The Drawback of Optimizing Air Cleaner Filters for the Adsorption of Formaldehyde
Abstract
1. Introduction
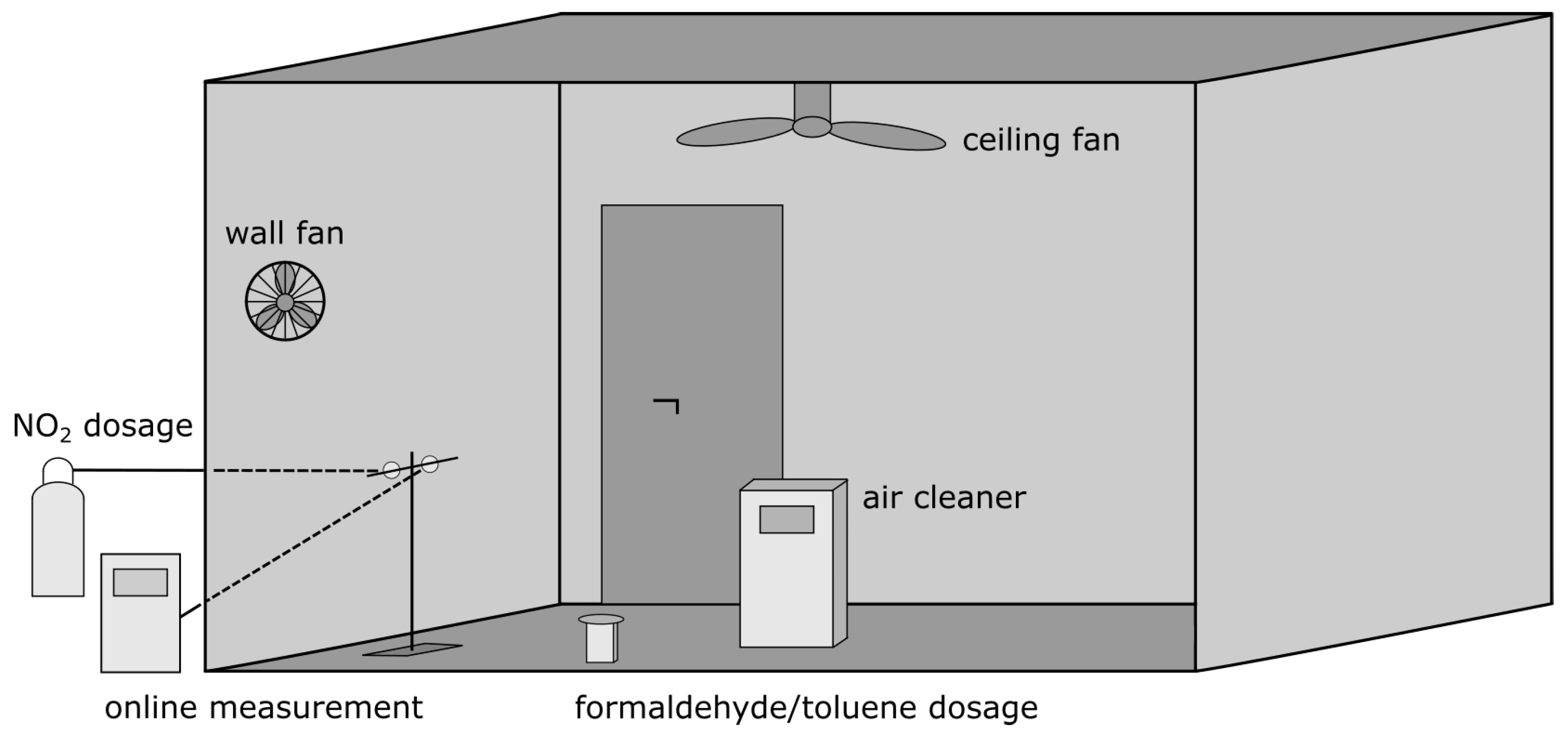
2. Materials and Methods
- The initial formaldehyde concentration of about 815 ppb (1.0 mg m−3 at 1013.25 hPa and 25 °C) was generated by the sublimation of paraformaldehyde (>95% purity) at 200 °C. The concentration was measured with a continuous formaldehyde monitor (model AeroLaser AL 4021). The detection was based on the fluorescence of the product formed in the reaction of gaseous formaldehyde trapped in an aqueous solution with different liquids (Hantzsch reaction).
- The initial toluene concentration of about 530 ppb (2.0 mg m−3 at 1013.25 hPa and 25 °C) was generated by the evaporation of liquid toluene (99.8 % purity) on a glass tray at an ambient temperature of 25 °C. The concentration was measured with a compact proton-transfer-reaction mass spectrometer with a quadrupole mass analyzer (PTR-MS, model IONICON Compact PTR-MS).
