Comparative Experimental Assessment of Pollutant Emission Behavior in Combustion of Untreated and Thermally Treated Solid Biofuels from Spruce Chips and Rapeseed Straw
Abstract
1. Introduction
2. Materials and Methods
2.1. Wood Chips
2.2. Rapeseed Straw
2.3. Sample Pre-Treatment
2.4. Thermal Treatment
2.5. Elemental and Proximate Analysis
2.6. Combustion Tests
2.7. Emission Measurement
3. Results and Discussions
3.1. Fuel Analysis
3.2. Emission Concentrations of Carbon Monoxide as a Function of the Coefficient of Excess Air and the Flue Gas Temperature
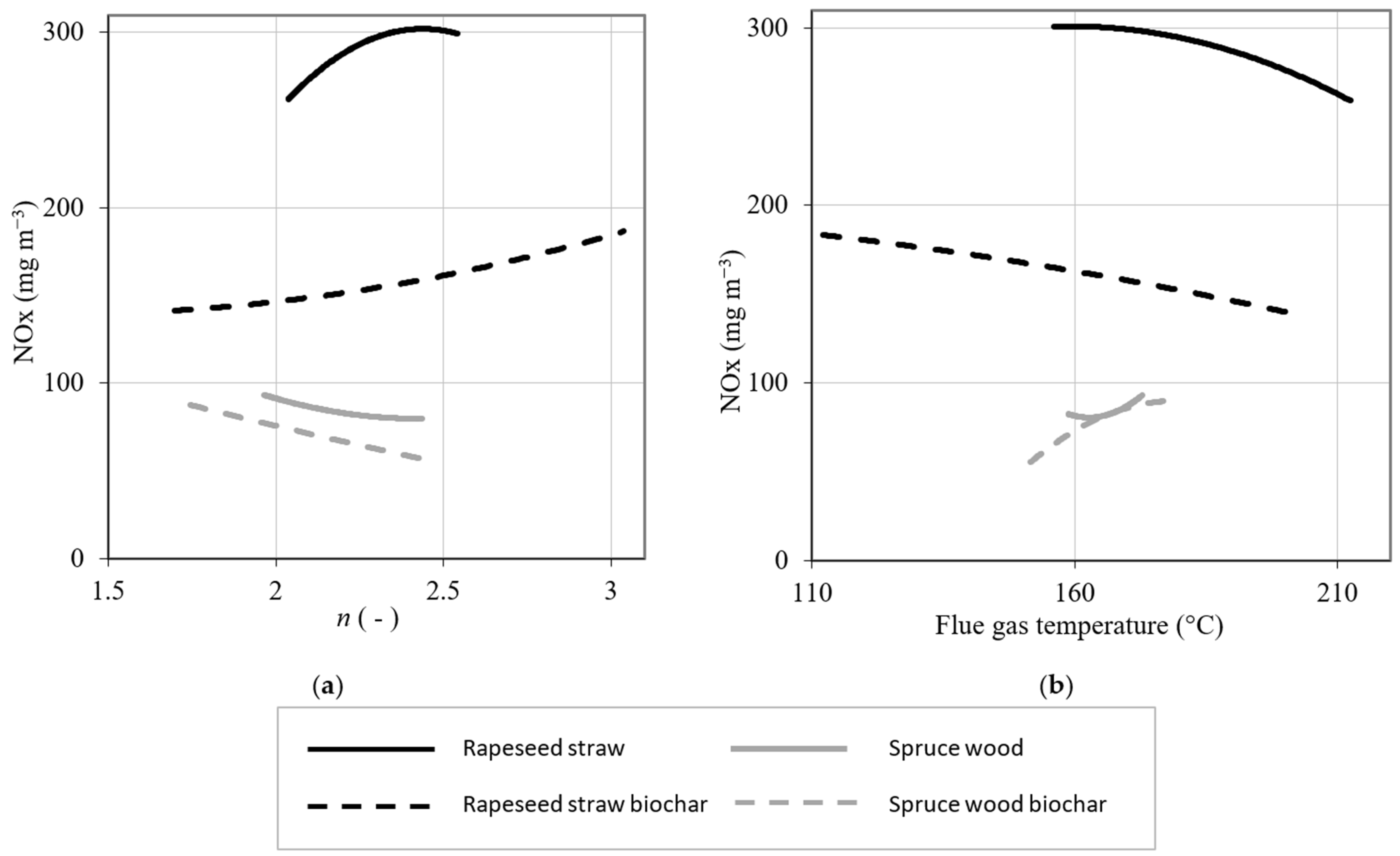
3.3. Emission Concentrations of Nitrogen Oxides as a Function of the Coefficient of Excess Air and the Flue Gas Temperature
3.4. Comparison of Emissions Per Unit of Heat Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A Review on Biomass as a Fuel for Boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- McIlveen-Wright, D.R.; Huang, Y.; Rezvani, S.; Mondol, J.D.; Redpath, D.; Anderson, M.; Hewitt, N.J.; Williams, B.C. A Techno-Economic Assessment of the Reduction of Carbon Dioxide Emissions through the Use of Biomass Co-Combustion. Fuel 2011, 90, 11–18. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Bonifacio, S.; Clowes, J.; Foulds, A.; Holland, R.; Matthews, J.C.; Percival, C.J.; Shallcross, D.E. Investigation of Biofuel as a Potential Renewable Energy Source. Atmosphere 2021, 12, 1289. [Google Scholar] [CrossRef]
- Monjardino, J.; Dias, L.; Fortes, P.; Tente, H.; Ferreira, F.; Seixas, J. Carbon Neutrality Pathways Effects on Air Pollutant Emissions: The Portuguese Case. Atmosphere 2021, 12, 324. [Google Scholar] [CrossRef]
- Tarín-Carrasco, P.; Im, U.; Geels, C.; Palacios-Peña, L.; Jiménez-Guerrero, P. Reducing Future Air-Pollution-Related Premature Mortality over Europe by Mitigating Emissions from the Energy Sector: Assessing an 80% Renewable Energies Scenario. Atmos. Chem. Phys. 2022, 22, 3945–3965. [Google Scholar] [CrossRef]
- de Albuquerque, Y.L.; Berger, E.; Li, C.; Pardo, M.; George, C.; Rudich, Y.; Géloën, A. The Toxic Effect of Water-Soluble Particulate Pollutants from Biomass Burning on Alveolar Lung Cells. Atmosphere 2021, 12, 1023. [Google Scholar] [CrossRef]
