Culturable Microorganisms of Aerosols Sampled during Aircraft Sounding of the Atmosphere over the Russian Arctic Seas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flight Route
2.2. Aerosol Sampling
2.3. The Concentration of Culturable Microorganisms
2.4. The Phenotypic Characteristics of the Isolated Microorganisms
2.5. The Enzymatic Activity of the Isolated Microorganisms
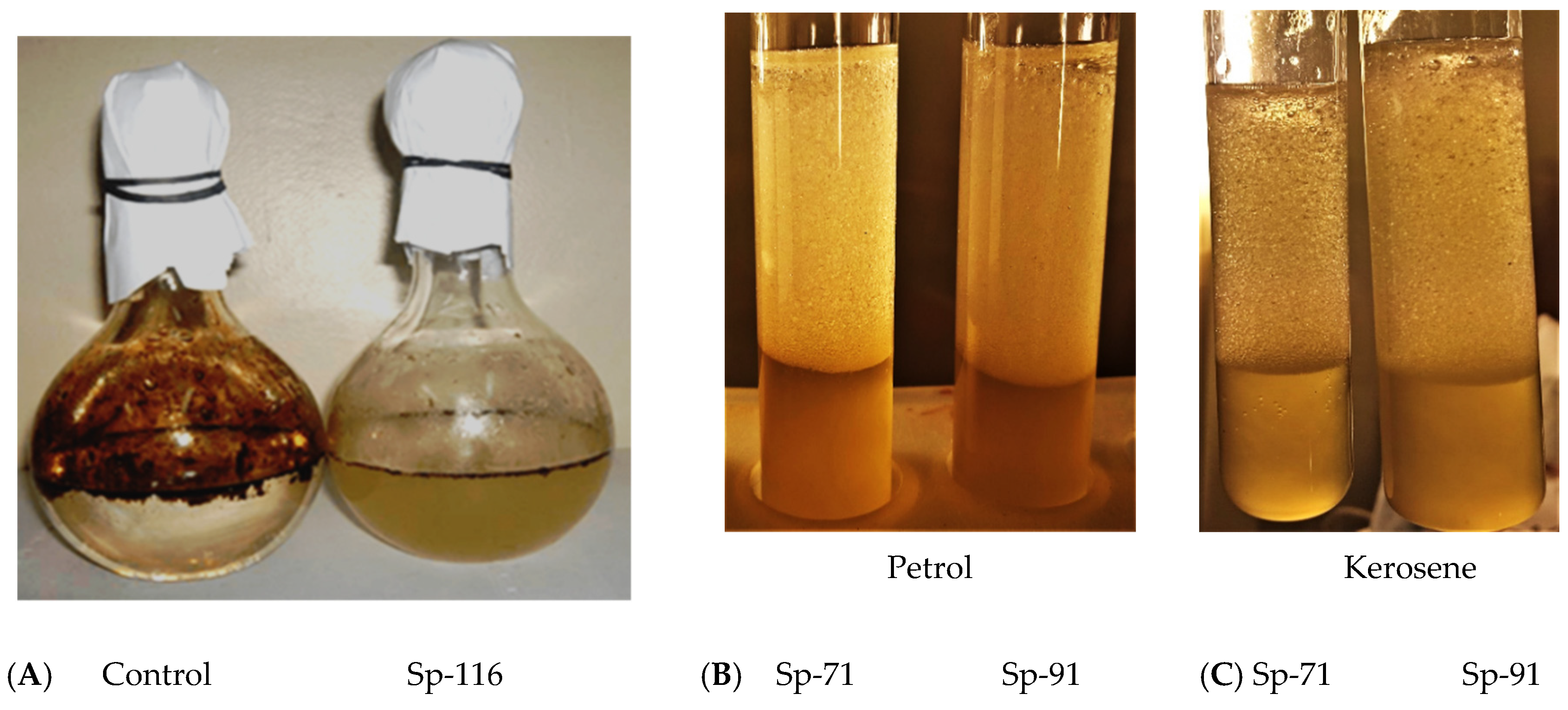
2.6. Destruction of Oil
2.7. Microorganisms’ Antibiotic Properties
2.8. Microorganisms’ Taxonomy
3. Results
3.1. Microorganisms Concentrations and Diversity
3.2. Antibiotic Activities of Isolates
3.3. Bacterial Growth at Different Temperatures
3.4. Biotechnological Properties of Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Issatchenko, B.L. Research on Bacteria in the Arctic Ocean; Tipografia V.O. Kirshbauma: Petrograd, Russia, 1914; Available online: https://elib.rgo.ru/safe-view/123456789/218205/1/UnVQUkxJQjEyMDg0Mjc5LlBERg (accessed on 16 January 2024). (In Russian)
- Kriss, A.E. Marine Microbiology: (Deep Sea); Publishing House of the USSR Academy of Sciences: Moscow, Russia, 1955. (In Russian) [Google Scholar]
- Kelly, C.D.; Layn, S. Bacteria found in the air over Canada and the American Arctic. Can. J. Microbiol. 1957, 3, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Pady, S.M.; Kapica, L. Air-borne fungi in the Arctic and other parts of Canada. Can. J. Bot. 1953, 31, 309–323. [Google Scholar] [CrossRef]
- Pady, S.M.; Kelly, C.D.; Polunin, N. Arctic aerobiology II: Preliminary report on fungi and bacteria isolated from the air in 1947. Nature 1948, 162, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Overpeck, J.; Hughen, K.; Hardy, D.; Bradley, R.; Case, R. Arctic environmental change of the last four centuries. Science 1997, 278, 1251–1256. [Google Scholar] [CrossRef]
- Schmale, J.; Zieger, P.; Ekman, A.M.L. Aerosols in current and future Arctic climate. Nat. Clim. Chang. 2021, 11, 95–105. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Serreze, M.C.; Barrett, A.P.; Stroeve, J.C.; Kindig, D.N.; Holland, M.M. The emergence of surface-based Arctic amplification. Cryosphere 2009, 3, 11–19. [Google Scholar] [CrossRef]
- Johansen, S.; Hafsten, U. Airborne pollen and spore registrations at Ny-Ålesund, Svalbard, summer 1986. Polar Res. 1988, 6, 11–17. [Google Scholar] [CrossRef]
- Feltracco, M.; Barbaro, E.; Hoppe, C.J.M.; Wolf, K.K.E.; Spolaor, A.; Layton, R.; Keuschnig, C.; Barbante, C.; Gambaro, A.; Larose, C. Airborne bacteria and particulate chemistry capture Phytoplankton bloom dynamics in an Arctic fjord. Atmos. Environ. 2021, 256, 118458. [Google Scholar] [CrossRef]
- Jensen, L.Z.; Glasius, M.; Gryning, S.-E.; Massling, A.; Finster, K.; Šantl-Temkiv, T. Seasonal variation of the atmospheric bacterial community in the Greenlandic High Arctic is influenced by weather events and local and distant sources. Front. Microbiol. 2022, 13, 909980. [Google Scholar] [CrossRef]
- Kawana, K.; Taketani, F.; Matsumoto, K.; Miyakawa, T.; Tobo, Y.; Iwamoto, Y.; Ito, A.; Kanaya, Y. Roles of marine biota in the formation of atmospheric bioaerosols, cloud condensation nuclei, and ice-nucleating particles over the North Pacific Ocean, Bering Sea, and Arctic Ocean. Atmos. Chem. Phys. 2023, 24, 1777–1799. [Google Scholar] [CrossRef]
