Does Atmospheric Nitrogen Deposition Confer a Competitive Advantage to Invasive Bidens pilosa L. over Native Pterocypsela laciniata (Houtt.) Shih?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
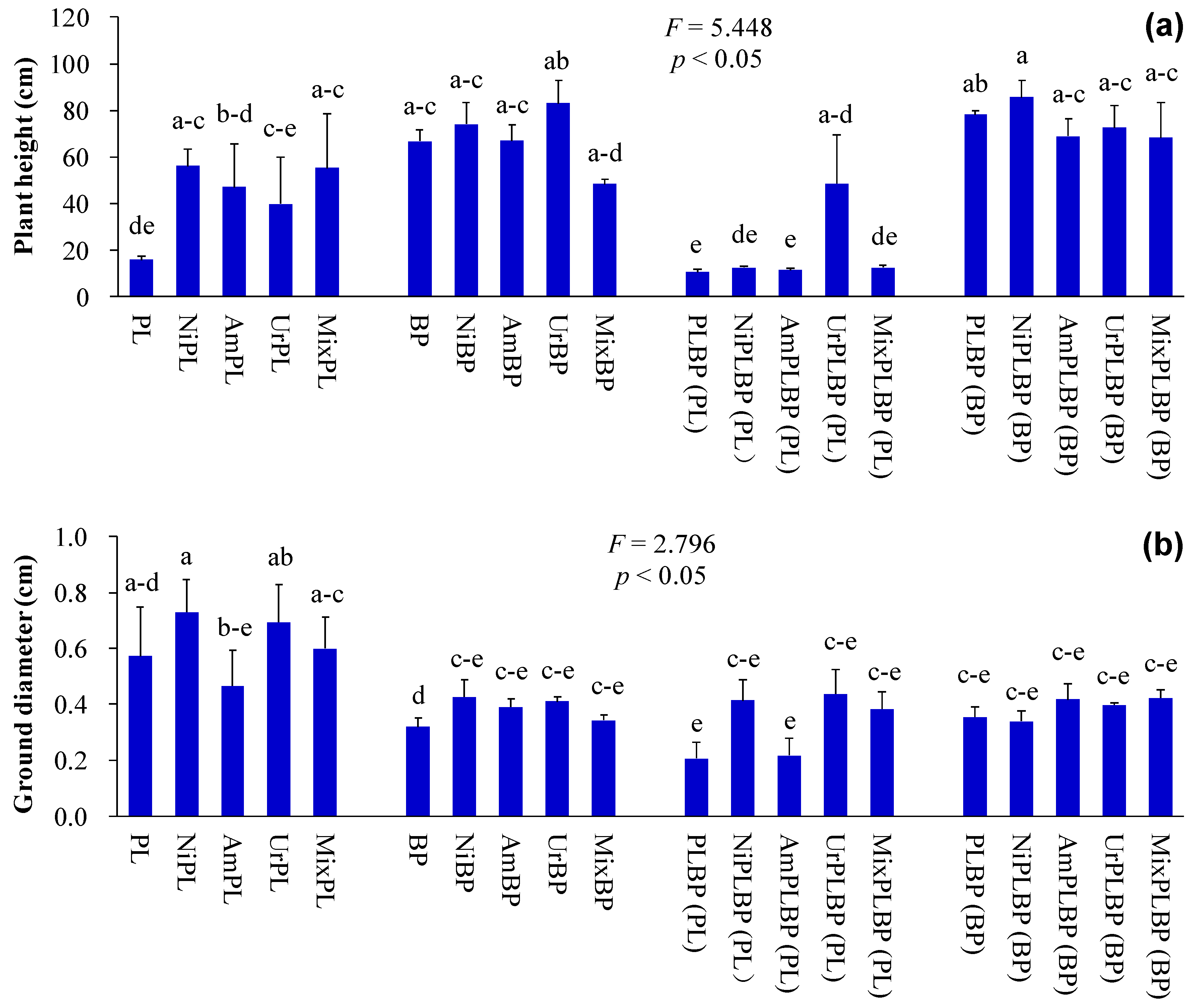
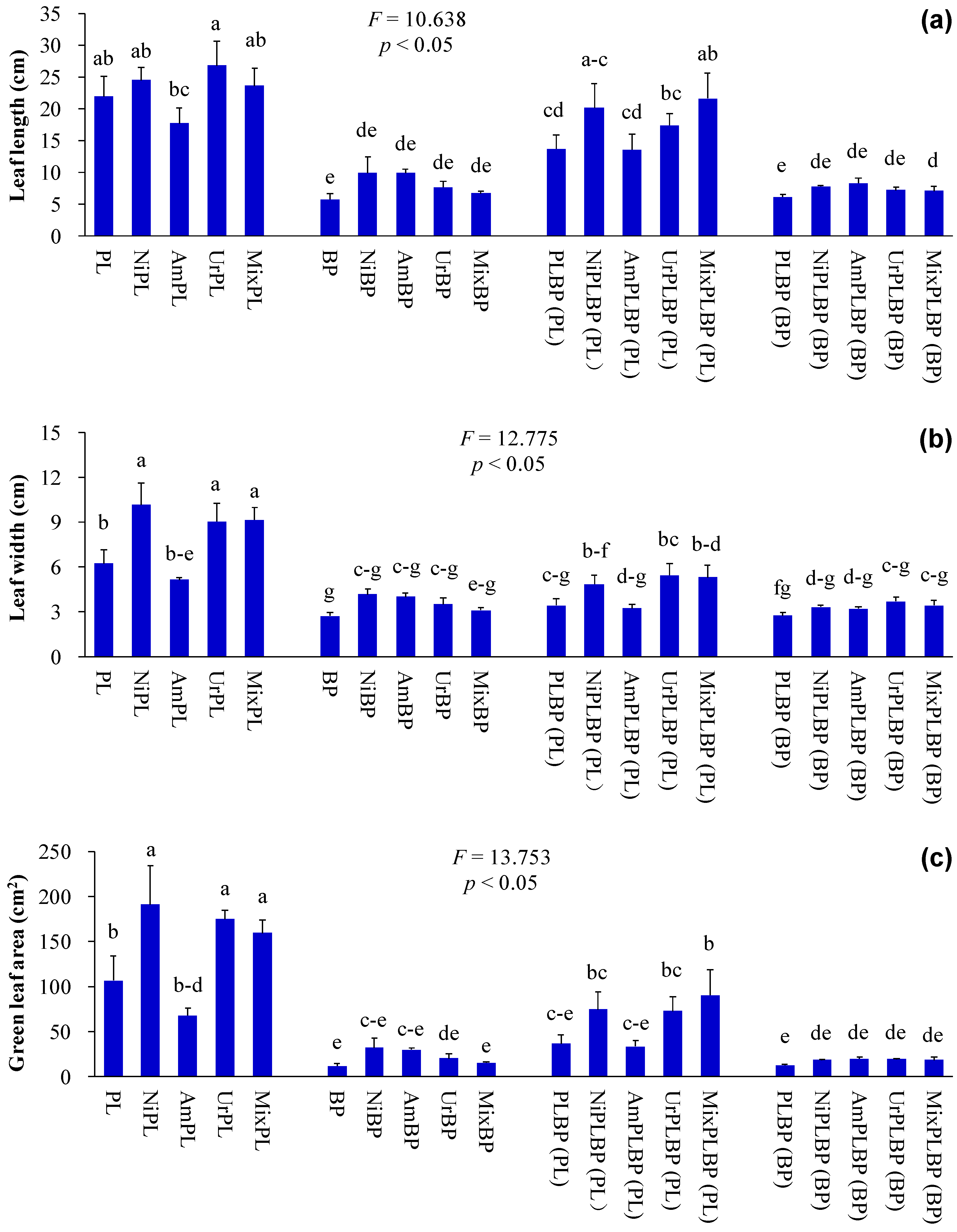
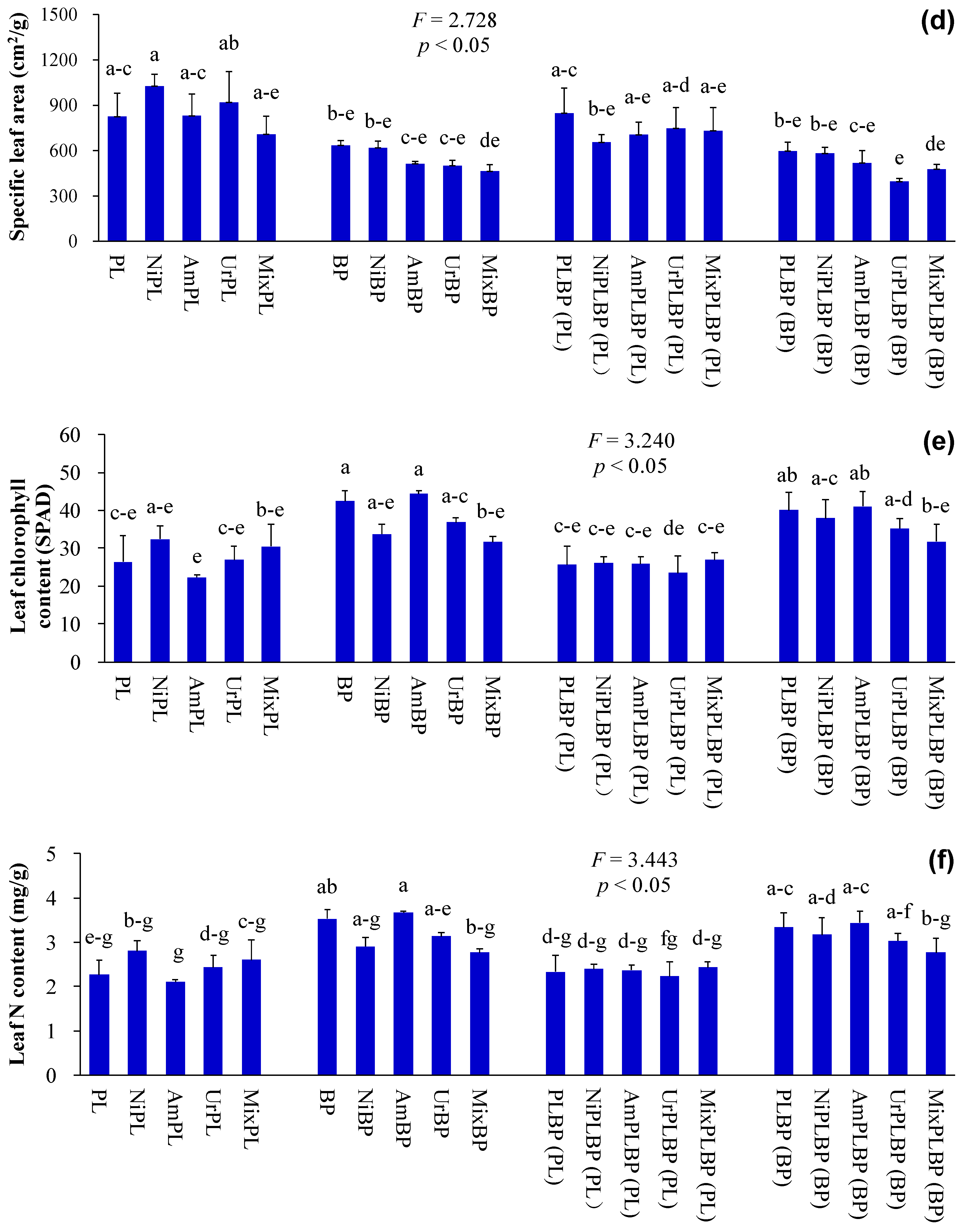
