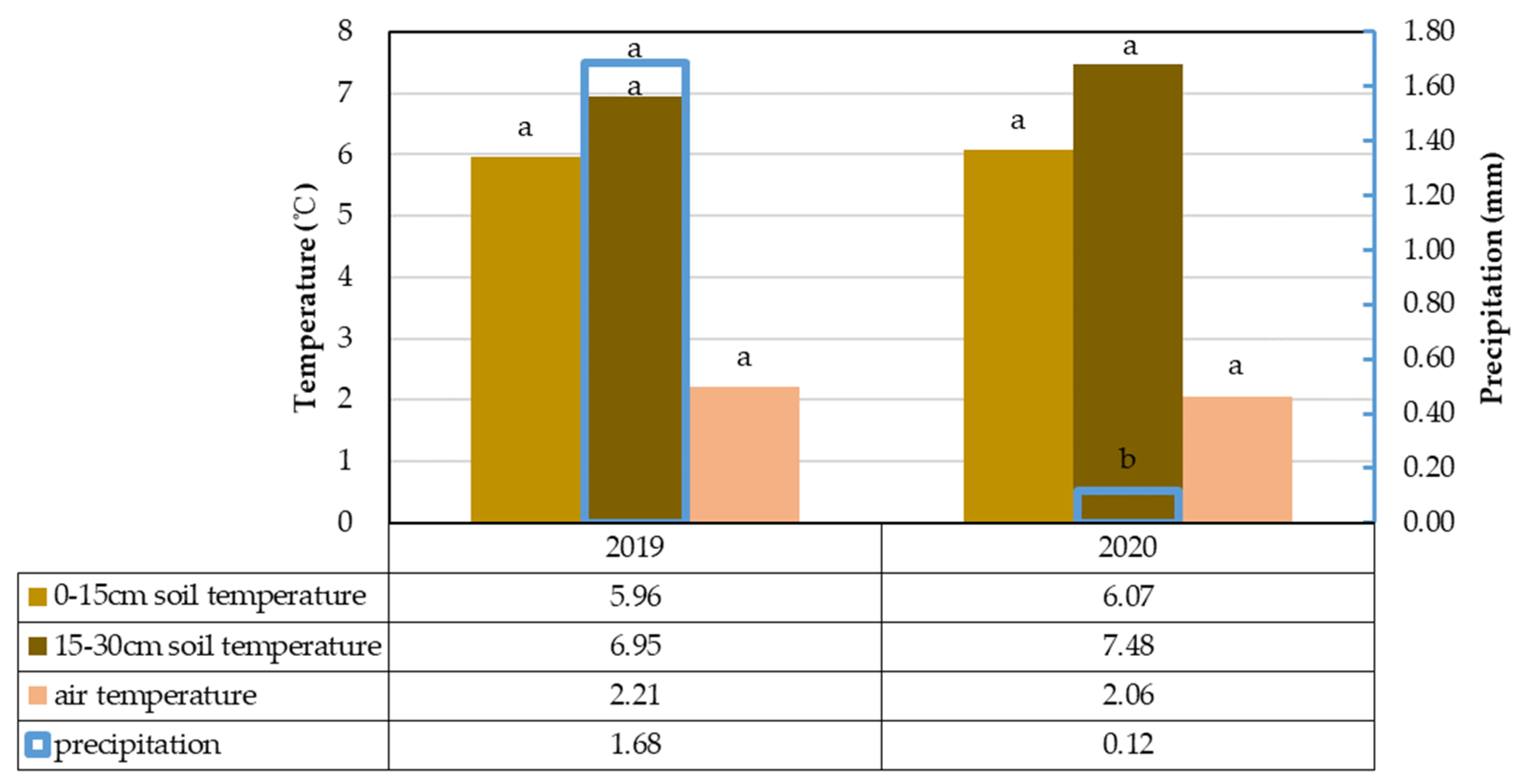
3.1. Climate Change Analysis and Its Impact on Soil Carbon
3.2. Soil Carbon Changes under Different Nitrogen Addition Conditions
3.3. Changes in Soil Physicochemical Properties and Their Effects on Soil Carbon under Nitrogen Addition Conditions
3.4. Soil Microbial Changes and Their Effect on Soil Carbon under Nitrogen Addition Conditions
4. Discussion
4.1. Effects of Climate Change and N Addition on SOC
4.2. Influence of Soil Physicochemical Properties on SOC
4.3. Influence of Soil Microorganisms on SOC
4.4. Research Shortcomings and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, P.; Zeng, Z.; Moore, D.J.P.; Zeng, X. Soil microbial respiration from observations and earth system models. Environ. Res. Lett. 2013, 8, 034034. [Google Scholar] [CrossRef]
- Carvalhais, N.; Forkel, M.; Khomik, M.; Bellarby, J.; Jung, M.; Migliavacca, M.; Mu, M.; Saatchi, S.; Santoro, M.; Thurner, M.; et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 2014, 514, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Lajtha, K.; Bowden, R.D.; Crow, S.; Fekete, I.; Kotroczó, Z.; Plante, A.; Simpson, M.J.; Nadelhoffer, K.J. The detrital input and removal treatment (DIRT) network: Insights into soil carbon stabilization. Sci. Total Environ. 2018, 640–641, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Hou, E.; Guo, J.; Gilliam, F.S.; Li, J.; Tang, S.; Kuang, Y. Nitrogen addition stimulates soil aggregation and enhances carbon storage in terrestrial ecosystems of China: A meta-analysis. Glob. Chang. Biol. 2021, 27, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.P.; Kang, S.X.; Apip; Zhou, M.M.; Lyu, J.Q.; Aisyah, S.; Binaya, M.; Regmi, R.K.; Nover, D. Water quality trend assessment in Jakarta: A rapidly growing Asian megacity. PLoS ONE 2019, 14, e0219009. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Rao, C.S.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil organic carbon dynamics: Impact of land use changes and management practices: A review. Adv. Agron. 2019, 156, 1–107. [Google Scholar]
- Fantappiè, M.; L’Abate, G.; Costantini, E. The influence of climate change on the soil organic carbon content in Italy from 1961 to 2008. Geomorphology 2011, 135, 343–352. [Google Scholar] [CrossRef]
- Bradford, M.A.; Wieder, W.R.; Bonan, G.B.; Fierer, N.; Raymond, P.A.; Crowther, T.W. Managing uncertainty in soil carbon feedbacks to climate change. Nat. Clim. Chang. 2016, 6, 751–758. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J.; Rivas-Ubach, A.; Janssens, I.A. The human-induced imbalance between C, N and P in earth’s life system. Glob. Chang. Biol. 2012, 18, 3–6. [Google Scholar] [CrossRef]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Bala, G.; Devaraju, N.; Chaturvedi, R.; Caldeira, K.; Nemani, R. Nitrogen deposition: How important is it for global terrestrial carbon uptake? Biogeosciences 2013, 10, 7147–7160. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, B. A global meta-analysis of soil respiration and its components in response to phosphorus addition. Soil Biol. Biochem. 2019, 135, 38–47. [Google Scholar] [CrossRef]
- Xu, C.; Xu, X.; Ju, C.; Chen, H.; Wilsey, B.; Luo, Y.; Fan, W. Long-term, amplified responses of soil organic carbon to nitrogen addition worldwide. Glob. Chang. Biol. 2021, 27, 1170–1180. [Google Scholar] [CrossRef]