- Schimel, D.S.; House, J.I.; Hibbard, K.A.; Bousquet, P.; Ciais, P.; Peylin, P.; Braswell, B.H.; Apps, M.J.; Baker, D.; Bondeau, A.; et al. Recent Patterns and Mechanisms of Carbon Exchange by Terrestrial Ecosystems. Nature 2001, 414, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Malaťák, J.; Jankovský, M.; Malaťáková, J.; Velebil, J.; Gendek, A.; Aniszewska, M. Substituting Solid Fossil Fuels with Torrefied Timber Products. Materials 2023, 16, 7569. [Google Scholar] [CrossRef] [PubMed]
- Houshfar, E.; Wang, L.; Vähä-Savo, N.; Brink, A.; Løvås, T. Characterisation of CO/NO/SO2 Emission and Ash-Forming Elements from the Combustion and Pyrolysis Process. Clean Technol. Environ. Policy 2014, 16, 1339–1351. [Google Scholar] [CrossRef]
- Čuček, L.; Klemeš, J.J.; Kravanja, Z. Carbon and Nitrogen Trade-Offs in Biomass Energy Production. Clean Technol. Environ. Policy 2012, 14, 389–397. [Google Scholar] [CrossRef]
- Malaťák, J.; Gendek, A.; Aniszewska, M.; Velebil, J. Emissions from Combustion of Renewable Solid Biofuels from Coniferous Tree Cones. Fuel 2020, 276, 118001. [Google Scholar] [CrossRef]
- Barmina, I.; Lickrastina, A.; Zake, M.; Arshanitsa, A.; Solodovnik, V.; Telisheva, G. Effect of Main Characteristics of Pelletized Renewable Energy Resources on Combustion Characteristics and Heat Energy Production. Chem. Eng. 2012, 29, 901–906. [Google Scholar]
- Glarborg, P.; Jensen, A.D.; Johnsson, J.E. Fuel Nitrogen Conversion in Solid Fuel Fired Systems. Prog. Energy Combust. Sci. 2003, 29, 89–113. [Google Scholar] [CrossRef]
- Gürdil, G.; Selvi, K.; Malaťák, J.; Pinar, Y. Biomass Utilization for Thermal Energy. Ama Agric. Mech. Asia Afr. Lat. Am. 2009, 40, 80–85. [Google Scholar]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Goos, E.; Sickfeld, C.; Mauß, F.; Seidel, L.; Ruscic, B.; Burcat, A.; Zeuch, T. Prompt NO Formation in Flames: The Influence of NCN Thermochemistry. Proc. Combust. Inst. 2013, 34, 657–666. [Google Scholar] [CrossRef]
- Harding, L.B.; Klippenstein, S.J.; Miller, J.A. Kinetics of CH + N2 Revisited with Multireference Methods. J. Phys. Chem. A 2008, 112, 522–532. [Google Scholar] [CrossRef]
- Winter, F.; Wartha, C.; Hofbauer, H. NO and N2O Formation during the Combustion of Wood, Straw, Malt Waste and Peat. Bioresour. Technol. 1999, 70, 39–49. [Google Scholar] [CrossRef]
- Liu, H.; Chaney, J.; Li, J.; Sun, C. Control of NOx Emissions of a Domestic/Small-Scale Biomass Pellet Boiler by Air Staging. Fuel 2013, 103, 792–798. [Google Scholar] [CrossRef]