- Ferrero, L.; Sangiorgi, G.; Perrone, M.G.; Rizzi, C.; Cataldi, M.; Markuszewski, P.; Pakszys, P.; Makuch, P.; Petelski, T.; Becagli, S.; et al. Chemical composition of aerosol over the Arctic Ocean from Summer ARctic EXpedition (AREX) 2011–2012 cruises: Ions, amines, elemental carbon, organic matter, polycyclic aromatic hydrocarbons, n-alkanes, metals, and rare earth elements. Atmosphere 2019, 10, 54. [Google Scholar] [CrossRef]
- Yttri, K.E.; Bäcklund, A.; Conen, F.; Eckhardt, S.; Evangeliou, N.; Fiebig, M.; Kasper-Giebl, A.; Gold, A.; Gundersen, H.; Myhre, C.L.; et al. Composition and sources of carbonaceous aerosol in the European Arctic at Zeppelin Observatory, Svalbard. Atmos. Chem. Phys. 2023, 24, 2731–2758. [Google Scholar] [CrossRef]
- Kravchishina, M.D.; Klyuvitkin, A.A.; Novigatsky, A.N.; Glukhovets, D.I.; Shevchenko, V.P.; Belan, B.D. Cruise 89 (First Leg) of the R/V Akademik Mstislav Keldysh: Climate experiment in interaction with the Tu-134 Optik Flying Laboratory. Oceanology 2023, 63, 428–431. [Google Scholar] [CrossRef]
- Duncan, S.M.; Farrell, R.L.; Jordan, N.; Jurgens, J.A.; Blanchette, R.A. Monitoring and identification of airborne fungi at historic locations on Ross Island, Antarctic. Polar Sci. 2010, 4, 275–283. [Google Scholar] [CrossRef]
- Li, D.; Kendrick, B. A year-round outdoor aeromycological study in Waterloo, Ontario, Canada. Grana 1995, 34, 199–207. [Google Scholar] [CrossRef]
- Malard, L.A.; Avila-Jimenez, M.-L.; Schmale, J.; Cuthbertson, L.; Cockerton, L.; Pearce, D.A. Aerobiology over the Southern Ocean—Implications for bacterial colonization of Antarctica. Environ. Int. 2022, 169, 107492. [Google Scholar] [CrossRef]
- Kobayashi, F. Direct sampling and bioanalyses of atmospheric bioaerosols using a tethered balloon over Syowa Station, Antarctica. Polar Sci. 2022, 32, 100842. [Google Scholar] [CrossRef]
- Feltracco, M.; Zangrando, R.; Barbaro, E.; Becagli, S.; Park, K.-T.; Vecchiato, M.; Caiazzo, L.; Traversi, R.; Severi, M.; Barbante, C.; et al. Characterization of free L- and D-amino acids in size-segregated background aerosols over the Ross Sea, Antarctica. Sci. Total Environ. 2023, 879, 163070. [Google Scholar] [CrossRef]
- Holmberg, S.M.; Jørgensen, N.O.G. Insights into abundance, adaptation and activity of prokaryotes in arctic and Antarctic environments. Polar Biol. 2023, 46, 381–396. [Google Scholar] [CrossRef]
- Duncan, B.N.; Ott, L.E.; Abshire, J.B.; Brucker, L.; Carroll, M.L.; Carton, J.; Comiso, J.C.; Dinnat, E.P.; Forbes, B.C.; Gonsamo, A.; et al. Space-based observations for understanding changes in the Arctic-Boreal Zone. Rev. Geophys. 2020, 58, e2019RG000652. [Google Scholar] [CrossRef]
- Cusworth, D.H.; Duren, R.M.; Yadav, V.; Thorpe, A.K.; Verhulst, K.; Sander, S.; Hopkins, F.; Rafiq, T.; Miller, C.E. Synthesis of methane observations across scales: Strategies for deploying a multitiered observing network. Geophys. Res. Lett. 2020, 47, GL087869. [Google Scholar] [CrossRef]