- Yue, K.; Fornara, D.A.; Yang, W.; Peng, Y.; Peng, C.; Liu, Z.; Wu, F. Influence of multiple global change drivers on terrestrial carbon storage: Additive effects are common. Ecol. Lett. 2017, 20, 663–672. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.Q.; Groenigen, K.J.; Hungate, B.A.; Cao, J.J.; Zhou, X.H.; Wang, R.W. A keystone microbial enzyme for nitrogen control of soil carbon storage. Sci. Adv. 2018, 4, eaaq1689. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; Burton, A.J.; Talhelm, P.A.F. Simulated atmospheric no3- deposition increases soil organic matter by slowing decomposition. Ecol. Appl. 2008, 18, 2016–2027. [Google Scholar] [CrossRef]
- Crowther, T.W.; Riggs, C.; Lind, E.; Borer, E.T.; Seabloom, E.W.; Hobbie, S.E.; Wubs, J.; Adler, P.B.; Firn, J.; Gherardi, L.; et al. Sensitivity of global soil carbon stocks to combined nutrient enrichment. Ecol. Lett. 2019, 22, 936–945. [Google Scholar] [CrossRef]
- Lu, X.; Gilliam, F.S.; Guo, J.; Hou, E.; Kuang, Y. Decrease in soil pH has greater effects than increase in above-ground carbon inputs on soil organic carbon in terrestrial ecosystems of China under nitrogen enrichment. J. Appl. Ecol. 2022, 59, 768–778. [Google Scholar] [CrossRef]
- Mack, M.C.; Schuur, E.A.G.; Bret-Harte, M.S.; Shaver, G.R.; Chapin, F.S. Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature 2004, 431, 440–443. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R. Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc. Natl. Acad. Sci. USA 2006, 103, 10316–10321. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.J.; Cheng, S.L.; Yu, G.R.; Yang, X.M.; Xu, M.J.; Wang, Y.S.; Li, L.S.; Dang, X.S.; Wang, L.; Li, Y.N. Nitrogen deposition impacts on the amount and stability of soil organic matter in an alpine meadow ecosystem depend on the form and rate of applied nitrogen. Eur. J. Soil Sci. 2014, 65, 510–519. [Google Scholar] [CrossRef]