- The initial NO2 concentration of about 1280 ppb (2.4 mg m−3 at 1013.25 hPa and 25 °C) was generated by feeding NO2 from a gas cylinder (10,000 ppm NO2 in nitrogen 5.0) into the test chamber. NO2 was measured with a chemiluminescence detector (CLD, model Environnement AC 32M). Besides NO2, the CLD detector also measures the concentration of nitrogen monoxide (NO).
3. Results
3.1. Reference Measurements with Cigarette Smoke Particles
3.2. Measurements with Formaldehyde
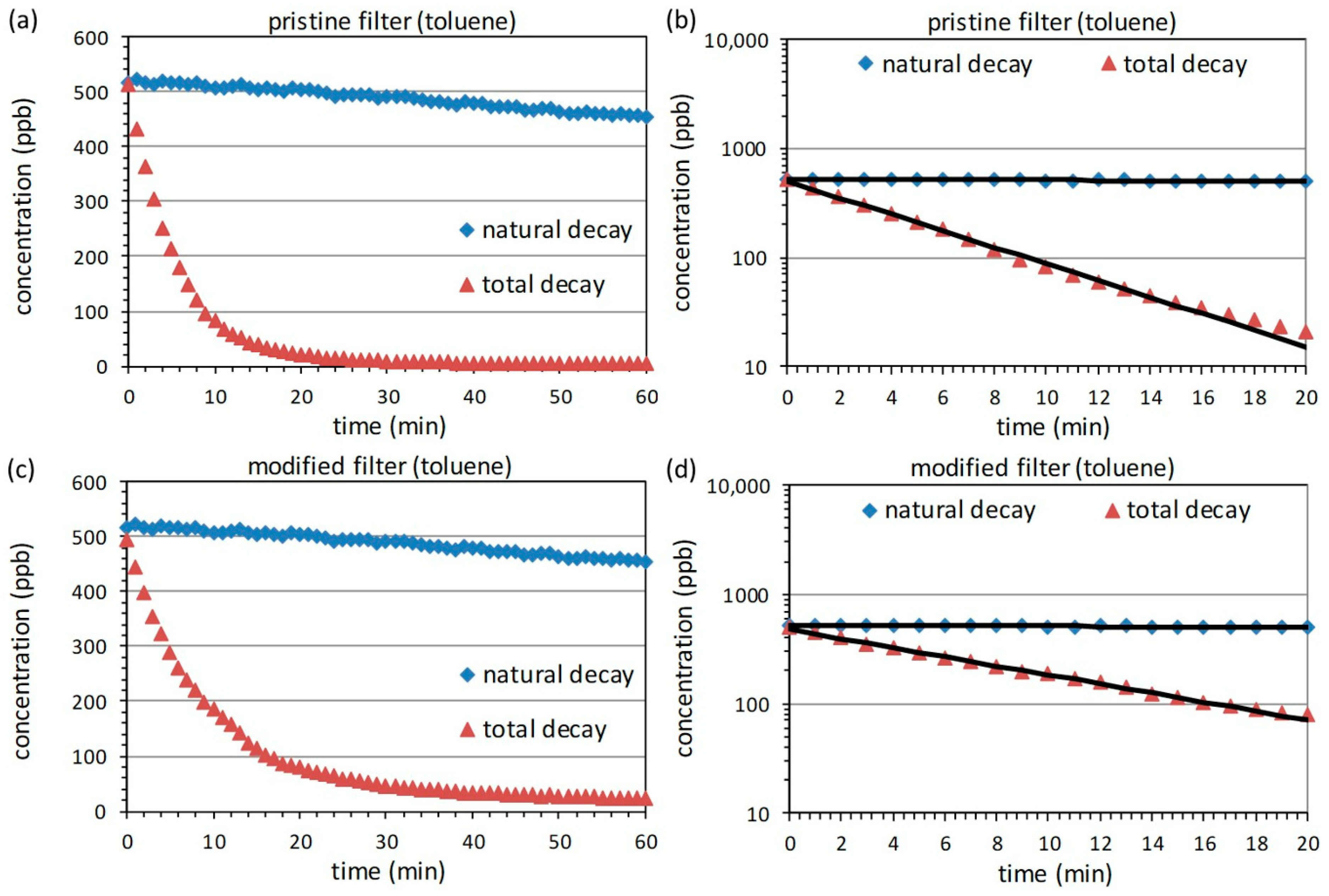
3.3. Measurements with Toluene
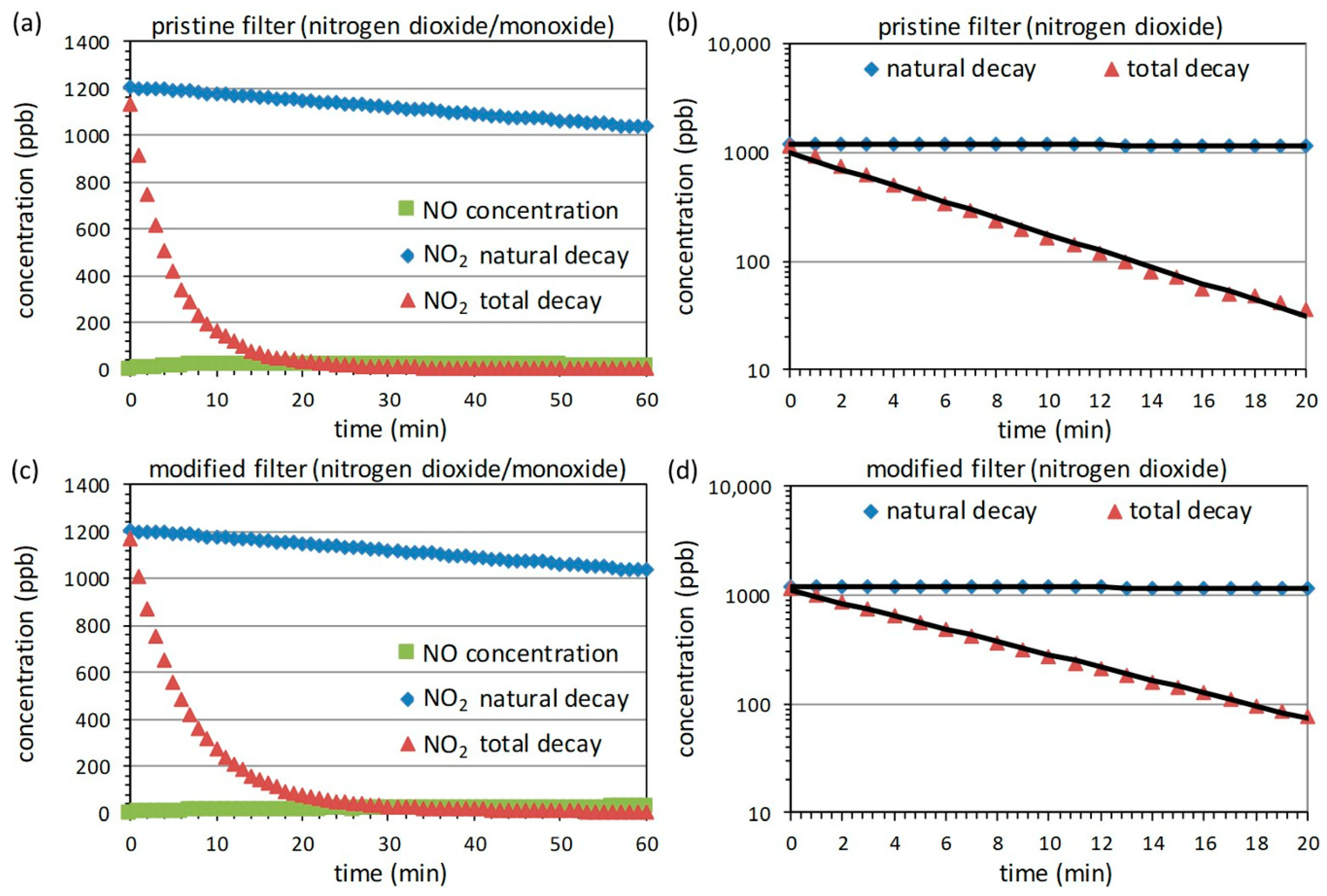
3.4. Measurements with Nitrogen Dioxide
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Alexis, N.; Bacchus, H.; Bernstein, I.L.; Fritz, P.; Horner, E.; Li, N.; Mason, S.; Nel, A.; Oullette, J.; et al. The health effects of nonindustrial indoor air pollution. J. Allergy Clin. Immunol. 2008, 121, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Halios, C.H.; Landeg-Cox, C.; Lowther, S.D.; Middleton, A.; Marczylo, T.; Dimitroulopoulou, S. Chemicals in European residences—Part I: A review of emissions, concentrations and health effects of volatile organic compounds (VOCs). Sci. Total Environ. 2022, 839, 156201. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.D.; Wolkoff, P. Cancer effects of formaldehyde: A proposal for an indoor air guideline value. Arch. Toxicol. 2010, 84, 423–446. [Google Scholar] [CrossRef]
- Kang, Y.J.; Jo, H.K.; Jang, M.H.; Ma, X.; Jeon, Y.; Oh, K.; Park, J.I. A brief review of formaldehyde removal through AC adsorption. Appl. Sci. 2022, 12, 5025. [Google Scholar] [CrossRef]
- Thompson, C.M.; Subramaniam, R.P.; Grafström, R.C. Mechanistic and dose considerations for supporting adverse pulmonary physiology in response to formaldehyde. Toxicol. Appl. Pharmacol. 2008, 233, 355–359. [Google Scholar] [CrossRef] [PubMed]
- McGwin, G., Jr.; Lienert, J.; Kennedy, J.I., Jr. Formaldehyde exposure and asthma in children: A systematic review. Environ. Health Perspect. 2010, 118, 313–317. [Google Scholar] [CrossRef]
- Salthammer, T. The reliability of models for converting formaldehyde emissions from wood-based materials to different environmental conditions. Build. Environ. 2023, 247, 111041. [Google Scholar] [CrossRef]
- Salthammer, T. Formaldehyde sources, formaldehyde concentrations and air exchange rates in European housings. Build. Environ. 2019, 150, 219–232. [Google Scholar] [CrossRef]