- Bai, J.; Yu, C.; Li, L.; Wu, P.; Luo, Z.; Ni, M. Experimental Study on the NO and N2O Formation Characteristics during Biomass Combustion. Energy Fuels 2012, 27, 515–522. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, X.; Liu, J.; Han, X. NOx Emission of Fine- and Superfine- Pulverized Coal Combustion in O2/CO2 Atmosphere. Energy Fuels 2010, 24, 6307–6313. [Google Scholar] [CrossRef]
- Malaťák, J.; Velebil, J.; Bradna, J.; Gendek, A.; Tamelová, B. Evaluation of Co and NoxEmissions in Real-Life Operating Conditions of Herbaceous Biomass Briquettes Combustion. Acta Technol. Agric. 2020, 23, 53–59. [Google Scholar]
- Houshfar, E.; Skreiberg, Ø.; Løvås, T.; Todorović, D.; Sørum, L. Effect of Excess Air Ratio and Temperature on NOx Emission from Grate Combustion of Biomass in the Staged Air Combustion Scenario. Energy Fuels 2011, 25, 4643–4654. [Google Scholar] [CrossRef]
- Malaťák, J.; Jevic, P.; Gürdil, G.; Selvi, K. Biomass Heat-Emission Characteristics of Energy Plants. Ama Agric. Mech. Asia Afr. Lat. Am. 2008, 39, 9–13. [Google Scholar]
- Wei, X.; Schnell, U.; Han, X.; Hein, K.R.G. Interactions of CO, HCl, and SOx in Pulverised Coal Flames. Fuel 2004, 83, 1227–1233. [Google Scholar] [CrossRef]
- Li, J.; Brzdekiewicz, A.; Yang, W.; Blasiak, W. Co-Firing Based on Biomass Torrefaction in a Pulverized Coal Boiler with Aim of 100% Fuel Switching. Appl. Energy 2012, 99, 344–354. [Google Scholar] [CrossRef]
- Phanphanich, M.; Mani, S. Impact of Torrefaction on the Grindability and Fuel Characteristics of Forest Biomass. Bioresour. Technol. 2011, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Repellin, V.; Govin, A.; Rolland, M.; Guyonnet, R. Energy Requirement for Fine Grinding of Torrefied Wood. Biomass Bioenergy 2010, 34, 923–930. [Google Scholar] [CrossRef]
- Jeníček, L.; Tunklová, B.; Malaťák, J.; Velebil, J.; Malaťáková, J.; Neškudla, M.; Hnilička, F. The Impact of Nutshell Biochar on the Environment as an Alternative Fuel or as a Soil Amendment. Materials 2023, 16, 2074. [Google Scholar] [CrossRef]
- Lin, F.; Wang, H.; Shaghaleh, H.; Ali Adam Hamad, A.; Zhang, Y.; Yang, B.; Alhaj Hamoud, Y. Effects of Biochar Amendment on N2O Emissions from Soils with Different PH Levels. Atmosphere 2024, 15, 68. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerman, J. Effects of Torrefaction Process Parameters on Biomass Feedstock Upgrading. Fuel 2012, 91, 147–154. [Google Scholar] [CrossRef]