- Miller, C.E.; Griffith, P.C.; Goetz, S.J.; Hoy, E.E.; Pinto, N.; McCubbin, I.B.; Thorpe, A.K.; Hofton, M.; Hodkinson, D.; Hansen, C.; et al. An overview of ABoVE airborne campaign data acquisitions and science opportunities. Environ. Res. Lett. 2019, 14, 080201. [Google Scholar] [CrossRef]
- Belan, B.D.; Ancellet, G.; Andreeva, I.S.; Antokhin, P.N.; Arshinova, V.G.; Arshinov, M.Y.; Balin, Y.S.; Barsuk, V.E.; Belan, S.B.; Chernov, D.G.; et al. Integrated airborne investigation of the air composition over the Russian Sector of the Arctic. Atmos. Meas. Tech. 2022, 15, 3941–3967. [Google Scholar] [CrossRef]
- Kirtsideli, I.Y.; Vlasov, D.Y.; Krylenkov, V.A.; Sokolov, V.T. Airborne fungal in the areas of Russian arctic station near White, Barents and Kara sea. Micol. Phytopathol. 2011, 45, 228–239. (In Russian) [Google Scholar]
- Kirtsideli, I.Y.; Abakumov, E.V.; Teshebaev, S.B.; Zelenskaya, M.S.; Vlasov, D.Y.; Krylenkov, V.A.; Ryabusheva, Y.V.; Sokolov, V.T.; Barantsevich, E.P. Microbial communities in regions of Arctic settlements. Hyg. Sanit. 2016, 95, 923–929. [Google Scholar] [CrossRef]
- Anokhin, G.G.; Antokhin, P.N.; Arshinov, M.Y.; Barsuk, V.E.; Belan, B.D.; Belan, S.B.; Davydov, D.K.; Ivlev, G.A.; Kozlov, A.V.; Kozlov, V.S.; et al. OPTIK Tu-134 aicraft laboratory. Atmos. Ocean. Opt. 2011, 24, 805–816. (In Russian) [Google Scholar]
- Gerhardt, F.; Murray, R.G.E.; Wood, W.A.; Krieg, N.R. Methods of General Bacteriology, 2nd ed.; Publisher American Society for Microbiology: Washington, DC, USA, 1994. [Google Scholar]
- Saggie, J. Methods of Soil Microbiology; Kolos Publishers: Moscow, Russia, 1983. (In Russian) [Google Scholar]
- Ashmarin, I.P.; Vorobyov, A.A. Statistical Methods in Microbiological Studies; Medgiz: Leningrad, Russia, 1962. (In Russian) [Google Scholar]
- Labinskaya, A.C.; Volina, E.G. (Eds.) Guide to Medical Microbiology. General and Sanitary Microbiology. Book I; Binom: Moscow, Russia, 2020. (In Russian) [Google Scholar]
- Andreeva, I.S.; Emelyanova, E.K.; Malinkin, A.A.; Rebus, M.E.; Safatov, A.S. Screening of bacteria isolated from Arctic atmospheric aerosols for oil degradation ability. Bull. Nizhnevartovsk State Univ. 2023, 3, 4–17. (In Russian) [Google Scholar] [CrossRef]
- Federal Center for State Sanitary and Epidemiological Surveillance of the Ministry of Health of Russia. Determination of the Sensitivity of Microorganisms to Antibacterial Drugs: Guidelines; Federal Center for State Sanitary and Epidemiological Surveillance of the Ministry of Health of Russia: Moscow, Russia, 2004. (In Russian) [Google Scholar]
- Federal Center for Hygiene and Epidemiology of Rospotrebnadzor. Guidelines for Sanitary and Epidemiological Assessing the Safety and Functional Potential of Probiotic Microorganisms Used for Food Production Products: Guidelines; Federal Center for Hygiene and Epidemiology of Rospotrebnadzor: Moscow, Russia, 2011. (In Russian) [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Osterman, L.A. Methods for Studying Proteins and Nucleic Acids: Electrophoresis and Ultracentrifugation; Nauka: Moscow, Russia, 1996. (In Russian) [Google Scholar]
- Watts, D.; MacBeath, J.R. Automated fluorescent DNA sequencing on the ABI PRISM 310 Genetic Analyzer. Methods Mol. Biol. 2001, 167, 153–170. [Google Scholar] [CrossRef]