- Carter, E.M.; Katz, L.E.; Speitel, G.E., Jr.; Ramirez, D. Gas-phase formaldehyde adsorption isotherm studies on activated carbon: Correlations of adsorption capacity to surface functional group density. Environ. Sci. Technol. 2011, 45, 6498–6503. [Google Scholar] [CrossRef]
- Cavalcante, R.M.; Seyffert, B.H.; D’Oca, M.G.M.; Nascimento, R.F.; Campelo, C.S.; Pinto, I.S.; Anjos, F.B.; Costa, A.H. Exposure assessment for formaldehyde and acetaldehyde in the workplace. Indoor Built Environ. 2005, 14, 165–172. [Google Scholar] [CrossRef]
- Cammalleri, V.; Pocino, R.N.; Marotta, D.; Protano, C.; Sinibaldi, F.; Simonazzi, S.; Petyx, M.; Iavicoli, S.; Vitali, M. Occupational scenarios and exposure assessment to formaldehyde: A systematic review. Indoor Air 2022, 32, e12949. [Google Scholar] [CrossRef] [PubMed]
- Morantes, G.; Jones, B.; Sherman, M.; Molina, C. A preliminary assessment of the health impacts of indoor air contaminants determined using the DALY metric. Int. J. Vent. 2023, 22, 307–316. [Google Scholar] [CrossRef]
- Logue, J.M.; Price, P.N.; Sherman, M.H.; Singer, B.C. A method to estimate the chronic health impact of air pollutants in US residences. Environ. Health Perspect. 2012, 120, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.D.; Larsen, S.T.; Wolkoff, P. Re-evaluation of the WHO (2010) formaldehyde indoor air quality guideline for cancer risk assessment. Arch. Toxicol. 2017, 91, 35–61. [Google Scholar] [CrossRef]
- Schumacher, S.; Spiegelhoff, D.; Schneiderwind, U.; Finger, H.; Asbach, C. Performance of new and artificially aged electret filters in indoor air cleaners. Chem. Eng. Technol. 2018, 41, 27–34. [Google Scholar] [CrossRef]
- Morawska, L.; Tang, J.; Bahnfleth, W.; Bluyssen, P.; Boerstra, A.; Buonanno, G.; Cao, J.; Dancer, S.; Floto, A.; Franchimon, F.; et al. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020, 142, 105832. [Google Scholar] [CrossRef]
- Curtius, J.; Granzin, M.; Schrod, J. Testing mobile air cleaners in a school classroom: Reducing the airborne transmission risk for SARS-CoV-2. Aerosol Sci. Technol. 2021, 55, 586–599. [Google Scholar] [CrossRef]
- Schumacher, S.; Schmid, H.-J.; Asbach, C. Effektivität von Luftreinigern zur Reduzierung des COVID-19-Infektionsrisikos. Gefahrstoffe Reinh. Luft 2021, 81, 16–28. [Google Scholar] [CrossRef]