- Pimchuai, A.; Dutta, A.; Basu, P. Torrefaction of Agriculture Residue To Enhance Combustible Properties. Energy Fuels 2010, 24, 4638–4645. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.J.; Kuang, J.H.; Zhang, Y.L.; Luo, Y.H. Pretreatment of Agricultural Residues for Co-Gasification via Torrefaction. J. Anal. Appl. Pyrolysis 2009, 86, 331–337. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Yan, W.; Hastings, J.T.; Acharjee, T.C.; Coronella, C.J.; Vásquez, V.R. Mass and Energy Balances of Wet Torrefaction of Lignocellulosic Biomass. Energy Fuels 2010, 24, 4738–4742. [Google Scholar] [CrossRef]
- Malaťák, J.; Dlabaja, T. Hydrothermal Carbonization of Kitchen Waste. Res. Agric. Eng. 2016, 62, 64–72. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Minaret, J. Review on Comparative Study of Dry and Wet Torrefaction. Sustain. Energy Technol. Assess. 2015, 12, 26–37. [Google Scholar] [CrossRef]
- Aguado, R.; Cuevas, M.; Pérez-Villarejo, L.; Martínez-Cartas, M.L.; Sánchez, S. Upgrading Almond-Tree Pruning as a Biofuel via Wet Torrefaction. Renew Energy 2020, 145, 2091–2100. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Assima, G.P.; Beaudoin, G.; Larachi, F. Biomass Torrefaction and CO2 Capture Using Mining Wastes—A New Approach for Reducing Greenhouse Gas Emissions of Co-Firing Plants. Fuel 2014, 115, 749–757. [Google Scholar] [CrossRef]
- Zwart, R.W.R.; Boerrigter, H.; van der Drift, A. The Impact of Biomass Pretreatment on the Feasibility of Overseas Biomass Conversion to Fischer−Tropsch Products. Energy Fuels 2006, 20, 2192–2197. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More Efficient Biomass Gasification via Torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- ISO 17225-8:2023; Solid Biofuels—Fuel Specifications and Classes—Part 8: Graded Thermally Treated and Densified Biomass Fuels for Commercial and Industrial Use. International Organization for Standardization: Geneva, Switzerland, 2023.
- Magalhaes, D.; Kazanc, F. Influence of Biomass Thermal Pre-Treatment on the Particulate Matter Formation during Pulverized Co-Combustion with Lignite Coal. Fuel 2022, 308, 122027. [Google Scholar] [CrossRef]
- Malaťáková, J.; Jankovský, M.; Malaťák, J.; Velebil, J.; Tamelová, B.; Gendek, A.; Aniszewska, M. Evaluation of Small-Scale Gasification for CHP for Wood from Salvage Logging in the Czech Republic. Forests 2021, 12, 1448. [Google Scholar] [CrossRef]
- Ministry of Agriculture of Czech Republic. Situační a Výhledová Zpráva Olejniny (Situation and Outlook Report—Oil Crops); Ministry of Agriculture: Prague, Czech Republic, 2022.