- Draxler, R.R.; Hess, G.D. An overview of the HYSPLIT_4 modelling system for trajectories, dispersion and deposition. Aust. Meteorol. Mag. 1998, 47, 295–308. [Google Scholar]
- Freitag, S.; Clarke, A.D.; Howell, S.G.; Kapustin, V.N.; Campos, T.; Brekhovskikh, V.L.; Zhou, J. Combining airborne gas and aerosol measurements with HYSPLIT: A visualization tool for simultaneous evaluation of air mass history and back trajectory consistency. Atmos. Meas. Tech. 2014, 7, 107–128. [Google Scholar] [CrossRef]
- Safatov, A.S.; Buryak, G.A.; Olkin, S.E.; Reznikova, I.K.; Makarov, V.I.; Popova, S.A. Analysis of monitoring data on organic/elemental carbon and total protein in ground air layer aerosol in the South of Western Siberia. Atmos. Ocean. Opt. 2014, 27, 164–168. [Google Scholar] [CrossRef]
- Safatov, A.; Andreeva, I.; Buryak, G.; Olkin, S.; Reznikova, I.; Belan, B.; Panchenko, M.; Simonenkov, D. Long-term studies of biological components of atmospheric aerosol: Trends and variability. Atmosphere 2022, 13, 651. [Google Scholar] [CrossRef]
- Klimko, N.N.; Kozlova, Y.I.; Khostelidi, S.N.; Shadrivova, O.V.; Borzova, Y.V.; Vasilyeva, N.V. The prevalence of serous and chronic fungal diseases in Russian Federation on Life Program Model. Probl. Med. Mycol. 2014, 1, 3–9. (In Russian) [Google Scholar]
- Kozlova, Y.I.; Sobolev, A.V.; Frolova, E.V.; Aak, O.V.; Burygina, E.V.; Klimko, N.N. Allergic bronchopulmonary aspergillosis in patients with asthma. Russ. Allergol. J. 2015, 2, 37–46. (In Russian) [Google Scholar] [CrossRef]
- Maturu, V.N.; Agarwal, R. Prevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis in cystic fibrosis: Systematic review and meta-analysis. Clin. Exp. Allergy 2015, 45, 1765–1778. [Google Scholar] [CrossRef] [PubMed]
- Alsante, A.N.; Thornton, D.C.O.; Brooks, S.D. Ocean aerobiology. Front. Microbiol. 2021, 12, 764178. [Google Scholar] [CrossRef]
- Purić, J.; Vieira, G.; Cavalca, L.B.; Sette, L.D.; Ferreira, H.; Vieira, M.L.C.; Sass, D.C. Activity of Antarctic fungi extracts against phytopathogenic bacteria. Lett. Appl. Microbiol. 2018, 66, 530–536. [Google Scholar] [CrossRef]
- Baricz, A.; Teban, A.; Chiriac, C.M.; Szekeres, E.; Farkas, A.; Nica, M.; Dascălu, A.; Oprișan, C.; Lavin, P.; Coman, C. Investigating the potential use of an Antarctic variant of Janthinobacterium lividum for tackling antimicrobial resistance in a One Health approach. Sci. Rep. 2018, 8, 15272. [Google Scholar] [CrossRef]
- Corte, A.M. Antibacterial activity of Penicillium spp. strains isolated in extreme environments. Polar Biol. 2000, 23, 294–297. [Google Scholar] [CrossRef]
- Giudice, A.L.; Fani, R. Antimicrobial potential of cold-adapted bacteria and fungi from Polar Regions. In Biotechnology of Extremophiles: Advances and Challenges; Rampelotto, P.H., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 83–115. [Google Scholar] [CrossRef]
- Krishnan, A.; Alias, S.A.; Wong, C.M.; Pang, K.L.; Convey, P. Extracellular hydrolase enzyme production by soil fungi from King George Island, Antarctica. Polar Biol. 2011, 34, 1535–1542. [Google Scholar] [CrossRef]