- Schumacher, S.; Banda Sanchez, A.; Caspari, A.; Staack, K.; Asbach, C. Testing filter-based air cleaners with surrogate particles for viruses and exhaled droplets. Atmosphere 2022, 13, 1538. [Google Scholar] [CrossRef]
- Siegel, J.A. Primary and secondary consequences of indoor air cleaners. Indoor Air 2016, 26, 88–96. [Google Scholar] [CrossRef]
- Zhu, X.; Lv, M.; Yang, X. Performance of sorption-based portable air cleaners in formaldehyde removal: Laboratory tests and field verification. Build. Environ. 2018, 136, 177–184. [Google Scholar] [CrossRef]
- Sørensen, S.B.; Feilberg, A.; Kristensen, K. Removal of volatile organic compounds by mobile air cleaners: Dynamics, limitations, and possible side effects. Build. Environ. 2023, 242, 110541. [Google Scholar] [CrossRef]
- An, K.; Wang, Z.; Yang, X.; Qu, Z.; Sun, F.; Zhou, W.; Zhao, H. Reasons of low formaldehyde adsorption capacity on activated carbon: Multi-scale simulation of dynamic interaction between pore size and functional groups. J. Environ. Chem. Eng. 2022, 10, 108723. [Google Scholar] [CrossRef]
- Pei, J.; Zhang, J.S. On the performance and mechanisms of formaldehyde removal by chemi-sorbents. Chem. Eng. J. 2011, 167, 59–66. [Google Scholar] [CrossRef]
- de Falco, G.; Li, W.; Cimino, S.; Bandosz, T.J. Role of sulfur and nitrogen surface groups in adsorption of formaldehyde on nanoporous carbons. Carbon 2018, 138, 283–291. [Google Scholar] [CrossRef]
- Lee, K.J.; Miyawaki, J.; Shiratori, N.; Yoon, S.H.; Jang, J. Toward an effective adsorbent for polar pollutants: Formaldehyde adsorption by activated carbon. J. Hazard. Mater. 2013, 260, 82–88. [Google Scholar] [CrossRef]
- Tanada, S.; Kawasaki, N.; Nakamura, T.; Araki, M.; Isomura, M. Removal of formaldehyde by activated carbons containing amino groups. J. Colloid Interface Sci. 1999, 214, 106–108. [Google Scholar] [CrossRef]
- Kim, D.I.; Park, J.H.; Do Kim, S.; Lee, J.Y.; Yim, J.H.; Jeon, J.K.; Park, S.H.; Park, Y.K. Comparison of removal ability of indoor formaldehyde over different materials functionalized with various amine groups. J. Ind. Eng. Chem. 2011, 17, 1–5. [Google Scholar] [CrossRef]
- Ma, C.; Li, X.; Zhu, T. Removal of low-concentration formaldehyde in air by adsorption on activated carbon modified by hexamethylene diamine. Carbon 2011, 49, 2873–2875. [Google Scholar] [CrossRef]