- Camenzind, T.; Hättenschwiler, S.; Treseder, K.K.; Lehmann, A.; Rillig, M.C. Nutrient limitation of soil microbial processes in tropical forests. Ecol. Monogr. 2018, 88, 4–21. [Google Scholar] [CrossRef]
- Mori, T.; Lu, X.K.; Aoyagi, R.; Mo, J. Reconsidering the phosphorus limitation of soil microbial activity in tropical forests. Func. Ecol. 2018, 32, 1145–1154. [Google Scholar] [CrossRef]
- Riggs, C.E.; Hobbie, S.E. Mechanisms driving the soil organic matter decomposition response to nitrogen enrichment in grassland soils. Soil Biol. Biochem. 2016, 99, 54–65. [Google Scholar] [CrossRef]
- Xin, H. A green fervor sweeps the Qinghai-Tibetan Plateau. Science 2008, 321, 633–635. [Google Scholar] [CrossRef]
- Ma, Y.J.; Li, X.Y.; Liu, L.; Huang, Y.M.; Li, Z.; Hu, X.; Wu, X.C.; Yang, X.F.; Wang, P.; Zhao, S.J.; et al. Measurements and modeling of the water budget in semiarid high-altitude Qinghai Lake Basin, Northeast Qinghai-Tibet Plateau. J. Geophys. Res. Atmos. 2018, 123, 10857–10871. [Google Scholar] [CrossRef]
- Li, J.; Gong, J.; Guldmann, J.M.; Li, S.; Zhu, J. Carbon dynamics in the northeastern qinghai–tibetan plateau from 1990 to 2030 using landsat land use/cover change data. Remote Sens. 2020, 12, 528. [Google Scholar] [CrossRef]
- Ma, Y.J.; Xie, T.; Li, X.Y. Spatial variation of soil organic carbon in the Qinghai Lake watershed, northeast Qinghai-Tibet Plateau. Catena 2022, 213, 106187. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, S.; Cao, G.; Lan, Y. Effects of vegetation phenology on vegetation productivity in the Qinghai Lake Basin of the Northeastern Qinghai–Tibet Plateau. Arab. J. Geosci. 2021, 14, 1030. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, S.; Cao, G.; Hou, Y.; Wang, Y.; Kang, L. The Partitioning of Catchment Evapotranspiration Fluxes as Revealed by Stable Isotope Signals in the Alpine Inland River Basin. Water 2022, 14, 790. [Google Scholar] [CrossRef]