- Liu, J.T.; Lu, X.L.; Liu, X.Y.; Gao, Y.; Hu, B.; Jiao, B.H.; Zheng, H. Bioactive natural products from the Antarctic and Arctic organisms. Mini Rev. Med. Chem. 2013, 13, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Abrashev, R.; Feller, G.; Kostadinova, N.; Krumova, E.; Alexieva, Z.; Gerginova, M.; Spasova, B.; Miteva-Staleva, J.; Vassilev, S.; Angelova, M. Production, purification, and characterization of a novel cold-active superoxide dismutase from the Antarctic strain Aspergillus glaucus 363. Fungal Biol. 2016, 120, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Cavello, I.; Garmendia, G.; Rufo, C.; Cavalitto, S.; Veroet, S. Yeasts from sub-Antarctic region: Biodiversity, enzymatic activities and their potential as oleaginous microorganisms. Extremophiles 2016, 20, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Bratchkova, A.; Ivanova, V. Bioactive metabolites produced by microorganisms collected in Antarctica and the Arctic. Biotechnol. Biotechnol. Equip. 2011, 25, 1–7. [Google Scholar] [CrossRef]
- Kirtsideli, I.Y.; Vlasov, D.Y.; Krylenkov, V.A.; Rolle, N.N.; Barantsevich, E.P.; Sokolov, V.T. Comparative study of airborne fungi at Arctic stations near water area of the Northern Sea Route. Hum. Ecol. 2018, 4, 16–21. (In Russian) [Google Scholar] [CrossRef]
- Kellogg, C.A.; Griffin, D.W. Aerobiology and the global transport of desert dust. Trends Ecol. Evol. 2006, 21, 638–644. [Google Scholar] [CrossRef]
- Barrie, L.A. Arctic air pollution: An overview of current knowledge. Atmos. Environ. 1986, 20, 643–663. [Google Scholar] [CrossRef]
- Brown, J.K.M.; Hovmøller, M.S. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef]
- Garcıa-Pando, C.P.; Stanton, M.C.; Diggle, P.J.; Trzaska, S.; Miller, R.L.; Perlwitz, J.P.; Baldasano, J.M.; Cuevas, E.; Ceccato, P.; Yaka, P.; et al. Soil dust aerosols and wind as predictors of seasonal meningitis incidence in Niger. Environ. Health Perspect. 2014, 122, 679–686. [Google Scholar] [CrossRef]
- Kollath, D.R.; Miller, K.J.; Barker, B.M. The mysterious desert dwellers: Coccidioides immitis and Coccidioides posadasii, causative fungal agents of coccidioidomycosis. Virulence 2019, 10, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Fahlgren, C.; Hagström, Å.; Nilsson, D.; Zweifel, U.L. Annual variations in the diver-sity, viability, and origin of airborne bacteria. Appl. Environ. Microbiol. 2010, 76, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Pearce, D.A.; Bridge, P.D.; Hughes, K.A.; Sattler, B.; Psenner, R.; Russell, N.J. Microorganisms in the atmosphere over Antarctica. FEMS Microbiol. Ecol. 2009, 69, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, L.Z.; Kolokolova, Y.V.; Kolesnikova, A.G.; Mishin, V.V.; Ananyeva, M.N. Role of acinetobacter in the occurrence of problematic infections. Med. Soc. Probl. Fam. 2014, 19, 122–127. (In Russian) [Google Scholar]
- Moreira, J.S.; Riccetto, A.G.L.; da Silva, M.T.N.; dos Santos Vilela, M.M. Endocarditis by Kocuria rosea in an immunocompetent child. Braz. J. Infect. Dis. 2015, 19, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-J.; Chou, J.-H.; Lin, K.-Y.; Lin, M.-C.; Wei, Y.-H.; Arun, A.B.; Young, C.-C.; Chen, W.-M. Rothia terrae sp. nov. isolated from soil in Taiwan. Int. J. Syst. Evol. Microbiol. 2008, 58, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Arda, B.; Aydemir, S.; Yamazhan, T.; Hassan, A.; Tunger, A.; Serter, D. Comamonas testosteroni meningitis in a patient with recurrent cholesteatoma. APMIS 2003, 111, 474–476. [Google Scholar] [CrossRef]