- Baur, G.B.; Spring, J.; Kiwi-Minsker, L. Amine functionalized activated carbon fibers as effective structured adsorbents for formaldehyde removal. Adsorption 2018, 24, 725–732. [Google Scholar] [CrossRef]
- Bellat, J.P.; Bezverkhyy, I.; Weber, G.; Royer, S.; Averlant, R.; Giraudon, J.M.; Lamonier, J.F. Capture of formaldehyde by adsorption on nanoporous materials. J. Hazard. Mater. 2015, 300, 711–717. [Google Scholar] [CrossRef]
- Lara-Ibeas, I.; Megías-Sayago, C.; Louis, B.; Le Calvé, S. Adsorptive removal of gaseous formaldehyde at realistic concentrations. J. Environ. Chem. Eng. 2020, 8, 103986. [Google Scholar] [CrossRef]
- Birmili, W.; Daniels, A.; Bethke, R.; Schechner, N.; Brasse, G.; Conrad, A.; Kolossa-Gehring, M.; Debiak, M.; Hurraß, J.; Uhde, E.; et al. Formaldehyde, aliphatic aldehydes (C2–C11), furfural, and benzaldehyde in the residential indoor air of children and adolescents during the German Environmental Survey 2014–2017 (GerES V). Indoor Air 2022, 32, e12927. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Liu, N.; Liu, W.; Mo, J.; Zhao, Z.; Kan, H.; Deng, F.; Huang, C.; Zhao, B.; Zeng, X.; et al. Indoor formaldehyde levels in residences, schools, and offices in China in the past 30 years: A systematic review. Indoor Air 2022, 32, e13141. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T. An overview of health hazards of volatile organic compounds regulated as indoor air pollutants. Environ. Health Rev. 2019, 34, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sarigiannis, D.A.; Karakitsios, S.P.; Gotti, A.; Liakos, I.L.; Katsoyiannis, A. Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ. Int. 2011, 37, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Vardoulakis, S.; Giagloglou, E.; Steinle, S.; Davis, A.; Sleeuwenhoek, A.; Galea, K.S.; Dixon, K.; Crawford, J.O. Indoor exposure to selected air pollutants in the home environment: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 8972. [Google Scholar] [CrossRef]
- Win-Shwe, T.T.; Fujimaki, H. Neurotoxicity of toluene. Toxicol. Lett. 2010, 198, 93–99. [Google Scholar] [CrossRef]
- ISO 10121-2; Test Methods for Assessing the Performance of Gas-Phase Air Cleaning Media and Devices for General Ventilation Part 2: Gas-Phase Air Cleaning Media. International Organization for Standardization: Geneva, Switzerland, 2013.