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhou, X.; Luo, Y.; Yang, Y.; Fang, C.; Chen, J.; Li, B. Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis. Agric. Ecosyst. Environ. 2011, 140, 234–244. [Google Scholar] [CrossRef]
- Amador, J.A.; Görres, J.H.; Savin, M.C. Role of soil water content in the carbon and nitrogen dynamics of Lumbricus terrestris L. burrow soil. Appl. Soil Ecol. 2005, 28, 15–22. [Google Scholar] [CrossRef]
- Zhong, X.L.; Li, J.T.; Li, X.J.; Ye, Y.C.; Liu, S.S.; Hallett, P.D.; Ogden, M.R.; Naveed, M. Physical protection by soil aggregates stabilizes soil organic carbon under simulated N deposition in a subtropical forest of China. Geoderma 2017, 285, 323–332. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, X.; Zhang, B.; Lu, M.; Luo, Y.; Liu, L.; Li, B. Different responses of soil respiration and its components to nitrogen addition among biomes: A meta-analysis. Glob. Chang. Biol. 2014, 20, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Harpole, W.S.; Potts, D.L.; Suding, K.N. Ecosystem responses to water and nitrogen amendment in a California grassland. Glob. Chang. Biol. 2007, 13, 2341–2348. [Google Scholar] [CrossRef]
- Liu, K.; Fang, Y.; Yu, F.; Liu, Q.; Li, F.; Peng, S. Soil acidification in response to acid deposition in three subtropical forests of subtropical China. Pedosphere 2010, 20, 399–408. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Zhao, L.; Ge, S.; Chen, D.; Dong, Q.; Zhao, X. Land-use effects on organic and inorganic carbon patterns in the topsoil around Qinghai Lake basin, Qinghai-Tibetan Plateau. Catena 2016, 147, 345–355. [Google Scholar] [CrossRef]