- Bradna, J.; Malaťák, J.; Velebil, J. Impact of Differences in Combustion Conditions of Rape Straw on the Amount of Flue Gases and Fly Ash Properties. Agron. Res. 2017, 15, 649–657. [Google Scholar]
- Havránek, F.; Pavliš, J.; Hučko, B.; Czudek, R. Alternative Utilisation of Agricultural Land Scientific Monograph; Rembrandt: Prague, Czech Republic, 2007; ISBN 978-80-902617-6-1. [Google Scholar]
- Lewandowski, I.; Weger, J.; van Hooijdonk, A.; Havlickova, K.; van Dam, J.; Faaij, A. The Potential Biomass for Energy Production in the Czech Republic. Biomass Bioenergy 2006, 30, 405–421. [Google Scholar] [CrossRef]
- ISO 18122:2022; Solid Biofuels—Determination of Ash Content. International Organization for Standardization: Geneva, Switzerland, 2022; p. 6.
- EN ISO 18134-3:2023; Solid Biofuels—Determination of Moisture Content—Oven Dry Method Part 3: Moisture in General Analysis Sample. International Organization for Standardization: Geneva, Switzerland, 2023; p. 5.
- ISO 1928:2020; Coal and Coke—Determination of Gross Calorific Value. International Organization for Standardization: Geneva, Switzerland, 2020; p. 62.
- ČSN 07 0240; Warm Water and Low-Pressure Steam Boilers. Basic Regulations. Czech Standards Institute: Praha, Czech Republic, 1993; pp. 1–64.
- Carroll, J.; Finnan, J. Emissions and Efficiencies from the Combustion of Agricultural Feedstock Pellets Using a Small Scale Tilting Grate Boiler. Biosyst. Eng. 2013, 115, 50–55. [Google Scholar] [CrossRef]
- Röser, D.; Mola-Yudego, B.; Sikanen, L.; Prinz, R.; Gritten, D.; Emer, B.; Väätäinen, K.; Erkkilä, A. Natural Drying Treatments during Seasonal Storage of Wood for Bioenergy in Different European Locations. Biomass Bioenergy 2011, 35, 4238–4247. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash. Part 1. Phase–Mineral and Chemical Composition and Classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Lindström, E.; Larsson, S.H.; Boström, D.; Öhman, M. Slagging Characteristics during Combustion of Woody Biomass Pellets Made from a Range of Different Forestry Assortments. Energy Fuels 2010, 24, 3456–3461. [Google Scholar] [CrossRef]
- Lamberg, H.; Sippula, O.; Tissari, J.; Jokiniemi, J. Effects of Air Staging and Load on Fine-Particle and Gaseous Emissions from a Small-Scale Pellet Boiler. Energy Fuels 2011, 25, 4952–4960. [Google Scholar] [CrossRef]
- Näzelius, I.L.; Fagerström, J.; Boman, C.; Boström, D.; Öhman, M. Slagging in Fixed-Bed Combustion of Phosphorus-Poor Biomass: Critical Ash-Forming Processes and Compositions. Energy Fuels 2015, 29, 894–908. [Google Scholar] [CrossRef]