- Echeverria, D.; Fine, L.; Langolf, G.; Schork, T.; Sampaio, C. Acute behavioural comparisons of toluene and ethanol in human subjects. Occup. Environ. Med. 1991, 48, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Filley, C.M.; Halliday, W.; Kleinschmidt-DeMasters, B.K. The effects of toluene on the central nervous system. J. Neuropathol. Exp. Neurol. 2004, 63, 1–12. [Google Scholar] [CrossRef]
- Rooseboom, M.; Kocabas, N.A.; North, C.; Radcliffe, R.J.; Segal, L. Recommendation for an occupational exposure limit for toluene. Regul. Toxicol. Pharmacol. 2023, 141, 105387. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, B. Relationship between indoor and outdoor NO2: A review. Build. Environ. 2020, 180, 106909. [Google Scholar] [CrossRef]
- Huangfu, P.; Atkinson, R. Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: A systematic review and meta-analysis. Environ. Int. 2020, 144, 105998. [Google Scholar] [CrossRef] [PubMed]
- Salonen, H.; Salthammer, T.; Morawska, L. Human exposure to NO2 in school and office indoor environments. Environ. Int. 2019, 130, 104887. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- GB/T 18801; Air Cleaner. Standardization Administration of China: Beijing, China, 2015.
- ANSI/AHAM AC-1; Method for Measuring Performance of Portable Household Electric Room Air Cleaners. Association of Home Appliance Manufacturers: Washington, DC, USA, 2020.
- IEC 63086-1; Household and Similar Electrical Air Cleaning Appliances—Methods for Measuring the Performance—Part 1: General Requirements. International Electrotechnical Commission: Geneva, Switzerland, 2020.
- Laskar, I.I.; Hashisho, Z.; Phillips, J.H.; Anderson, J.E.; Nichols, M. Modeling the effect of relative humidity on adsorption dynamics of volatile organic compound onto activated carbon. Environ. Sci. Technol. 2019, 53, 2647–2659. [Google Scholar] [CrossRef]
- Sager, U.; Schmidt, F. Adsorption of nitrogen oxides, water vapour and ozone onto activated carbon. Adsorpt. Sci. Technol. 2009, 27, 135–145. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Liu, B.; Xia, Q.; Xi, H. Effect of relative humidity on adsorption of formaldehyde on modified activated carbons. Chin. J. Chem. Eng. 2008, 16, 871–875. [Google Scholar] [CrossRef]
- Küpper, M.; Asbach, C.; Schneiderwind, U.; Finger, H.; Spiegelhoff, D.; Schumacher, S. Testing of an indoor air cleaner for particulate pollutants under realistic conditions in an office room. Aerosol Air Qual. Res. 2019, 19, 1655–1665. [Google Scholar] [CrossRef]
- Quintero, F.; Nagarajan, V.; Schumacher, S.; Todea, A.M.; Lindermann, J.; Asbach, C.; Luzzato, C.M.A.; Jilesen, J. Reducing Particle Exposure and SARS-CoV-2 Risk in Built Environments through Accurate Virtual Twins and Computational Fluid Dynamics. Atmosphere 2022, 13, 2032. [Google Scholar] [CrossRef]
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland, 2017.
- GB/T 18883; Indoor Air Quality Standard. Standardization Administration of China: Beijing, China, 2002.