- Matson, P.; Lohse, K.A.; Hall, S.J. The globalization of nitrogen deposition: Consequences for terrestrial ecosystems. Ambio 2002, 113–119. [Google Scholar] [CrossRef]
- Kaye, J.; Barrett, J.; Burke, I. Stable nitrogen and carbon pools in grassland soils of variable texture and carbon content. Ecosystems 2002, 5, 461–471. [Google Scholar] [CrossRef]
- Gao, W.; Cheng, S.; Fang, H.; Chen, Y.; Yu, G.; Zhou, M.; Zhang, P.; Xu, M. Effects of simulated atmospheric nitrogen deposition on inorganic nitrogen content and acidification in a cold-temperate coniferous forest soil. Acta Ecol. Sin. 2013, 33, 114–121. [Google Scholar] [CrossRef]
- Kooijman, A.; Morriën, E.; Jagers op Akkerhuis, G.; Missong, A.; Bol, R.; Klumpp, E.; Hall, R.; Til, M.; Kalbitz, K.; Bloem, J. Resilience in coastal dune grasslands: pH and soil organic matter effects on P nutrition, plant strategies, and soil communities. Ecosphere 2020, 11, e03112. [Google Scholar] [CrossRef]
- Wamelink, G.W.W.; Van Dobben, H.F.; Berendse, F. Vegetation succession as affected by decreasing nitrogen deposition, soil characteristics and site management: A modelling approach. For. Ecol. Manag. 2009, 258, 1762–1773. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Schuster, M.J.; Smith, N.G.; Dukes, J.S. Responses of aboveground C and N pools to rainfall variability and nitrogen deposition are mediated by seasonal precipitation and plant community dynamics. Biogeochemistry 2016, 129, 389–400. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Xia, Z.; Yang, J.; Sang, C.; Wang, X.; Sun, L.; Jiang, P.; Wang, C.; Bai, E. Phosphorus reduces negative effects of nitrogen addition on soil microbial communities and functions. Microorganisms 2020, 8, 1828. [Google Scholar] [CrossRef]