- Lee, M.R.; Huang, Y.T.; Liao, C.H.; Chuang, T.Y.; Lin, C.K.; Lee, S.W.; Lai, C.C.; Yu, C.J.; Hsueh, P.R. Bacteremia caused by Brevundimonas species at a tertiary care hospital in Taiwan, 2000–2010. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1185–1191. [Google Scholar] [CrossRef]
- Chen, Y.-G.; Zhang, Y.-Q.; Shi, J.-X.; Xiao, H.-D.; Tang, S.-K.; Liu, Z.-X.; Huang, K.; Cui, X.-L.; Li, W.-J. Jeotgalicoccus marinus sp. nov., a marine bacterium isolated from a sea urchin. Int. J. Syst. Evol. Microbiol. 2009, 59, 1625–1629. [Google Scholar] [CrossRef]
- Ghannoum, M.A. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 2000, 13, 122–143. [Google Scholar] [CrossRef]

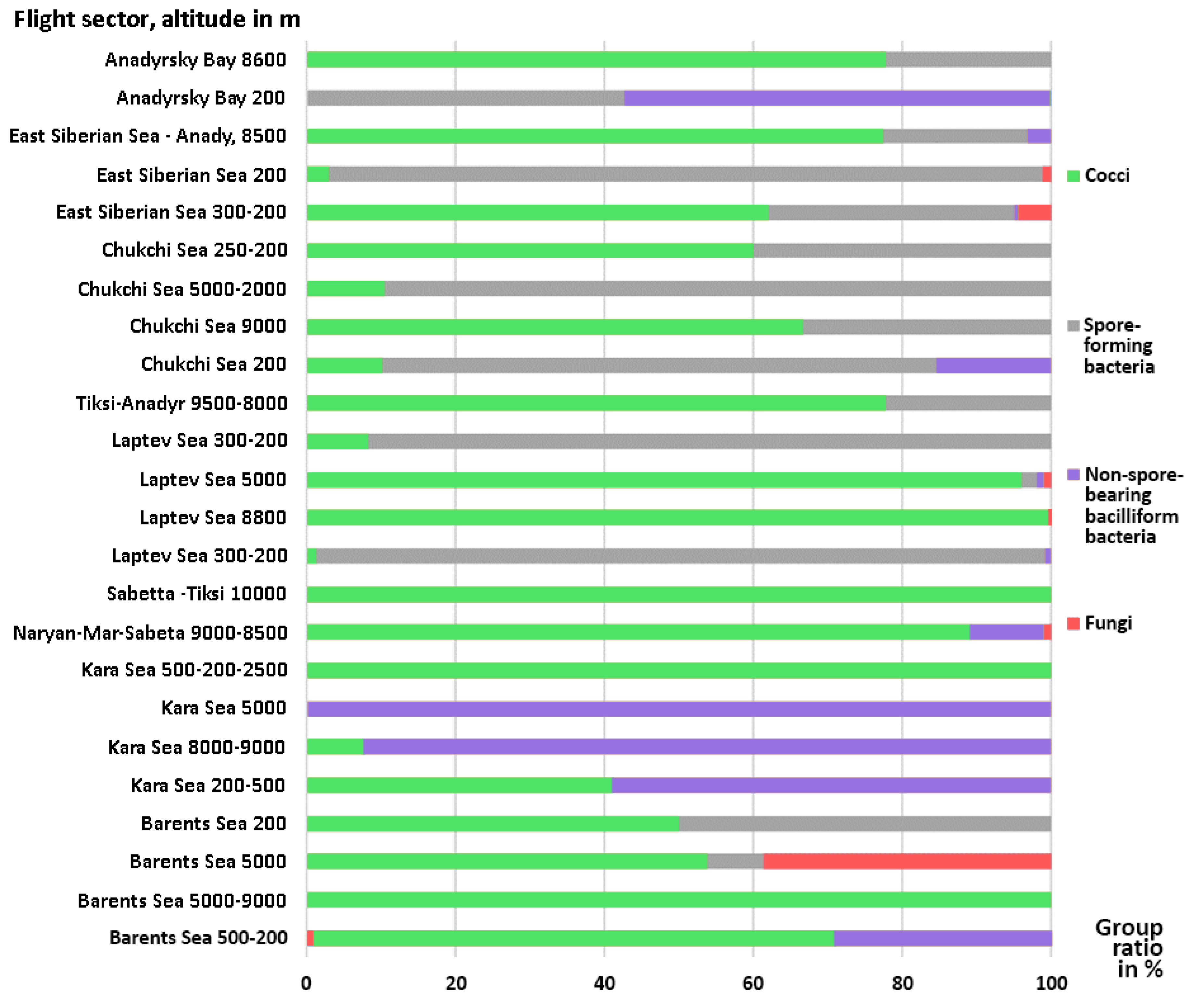
| Flight’s Day | Sampling | Flight’s Segment | Altitude, m | Sample Number | Sampling Duration, min. | Microorganisms Concentration, CFU/m3 | |
|---|---|---|---|---|---|---|---|
| Start | Finish | ||||||
| 4 September | 21:12 | 21:24 | Barents Sea | 500-200 | 1 | 10 | 5.50 × 103 |
| -«- | 21:35 | 22:05 | -«- | 5000-9000-8000 | 2 | 15 | 4.00 × 103 |
| -«- | 22:10 | 22:16 | -«- | 5000 | 3 | 16 | 4.06 × 103 |
| -«- | 22:25 | 22:39 | -«- | 200 | 4 | 10 | 5.00 × 103 |
| 6 September | 15:05 | 15:06 | Kara Sea | 500-200-500 | 5 | 9 | 5.55 × 103 |
| -«- | 15:35 | 15:53 | -«- | 8000-9000-8000 | 6 | 16 | 3.13 × 103 |
| -«- | 15:58 | 16:10 | -«- | 5000 | 7 | 11 | 4.54 × 103 |
| -«- | 16:15 | 16:30 | -«- | 500-200-2500 | 8 | 11 | 4.55 × 103 |
| 7 September | 8:25 | 9:03 | Naryan-Mar—Sabetta | 9000-8500 | 9 | 15 | 3.33 × 103 |
| -«- | 12:42 | 13:25 | Sabetta—Tiksi | 10,000 | 10 | 10 | 5.00 × 103 |
| 9 September | 13:12 | 13:24 | Laptev Sea | 300-200 | 11 | 9 | 5.55 × 103 |
| -«- | 13:43 | 13:55 | -«- | 8800 | 12 | 10 | 5.00 × 103 |
| -«- | 14:01 | 14:14 | -«- | 5000 | 13 | 12 | 4.17 × 103 |
| -«- | 14:22 | 14:32 | -«- | 300-200 | 14 | 12 | 4.17 × 103 |
| 11 September | 12:18 | 13:18 | Tiksi—Anadyr | 9500-8000 | 15 | 17 | 2.94 × 103 |
| 15 September | 12:36 | 12:50 | Chukchi Sea | 200 | 16 | 15 | 3.33 × 103 |
| -«- | 13:10 | 13:21 | -«- | 9000 | 17 | 10 | 5.00 × 103 |
| -«- | 13:27 | 13:34 | -«- | 5000-2000 | 18 | 6 | 8.33 × 103 |
| -«- | 13:40 | 13:50 | -«- | 250-200 | 19 | 10 | 5.00 × 103 |
| 16 September | 11:03 | 11:40 | East Siberian Sea | 300-200 | 20 | 10 | 5.00 × 103 |
| -«- | 12:12 | 12:23 | -«- | 200 | 21 | 10 | 5.00 × 103 |
| -«- | 12:42 | 13:17 | East Siberian Sea—Anadyr | 8500 | 22 | 15 | 3.33 × 103 |
| -«- | 15:48 | 16:00 | Bering Sea | 200 | 23 | 12 | 3.33 × 103 |
| 17 September | 17:09 | 17:24 | -«- | 8600 | 24 | 10 | 5.00 × 103 |
| Flight’s Segment | Altitude, km/Name of Culturable Microorganisms | ||
|---|---|---|---|
| 0.2–0.5 | 2–5 | 8–10 | |
| Barents Sea | Acinetobacter spp.; Staphylococcus spp.; Micrococcus spp.; Bacillus spp. | Lysinibacillus spp.; Penicillium ssp.; Aspergillus spp.; Pseudomonas spp. | Staphylococcus spp.; Cladosporium spp. |