- Nik Norizam, N.N.A.; Yang, X.; Ingham, D.; Szuhánszki, J.; Yang, W.; Rezende, J.; Ma, L.; Pourkashanian, M. An Improved Index to Predict the Slagging Propensity of Woody Biomass on High-Temperature Regions in Utility Boilers. J. Energy Inst. 2023, 109, 101272. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion Characteristics of Different Biomass Fuels. Prog. Energy Combust. Sci 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Malaťák, J.; Velebil, J.; Malaťáková, J.; Passian, L.; Bradna, J.; Tamelová, B.; Gendek, A.; Aniszewska, M. Reducing Emissions from Combustion of Grape Residues in Mixtures with Herbaceous Biomass. Materials 2022, 15, 7288. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Basu, P. Combustion of Coal in Circulating Fluidized-Bed Boilers: A Review. Chem. Eng. Sci. 1999, 54, 5547–5557. [Google Scholar] [CrossRef]
- Johansen, J.M.; Jakobsen, J.G.; Frandsen, F.J.; Glarborg, P. Release of K, Cl, and S during Pyrolysis and Combustion of High-Chlorine Biomass. Energy Fuels 2011, 25, 4961–4971. [Google Scholar] [CrossRef]
- Ren, X.; Sun, R.; Meng, X.; Vorobiev, N.; Schiemann, M.; Levendis, Y.A. Carbon, Sulfur and Nitrogen Oxide Emissions from Combustion of Pulverized Raw and Torrefied Biomass. Fuel 2017, 188, 310–323. [Google Scholar] [CrossRef]
- Poudel, J.; Ohm, T.I.; Oh, S.C. A Study on Torrefaction of Food Waste. Fuel 2015, 140, 275–281. [Google Scholar] [CrossRef]
- Chen, W.H.; Lu, K.M.; Tsai, C.M. An Experimental Analysis on Property and Structure Variations of Agricultural Wastes Undergoing Torrefaction. Appl. Energy 2012, 100, 318–325. [Google Scholar] [CrossRef]
- Saleh, S.B.; Flensborg, J.P.; Shoulaifar, T.K.; Sárossy, Z.; Hansen, B.B.; Egsgaard, H.; Demartini, N.; Jensen, P.A.; Glarborg, P.; Dam-Johansen, K. Release of Chlorine and Sulfur during Biomass Torrefaction and Pyrolysis. Energy Fuels 2014, 28, 3738–3746. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Lin, Y.Y.; Chu, Y.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.S.; Ho, S.H.; Culaba, A.B.; et al. Progress in Biomass Torrefaction: Principles, Applications and Challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Johansen, J.M.; Aho, M.; Paakkinen, K.; Taipale, R.; Egsgaard, H.; Jakobsen, J.G.; Frandsen, F.J.; Glarborg, P. Release of K, Cl, and S during Combustion and Co-Combustion with Wood of High-Chlorine Biomass in Bench and Pilot Scale Fuel Beds. Proc. Combust. Inst. 2013, 34, 2363–2372. [Google Scholar] [CrossRef]
- ISO 17225-2:2021; Solid Biofuels—Fuel Specifications and Classes—Part 2: Graded Wood Pellets. International Organization for Standardization: Geneva, Switzerland, 2021.
- ISO 17225-6:2021; Solid Biofuels—Fuel Specifications and Classes—Part 6: Graded Non-Woody Pellets. International Organization for Standardization: Geneva, Switzerland, 2021.