- Chu, H.Y.; Fierer, N.; Lauber, C.L.; Caporaso, J.G.; Knight, R.; Grogan, P. Soil bacterial diversity in the Arctic is not fundamentally different from that found in other biomes. Environ. Microbiol. 2010, 12, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.I.; Thomson, B.C.; James, P.; Bell, T.; Whiteley, A.S. The bacterial biogeography of british soils rid d-5237-2011 rid a-3395-2012. Environ. Microbiol. 2011, 13, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
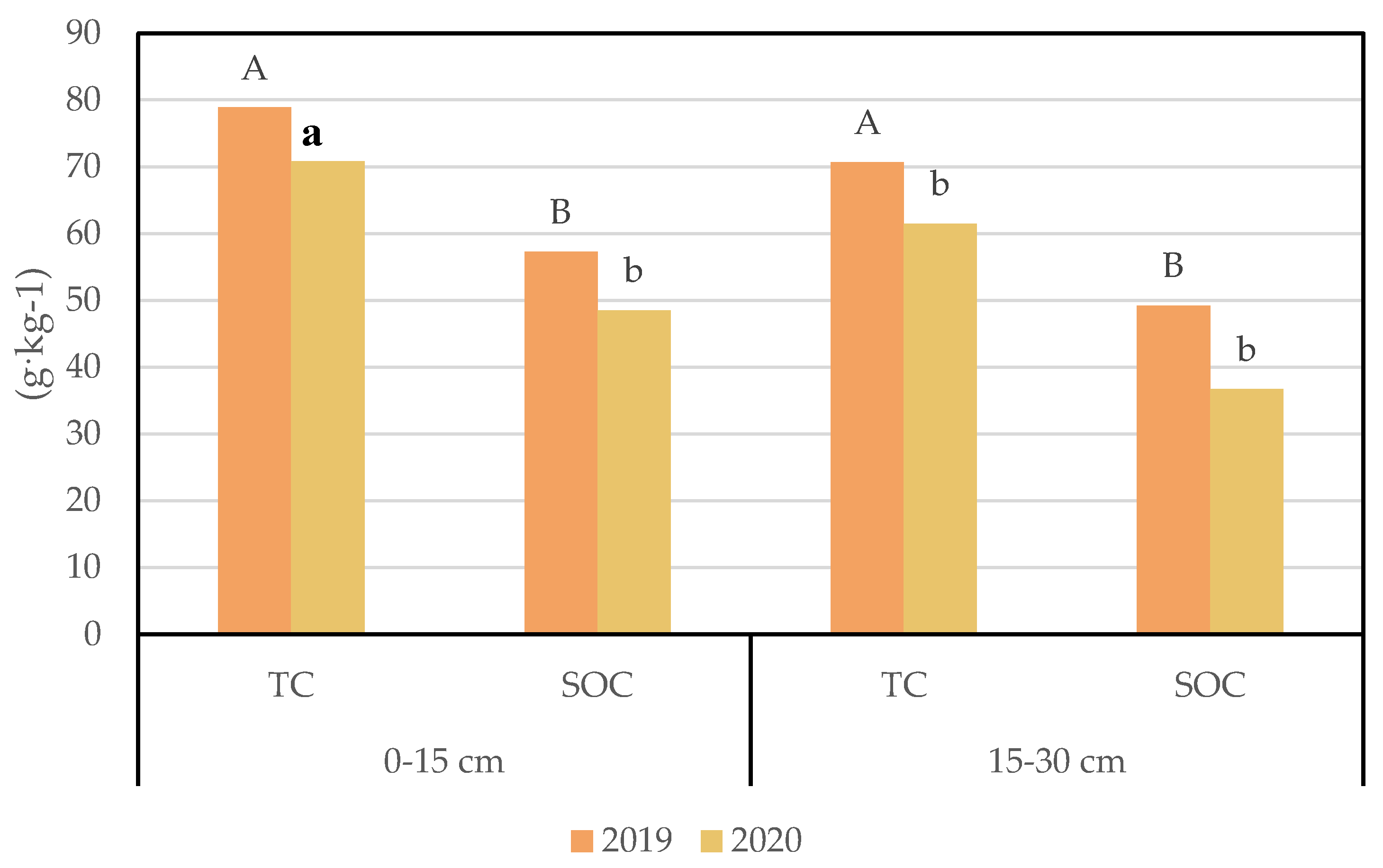
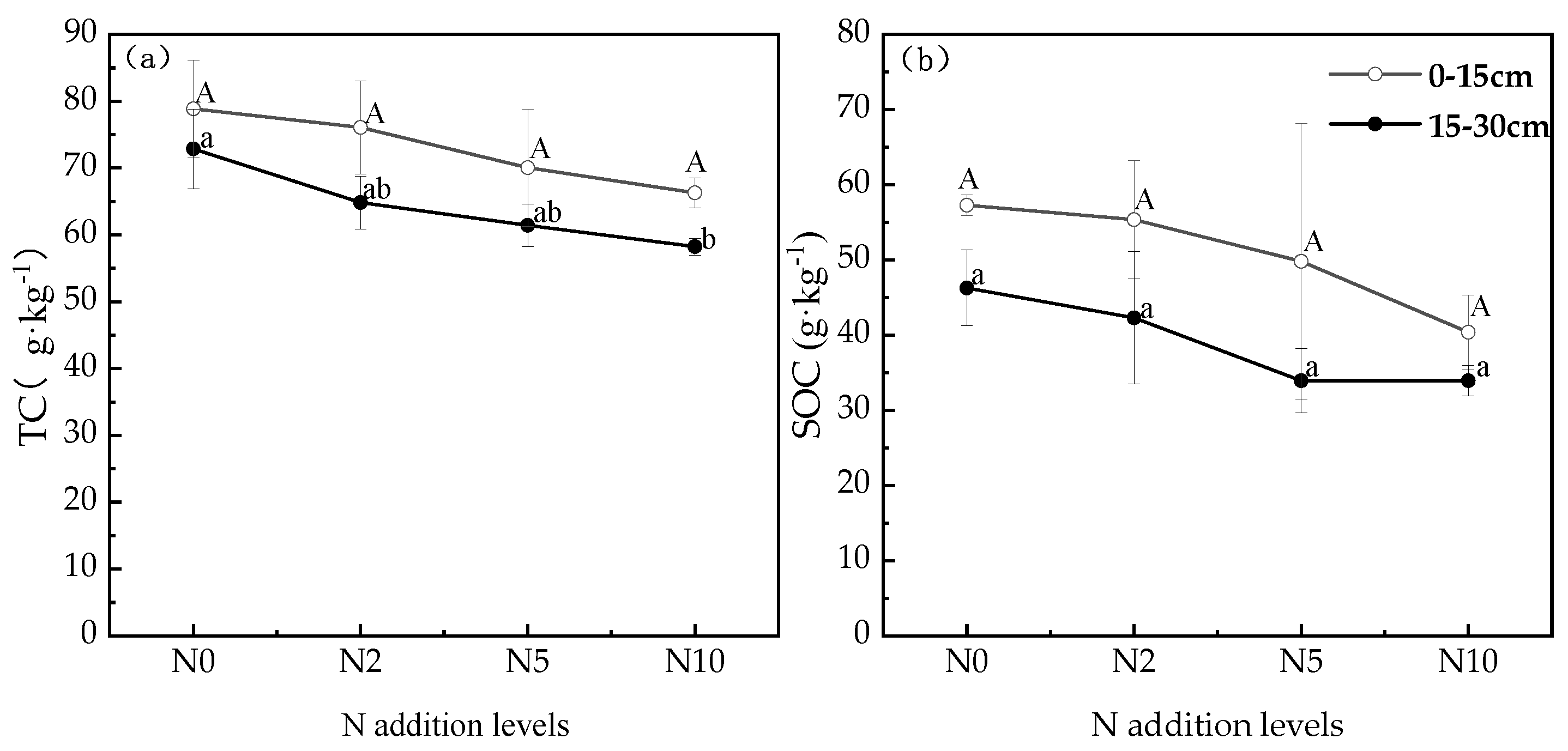


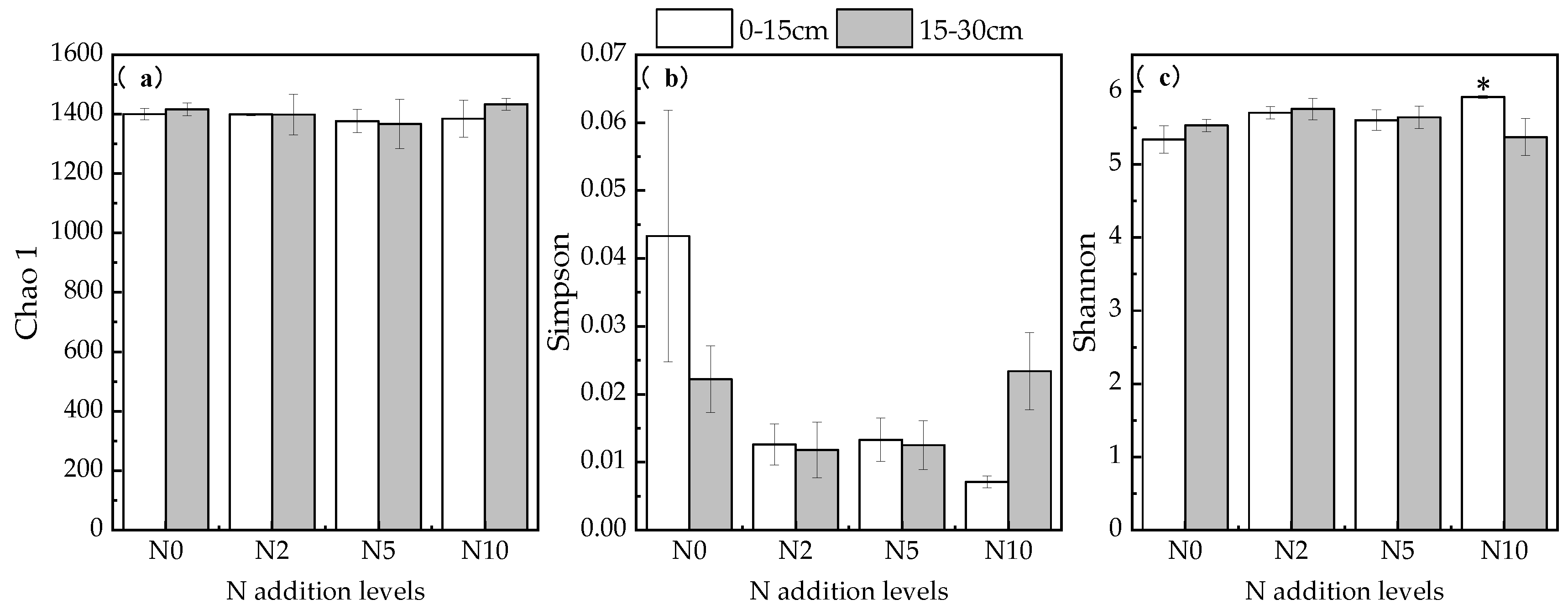
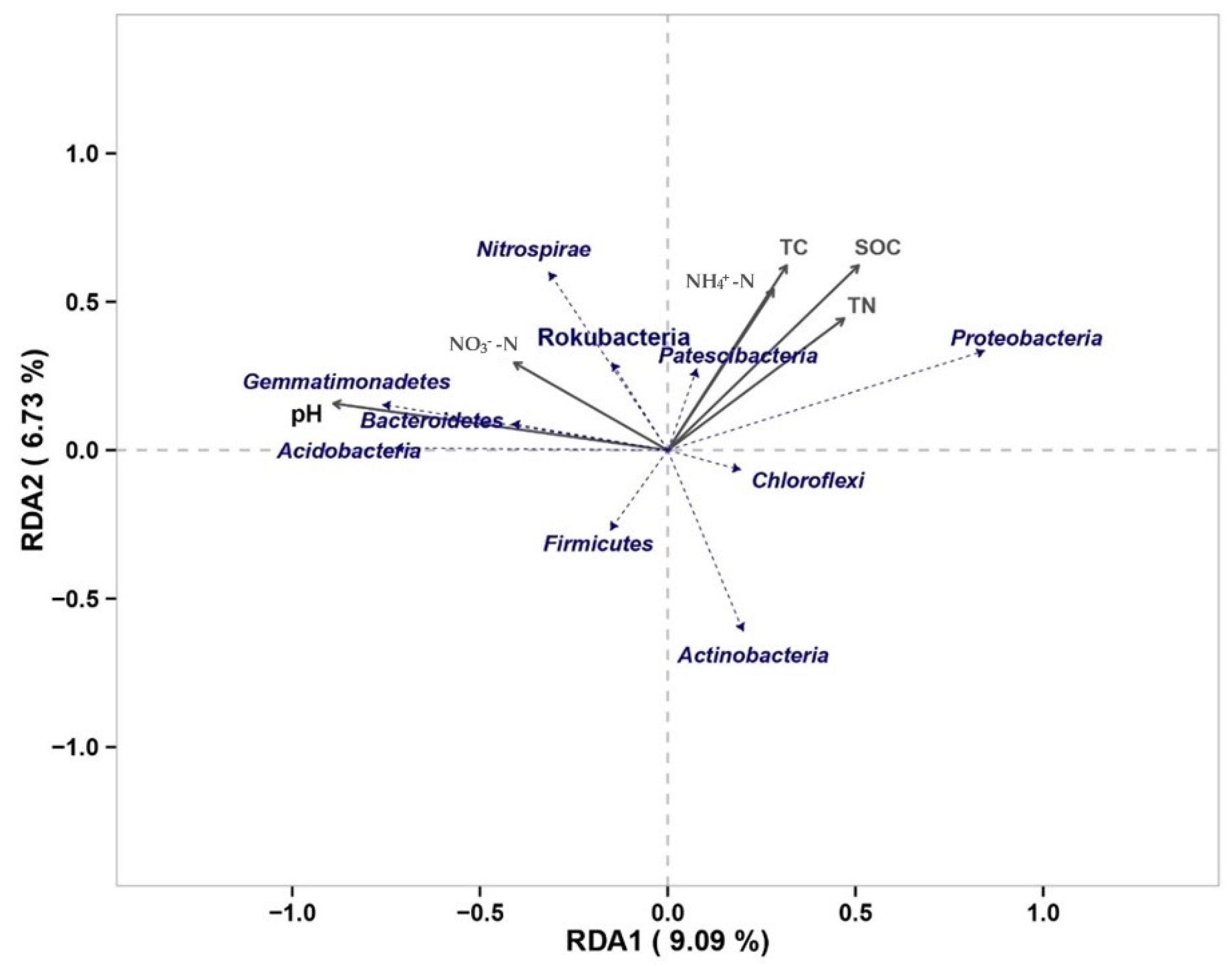
| Physical and Chemical Properties | Soil Depth (cm) | N0 | N2 | N5 | N10 |
|---|---|---|---|---|---|
| TN (g·kg−1) | 0–15 | 4.97 ± 0.31 a | 5.10 ± 0.82 a | 4.60 ± 1.14 a | 4.00 ± 0.37 a |
| 15–30 | 4.10 ± 0.45 a | 4.00 ± 0.62 a | 3.40 ± 0.31 a | 3.40 ± 0.09 a | |
| NH4+-N (mg·kg−1) | 0–15 | 10.39 ± 0.61 a | 10.25 ± 1.91 a | 9.92 ± 1.80 a | 8.67 ± 0.32 a |
| 15–30 | 10.21 ± 2.00 a | 9.61 ± 1.21 a | 8.95 ± 0.48 a | 10.42 ± 1.64 a | |
| NO3−-N (mg·kg−1) | 0–15 | 6.27 ± 0.88 b | 6.87 ± 1.09 b | 8.97 ± 3.24 ab | 13.40 ± 3.02 a |
| 15–30 | 7.43 ± 2.56 a | 7.07 ± 1.80 a | 5.60 ± 0.41 a | 7.43 ± 2.19 a | |
| SWC (%) | 0–15 | 75.03 ± 0.03 a | 79.50 ± 0.08 a | 59.63 ± 0.02 b | 55.46 ± 0.05 b |
| 15–30 | 71.64 ± 0.01 a | 76.74 ± 0.16 a | 56.86 ± 0.11 ab | 51.15 ± 0.02 b | |
| pH | 0–15 | 8.17 ± 0.48 b | 8.67 ± 0.09 ab | 8.77 ± 0.19 a | 9.13 ± 009 a |
| 15–30 | 8.3 ± 0.22 c | 8.57 ± 0.19 bc | 8.87 ± 0.24 ab | 9.00 ± 0.00 a |
| Physical and Chemical Properties | pH | TC | SOC | TN | NO3−-N | NH4+-N |
|---|---|---|---|---|---|---|
| TC | −0.409 * | |||||
| SOC | −0.454 * | 0.865 ** | ||||
| TN | −0.324 | 0.919 ** | 0.953 ** | |||