- Zhang, W.J.; Bagreev, A.; Rasouli, F. Reaction of NO2 with activated carbon at ambient temperature. Ind. Eng. Chem. Res. 2008, 47, 4358–4362. [Google Scholar] [CrossRef]
- Gao, X.; Liu, S.; Zhang, Y.; Luo, Z.; Ni, M.; Cen, K. Adsorption and reduction of NO2 over activated carbon at low temperature. Fuel Process. Technol. 2011, 92, 139–146. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Girgis, B.S. Impact of surface characteristics of activated carbon on adsorption of BTEX. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 181–193. [Google Scholar] [CrossRef]
- Deliyanni, E.; Bandosz, T.J. Effect of carbon surface modification with dimethylamine on reactive adsorption of NOx. Langmuir 2011, 27, 1837–1843. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.; Zhang, M.; Ma, C. Effect of N-doping on NO2 adsorption and reduction over activated carbon: An experimental and computational study. Fuel 2019, 258, 116109. [Google Scholar] [CrossRef]
- Yin, C.Y.; Aroua, M.K.; Daud, W.M.A.W. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 2007, 52, 403–415. [Google Scholar] [CrossRef]
- Yin, C.Y.; Aroua, M.K.; Daud, W.M.A.W. Polyethyleneimine impregnation on activated carbon: Effects of impregnation amount and molecular number on textural characteristics and metal adsorption capacities. Mater. Chem. Phys. 2008, 112, 417–422. [Google Scholar] [CrossRef]
- Zhang, R.; Zeng, L.; Wang, F.; Li, X.; Li, Z. Influence of pore volume and surface area on benzene adsorption capacity of activated carbons in indoor environments. Build. Environ. 2022, 216, 109011. [Google Scholar] [CrossRef]
- Jin, M.; Kurniawan, W.; Hinode, H. Development of zeolite/carbon composite adsorbent. J. Chem. Eng. Jpn. 2006, 39, 154–161. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. The environmental applications of activated carbon/zeolite composite materials. Adv. Colloid Interface Sci. 2011, 162, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Glover, T.G.; Dunne, K.I.; Davis, R.J.; LeVan, M.D. Carbon–silica composite adsorbent: Characterization and adsorption of light gases. Microporous Mesoporous Mater. 2008, 111, 1–11. [Google Scholar] [CrossRef]
- Mohammadi, A.; Moghaddas, J. Synthesis, adsorption and regeneration of nanoporous silica aerogel and silica aerogel-activated carbon composites. Chem. Eng. Res. Des. 2015, 94, 475–484. [Google Scholar] [CrossRef]
- Kim, W.K.; Vikrant, K.; Younis, S.A.; Kim, K.H.; Heynderickx, P.M. Metal oxide/activated carbon composites for the reactive adsorption and catalytic oxidation of formaldehyde and toluene in air. J. Clean. Prod. 2023, 387, 135925. [Google Scholar] [CrossRef]
- GB/T 18801; Air Cleaner. Standardization Administration of China: Beijing, China, 2022.
- AHAM AC-4; Method of Assessing the Reduction Rate of Chemical Gases by a Room Air Cleaner. Association of Home Appliance Manufacturers: Washington, DC, USA, 2022.