- Wang, K.; Nakao, S.; Thimmaiah, D.; Hopke, P.K. Emissions from In-Use Residential Wood Pellet Boilers and Potential Emissions Savings Using Thermal Storage. Sci. Total Environ. 2019, 676, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Win, K.M.; Persson, T.; Bales, C. Particles and Gaseous Emissions from Realistic Operation of Residential Wood Pellet Heating Systems. Atmos. Environ. 2012, 59, 320–327. [Google Scholar] [CrossRef]
- Johansson, L.S.; Leckner, B.; Gustavsson, L.; Cooper, D.; Tullin, C.; Potter, A. Emission Characteristics of Modern and Old-Type Residential Boilers Fired with Wood Logs and Wood Pellets. Atmos. Environ. 2004, 38, 4183–4195. [Google Scholar] [CrossRef]
- Juszczak, M. Concentrations of Carbon Monoxide and Nitrogen Oxides from a 15 KW Heating Boiler Supplied Periodically with a Mixture of Sunflower Husk and Wood Pellets. Environ. Prot. Eng. 2014, 40, 65–74. [Google Scholar] [CrossRef]
- Ferge, T.; Maguhn, J.; Hafner, K.; Mühlberger, F.; Davidovic, M.; Warnecke, R.; Zimmermann, R. On-Line Analysis of Gas-Phase Composition in the Combustion Chamber and Particle Emission Characteristics during Combustion of Wood and Waste in a Small Batch Reactor. Environ. Sci. Technol. 2005, 39, 1393–1402. [Google Scholar] [CrossRef]
- Juszczak, M. Comparison of CO and NOx Concentrations from a 20 KW Boiler for Periodic and Constant Wood Pellet Supply. Environ. Prot. Eng. 2016, 42, 95–107. [Google Scholar] [CrossRef]
- Johansson, L.S.; Tullin, C.; Leckner, B.; Sjövall, P. Particle Emissions from Biomass Combustion in Small Combustors. Biomass Bioenergy 2003, 25, 435–446. [Google Scholar] [CrossRef]
- Souček, J.; Jasinskas, A. Assessment of the Use of Potatoes as a Binder in Flax Heating Pellets. Sustainability 2020, 12, 10481. [Google Scholar] [CrossRef]
- Malaťák, J.; Bradna, J.; Velebil, J. The Dependence of COx and NOx Emission Concentrations on the Excess Air Coefficient during Combustion of Selected Agricultural Briquetted By-Products. Agron. Res. 2017, 15, 1084–1093. [Google Scholar]
- Díaz-Ramírez, M.; Sebastián, F.; Royo, J.; Rezeau, A. Influencing Factors on NOX Emission Level during Grate Conversion of Three Pelletized Energy Crops. Appl. Energy 2014, 115, 360–373. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Z.; Yu, C. Conversion of Char-N into NOx and N2O during Combustion of Biomass Char. Fuel 2019, 242, 389–397. [Google Scholar] [CrossRef]
- Ozgen, S.; Cernuschi, S.; Caserini, S. An Overview of Nitrogen Oxides Emissions from Biomass Combustion for Domestic Heat Production. Renew. Sustain. Energy Rev. 2021, 135, 110113. [Google Scholar] [CrossRef]
- Olave, R.J.; Forbes, E.G.A.; Johnston, C.R.; Relf, J. Particulate and Gaseous Emissions from Different Wood Fuels during Combustion in a Small-Scale Biomass Heating System. Atmos. Environ. 2017, 157, 49–58. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, R. A Comparison of Thermal Behaviors of Raw Biomass, Pyrolytic Biochar and Their Blends with Lignite. Bioresour. Technol. 2013, 146, 371–378. [Google Scholar] [CrossRef]


| Sample | Gravimetric Flow Rate of Material to the Combustion Unit | Nominal Heat Output | Nominal Combustion Efficiency | Amount of Combustion Air for 1 kg of Fuel at n = 2.1 |
|---|---|---|---|---|