| NO3−-N | 0.345 | 0.045 | 0.009 | 0.143 | ||
| NH4+-N | −0.203 | −0.017 | 0.069 | 0.003 | 0.018 | |
| SWC | −0.443 * | 0.668 * | 0.660 ** | 0.654 ** | −0.111 | 0.236 |
| Phylum | N0 | N2 | N5 | N10 |
|---|---|---|---|---|
| Proteobacteria | 43.50% | 35.16% | 31.82% | 32.32% |
| Chloroflexi | 16.76% | 16.51% | 14.46% | 17.36% |
| Acidobacteria | 10.65% | 15.59% | 18.87% | 15.55% |
| Actinobacteria | 13.10% | 12.29% | 9.90% | 12.27% |
| Gemmatimonadetes | 3.09% | 4.97% | 5.76% | 6.39% |
| Bacteroidetes | 2.77% | 3.60% | 4.63% | 3.40% |
| Rokubacteria | 2.50% | 3.57% | 2.64% | 3.59% |
| Patescibacteria | 2.24% | 2.77% | 2.41% | 2.33% |
| Nitrospirae | 1.54% | 1.81% | 2.41% | 1.76% |
| Firmicutes | 1.44% | 0.81% | 3.78% | 1.09% |
| Verrucomicrobia | 0.95% | 1.12% | 1.43% | 2.04% |
| Others | 1.44% | 1.77% | 1.85% | 1.89% |
| Unknown | 0.02% | 0.03% | 0.06% | 0.04% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Wang, Z.; Zhu, J. Effects of Climate Change and Nitrogen Addition on Carbon Loss in Alpine Wetland of Qinghai–Tibet Plateau. Atmosphere 2023, 14, 1342. https://doi.org/10.3390/atmos14091342
Xu R, Wang Z, Zhu J. Effects of Climate Change and Nitrogen Addition on Carbon Loss in Alpine Wetland of Qinghai–Tibet Plateau. Atmosphere. 2023; 14(9):1342. https://doi.org/10.3390/atmos14091342
Chicago/Turabian StyleXu, Runhong, Zhigang Wang, and Jinfu Zhu. 2023. "Effects of Climate Change and Nitrogen Addition on Carbon Loss in Alpine Wetland of Qinghai–Tibet Plateau" Atmosphere 14, no. 9: 1342. https://doi.org/10.3390/atmos14091342
APA StyleXu, R., Wang, Z., & Zhu, J. (2023). Effects of Climate Change and Nitrogen Addition on Carbon Loss in Alpine Wetland of Qinghai–Tibet Plateau. Atmosphere, 14(9), 1342. https://doi.org/10.3390/atmos14091342