| Kara Sea | Staphylococcus spp.; Paracoccus spp.; Rothia spp.; Pseudomonas spp.; Jeotgalicoccus spp.; Brevundimonas spp.; Acinetobacter radioresistens | Staphylococcus hominis; Pseudomonas spp.; Acinetobacter spp. | Microbacterium spp.; Staphylococcus spp.; Rhizobium spp.; Staphylococcus hominis |
| Laptev Sea | Curtobacterium spp.; Bacillus spp.; Pseudarthrobacter spp.; Rothia spp. | Bacillus spp.; Rothia spp.; Staphylococcus spp.; Penicillium spp. | Staphylococcus spp.; Penicillium ssp.; Staphylococcus hominis; Alternaria spp. |
| Chukchi Sea | Staphylococcus spp.; Micrococcus spp.; Bacillus spp.; Acinetobacter spp.; Staphylococcus epidermidis | Comamonas spp.; Micrococcus spp.; Bacillus spp.; Staphylococcus equorum | Bacillus spp. |
| East Siberian Sea | Staphylococcus spp.; Lysinibacillus spp.; Staphylococcus warneri; Aureobasidium spp.; Rothia terrae; Bacillus spp.; Aspergillus spp.; Kocuria spp.; | No data | No data |
| East Siberian Sea—Anadyr | No data | No data | Bacillus spp., Kocuria spp. Kocuria sediminis Staphylococcus epidermidis Staphylococcus warneri |
| Bering Sea | Bacillus spp., Nocardia spp. Bacillus pumilus Penicillium spp., Aspergillus spp. | No data | No data |
| Strain | Growth Temperature, °C | Strain | Growth Temperature, °C | Strain | Growth Temperature, °C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6–9 | 20–22 | 37 | 6–9 | 20–22 | 37 | 6–9 | 20–22 | 37 | |||
| Sp-1 | ++ | ++ | ++ | Sp-49 | + | Sp-97 | - | +++ | +++ | ||
| Sp-2 | ++ | ++ | ++ | Sp-50 | - | ++ | + | Sp-98 | - | +++ | +++ |
| Sp-3 | - | ++ | + | Sp-51 | - | ++ | + | Sp-99 | - | +++ | ++ |
| Sp-4 | - | ++++ | ± | Sp-52 | ±- | ++ | ++ | Sp-100 | - | ++++ | ++ |
| Sp-5 | - | + | ± | Sp-53 | -- | ++ | ++ | Sp-101 | - | +++ | +++ |
| Sp-6 | - | ++ | ++ | Sp-54 | - | + | ± | Sp-102 | - | ++++ | ++ |
| Sp-7 | - | ++ | + | Sp-55 | - | ++++ | ++++ | Sp-103 | - | ++++ | ± |
| Sp-8 | ± | ++++ | +++ | Sp-56 | - | ++++ | ++++ | Sp-104 | - | ++++ | ++++ |
| Sp-9 | - | ++ | ++ | Sp-57 | - | + | ± | Sp-105 | - | ++++ | ++++ |
| Sp-10 | - | ++ | ++ | Sp-58 | - | +++ | + | Sp-106 | - | ++++ | ++++ |
| Sp-11 | ± | ++ | ++ | Sp-59 | - | ++++ | ++++ | Sp-107 | - | ++++ | ++++ |
| Sp-12 | - | ++++ | ++++ | Sp-60 | - | ++++ | +++ | Sp-108 | ++ | ++++ | ++++ |
| Sp-13 | - | ++ | +++ | Sp-61 | - | +++ | + | Sp-109 | ++ | ++++ | ++++ |
| Sp-14 | ++ | ± | Sp-62 | - | ++++ | ++++ | Sp-110 | - | ++++ | ++++ | |
| Sp-15 | - | ++++ | ++ | Sp-63 | - | +++ | ++ | Sp-111 | - | ++++ | ++++ |
| Sp-16 | ++ | ++++ | +++ | Sp-64 | - | ++++ | ++++ | Sp-112 | - | + | ++ |
| Sp-17 | - | ++ | ++ | Sp-65 | ± | +++ | ++ | Sp-113 | - | ++ | ++ |
| Sp-18 | +++ | ++++ | +++ | Sp-66 | - | + | ± | Sp-114 | ++ | +++ | ++ |
| Sp-19 | - | ++ | ++ | Sp-67 | - | ++++ | +++ | Sp-115 | ++ | ++++ | ++ |
| Sp-20 | - | ++++ | ++++ | Sp-68 | - | ++++ | + | Sp-116 | ++ | ++++ | ± |
| Sp-21 | - | + | - | Sp-69 | ± | ++++ | +++ | Sp-117 | - | ++++ | ++++ |
| Sp-22 | ++ | ++++ | ++++ | Sp-70 | - | ++++ | +++ | Sp-118 | - | ++++ | ++++ |
| Sp-23 | + | ++ | +++ | Sp-71 | - | ++++ | +++ | Sp-119 | - | ++++ | ++++ |
| Sp-24 | ++ | +++ | ++ | Sp-72 | - | ++++ | +++ | Sp-120 | - | ++++ | ++++ |