| (kg h−1) | (kW) | (%) | (m3 kg−1) | |
| Spruce pellets | 5.72 | 25 | 90 | 9.58 |
| Spruce biochar | 3.62 | 25 | 90 | 15.01 |
| Rapeseed straw pellets | 6.20 | 25 | 90 | 8.56 |
| Rapeseed straw biochar | 3.94 | 25 | 90 | 14.23 |
| Sample | Ash | GCV | NCV | C | H | N | S | O | Cl |
|---|---|---|---|---|---|---|---|---|---|
| (% wt.) | (MJ kg−1) | (MJ kg−1) | (% wt.) | (% wt.) | (% wt.) | (% wt.) | (% wt.) | (% wt.) | |
| Spruce pellets | 0.55 | 18.65 | 17.49 | 51.31 | 5.32 | 0.10 | 0.03 | 42.67 | 0.02 |
| (±0.02) | (±0.01) | (±0.14) | (±0.04) | (±0.01) | (±0.001) | (±0.001) | |||
| Spruce biochar | 1.15 | 28.51 | 27.63 | 75.65 | 4.02 | 0.10 | 0.03 | 19.01 | 0.04 |
| (±0.02) | (±0.01) | (±0.24) | (±0.03) | (±0.01) | (±0.001) | (±0.001) | |||
| Rapeseed straw pellets | 5.11 | 17.25 | 16.12 | 46.24 | 5.18 | 0.76 | 0.24 | 42.24 | 0.23 |
| (±0.03) | (±0.01) | (±0.11) | (±0.05) | (±0.02) | (±0.002) | (±0.01) | |||
| Rapeseed straw biochar | 13.95 | 25.93 | 25.36 | 71.88 | 2.61 | 1.43 | 0.46 | 9.30 | 0.37 |
| (±0.04) | (±0.01) | (±0.15) | (±0.03) | (±0.03) | (±0.003) | (±0.01) |
| Sample | Ash | C | H | N | S | O | Cl |
|---|---|---|---|---|---|---|---|
| (g/MJ) | (g/MJ) | (g/MJ) | (g/MJ) | (g/MJ) | (g/MJ) | (g/MJ) | |
| Spruce pellets | 0.31 | 29.34 | 3.04 | 0.06 | 0.02 | 24.40 | 0.01 |
| Spruce biochar | 0.42 | 27.38 | 1.45 | 0.04 | 0.01 | 6.88 | 0.01 |
| Rapeseed straw pellets | 3.17 | 28.68 | 3.21 | 0.47 | 0.15 | 26.2 | 0.14 |
| Rapeseed straw biochar | 5.50 | 28.34 | 1.03 | 0.56 | 0.18 | 3.67 | 0.15 |
| Sample | CO | NOx | CO | NOx |
|---|---|---|---|---|
| (mg m−3) | (mg m−3) | (g/GJ) | (g/GJ) | |
| Spruce pellets | 1000 | 84 | 550 | 46 |
| Spruce biochar | 880 | 77 | 470 | 41 |
| Rapeseed straw pellets | 2640 | 288 | 1410 | 153 |
| Rapeseed straw biochar | 1820 | 158 | 1010 | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaťák, J.; Velebil, J.; Bradna, J.; Kučera, M.; Gendek, A.; Aniszewska, M.; Alexiou Ivanova, T. Comparative Experimental Assessment of Pollutant Emission Behavior in Combustion of Untreated and Thermally Treated Solid Biofuels from Spruce Chips and Rapeseed Straw. Atmosphere 2024, 15, 452. https://doi.org/10.3390/atmos15040452
Malaťák J, Velebil J, Bradna J, Kučera M, Gendek A, Aniszewska M, Alexiou Ivanova T. Comparative Experimental Assessment of Pollutant Emission Behavior in Combustion of Untreated and Thermally Treated Solid Biofuels from Spruce Chips and Rapeseed Straw. Atmosphere. 2024; 15(4):452. https://doi.org/10.3390/atmos15040452
Chicago/Turabian StyleMalaťák, Jan, Jan Velebil, Jiří Bradna, Marián Kučera, Arkadiusz Gendek, Monika Aniszewska, and Tatiana Alexiou Ivanova. 2024. "Comparative Experimental Assessment of Pollutant Emission Behavior in Combustion of Untreated and Thermally Treated Solid Biofuels from Spruce Chips and Rapeseed Straw" Atmosphere 15, no. 4: 452. https://doi.org/10.3390/atmos15040452
APA StyleMalaťák, J., Velebil, J., Bradna, J., Kučera, M., Gendek, A., Aniszewska, M., & Alexiou Ivanova, T. (2024). Comparative Experimental Assessment of Pollutant Emission Behavior in Combustion of Untreated and Thermally Treated Solid Biofuels from Spruce Chips and Rapeseed Straw. Atmosphere, 15(4), 452. https://doi.org/10.3390/atmos15040452