- IEC/CD 63086-2-2; Household and Similar Electrical Air Cleaning Appliances Methods for Measuring the Performance—Part 2-2: Particular Requirements for Determination of Gas-Phase Pollutant Reduction. International Electrotechnical Commission: Geneva, Switzerland, 2023.
- Rengga, W.D.P.; Chafidz, A.; Sudibandriyo, M.; Nasikin, M.; Abasaeed, A.E. Silver nano-particles deposited on bamboo-based activated carbon for removal of formaldehyde. J. Environ. Chem. Eng. 2017, 5, 1657–1665. [Google Scholar] [CrossRef]
- Chang, S.M.; Hu, S.C.; Shiue, A.; Lee, P.Y.; Leggett, G. Adsorption of silver nano-particles modified activated carbon filter media for indoor formaldehyde removal. Chem. Phys. Lett. 2020, 757, 137864. [Google Scholar] [CrossRef]
- Khaleghi, H.; Esmaeili, H.; Jaafarzadeh, N.; Ramavandi, B. Date seed activated carbon decorated with CaO and Fe3O4 nanoparticles as a reusable sorbent for removal of formaldehyde. Korean J. Chem. Eng. 2022, 39, 146–160. [Google Scholar] [CrossRef]
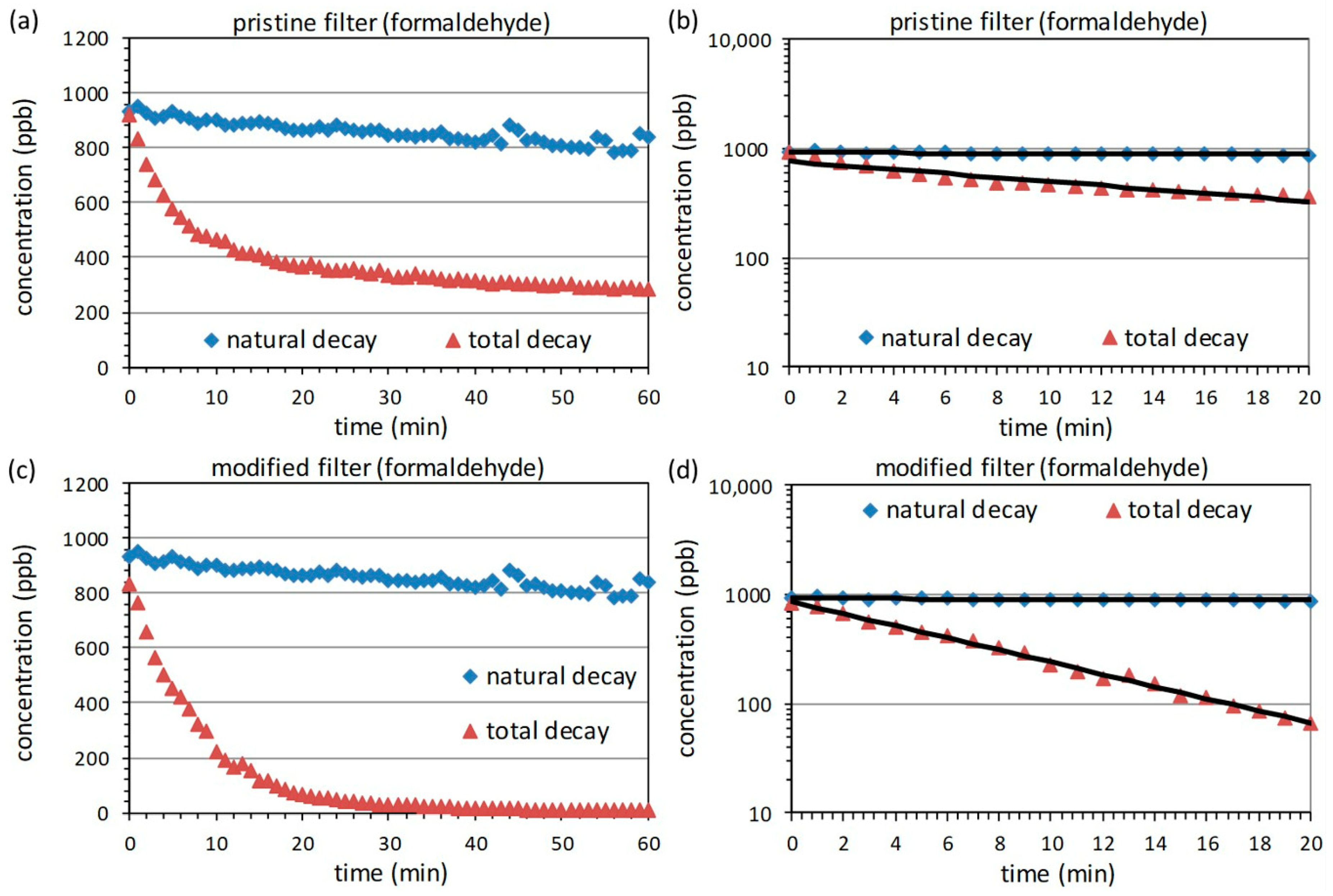
| Filter | Adsorption Efficiency | ||||
|---|---|---|---|---|---|
| Pristine | 0.0037 | 0.0438 | 0.9081 | 71 | ~21% |
| Modified | 0.0037 | 0.1280 | 0.9915 | 219 | ~62% |
| Filter | Adsorption Efficiency | ||||
|---|---|---|---|---|---|
| Pristine | 0.0016 | 0.1661 | 0.9924 | 289 | ~85% |
| Modified | 0.0016 | 0.0956 | 0.9952 | 165 | ~46% |
| Filter | Adsorption Efficiency | ||||
|---|---|---|---|---|---|
| Pristine | 0.0024 | 0.1773 | 0.9958 | 308 | ~90% |
| Modified | 0.0024 | 0.1382 | 0.9987 | 239 | ~67% |
| Parameter | Formaldehyde | Toluene | Nitrogen Dioxide |
|---|---|---|---|
| CADR pristine filter | 71 m3h−1 | 289 m3h−1 | 308 m3h−1 |
| CADR modified filter | 219 m3h−1 | 165 m3h−1 | 239 m3h−1 |
| Change with modification | +208% | −42% | −22% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schumacher, S.; Caspari, A.; Schneiderwind, U.; Staack, K.; Sager, U.; Asbach, C. The Drawback of Optimizing Air Cleaner Filters for the Adsorption of Formaldehyde. Atmosphere 2024, 15, 109. https://doi.org/10.3390/atmos15010109
Schumacher S, Caspari A, Schneiderwind U, Staack K, Sager U, Asbach C. The Drawback of Optimizing Air Cleaner Filters for the Adsorption of Formaldehyde. Atmosphere. 2024; 15(1):109. https://doi.org/10.3390/atmos15010109
Chicago/Turabian StyleSchumacher, Stefan, Anna Caspari, Ute Schneiderwind, Katharina Staack, Uta Sager, and Christof Asbach. 2024. "The Drawback of Optimizing Air Cleaner Filters for the Adsorption of Formaldehyde" Atmosphere 15, no. 1: 109. https://doi.org/10.3390/atmos15010109
APA StyleSchumacher, S., Caspari, A., Schneiderwind, U., Staack, K., Sager, U., & Asbach, C. (2024). The Drawback of Optimizing Air Cleaner Filters for the Adsorption of Formaldehyde. Atmosphere, 15(1), 109. https://doi.org/10.3390/atmos15010109