| Sp-25 | ++ | ++++ | ++ | Sp-73 | - | +++ | + | Sp-121 | - | + | ++ |
| Sp-26 | ++ | +++ | ± | Sp-74 | - | ++++ | ++ | Sp-122 | - | +++ | ++ |
| Sp-27 | + | ++ | ± | Sp-75 | - | ++++ | +++ | Sp-123 | - | + | ± |
| Sp-28 | - | ++ | ± | Sp-76 | - | ++ | + | Sp-124 | - | +++ | ± |
| Sp-29 | ± | +++ | ± | Sp-77 | - | + | ± | Sp-125 | - | ++ | + |
| Sp-30 | ++ | +++ | ++ | Sp-78 | - | + | + | Sp-126 | - | +++ | ++ |
| Sp-31 | ± | +++ | ++ | Sp-79 | - | ++++ | +++ | Sp-127 | - | ++ | ± |
| Sp-32 | + | ++ | + | Sp-80 | - | ++++ | +++ | Sp-128 | - | +++ | - |
| Sp-33 | - | ++ | + | Sp-81 | - | ++++ | ++++ | Sp-129 | - | +++ | - |
| Sp-34 | - | + | + | Sp-82 | - | ++++ | ++ | Sp-130 | ± | ++++ | - |
| Sp-35 | - | ++ | + | Sp-83 | - | ++++ | ++++ | Sp-131 | ± | ++++ | - |
| Sp-36 | - | ++ | ± | Sp-84 | - | +++ | +++ | Sp-132 | - | ++++ | ± |
| Sp-37 | - | ++ | + | Sp-85 | - | +++ | +++ | Sp-133 | - | +++ | ++ |
| Sp-38 | - | + | + | Sp-86 | - | +++ | +++ | Sp-134 | + | ++++ | ++ |
| Sp-39 | - | ++ | ++ | Sp-87 | - | +++ | ± | Sp-135 | - | +++ | + |
| Sp-40 | - | ++ | ++ | Sp-88 | - | + | ± | Sp-136 | - | +++ | ++ |
| Sp-41 | - | ++ | ++ | Sp-89 | - | ++ | ± | Sp-137 | - | +++ | ± |
| Sp-42 | - | ++ | ± | Sp-90 | - | ++++ | ++++ | Sp-138 | ++ | ++++ | +++ |
| Sp-43 | - | ++ | ++ | Sp-91 | - | ++++ | ++++ | Sp-139 | - | ++++ | +++ |
| Sp-44 | - | ++ | ± | Sp-92 | - | + | ± | Sp-140 | - | ++++ | ++++ |
| Sp-45 | ++ | ++ | ± | Sp-93 | - | + | ± | Sp-141 | - | ++++ | ++++ |
| Sp-46 | - | ++ | ± | Sp-94 | - | +++ | ++++ | Sp-142 | - | +++ | ++ |
| Sp-47 | ++ | ++ | ± | Sp-95 | - | ++++ | ++++ | Sp-143, | + | ++ | ± |
| Sp-48 | ++ | ++++ | ++++ | Sp-96 | + | ++++ | ++++ | Sp-144 | - | ++ | |
| Sp-146 | ± | +++ | +++ | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, I.S.; Safatov, A.S.; Puchkova, L.I.; Solovyanova, N.A.; Okhlopkova, O.V.; Rebus, M.E.; Buryak, G.A.; Belan, B.D.; Simonenkov, D.V. Culturable Microorganisms of Aerosols Sampled during Aircraft Sounding of the Atmosphere over the Russian Arctic Seas. Atmosphere 2024, 15, 365. https://doi.org/10.3390/atmos15030365
Andreeva IS, Safatov AS, Puchkova LI, Solovyanova NA, Okhlopkova OV, Rebus ME, Buryak GA, Belan BD, Simonenkov DV. Culturable Microorganisms of Aerosols Sampled during Aircraft Sounding of the Atmosphere over the Russian Arctic Seas. Atmosphere. 2024; 15(3):365. https://doi.org/10.3390/atmos15030365
Chicago/Turabian StyleAndreeva, Irina S., Aleksandr S. Safatov, Larisa I. Puchkova, Nadezhda A. Solovyanova, Olesya V. Okhlopkova, Maksim E. Rebus, Galina A. Buryak, Boris D. Belan, and Denis V. Simonenkov. 2024. "Culturable Microorganisms of Aerosols Sampled during Aircraft Sounding of the Atmosphere over the Russian Arctic Seas" Atmosphere 15, no. 3: 365. https://doi.org/10.3390/atmos15030365
APA StyleAndreeva, I. S., Safatov, A. S., Puchkova, L. I., Solovyanova, N. A., Okhlopkova, O. V., Rebus, M. E., Buryak, G. A., Belan, B. D., & Simonenkov, D. V. (2024). Culturable Microorganisms of Aerosols Sampled during Aircraft Sounding of the Atmosphere over the Russian Arctic Seas. Atmosphere, 15(3), 365. https://doi.org/10.3390/atmos15030365