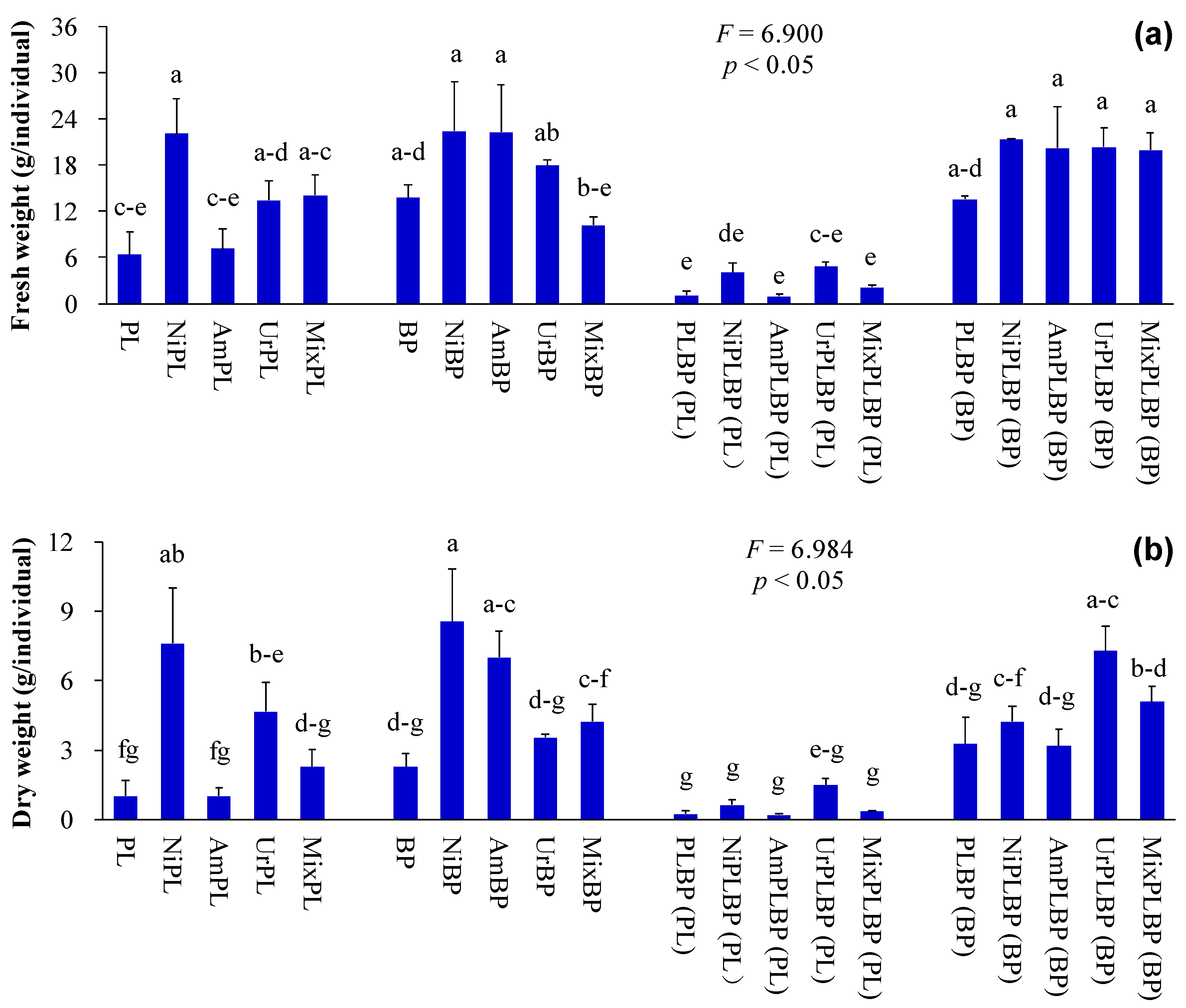
2.2. Determination of Plant Indices
2.3. Statistical Analysis
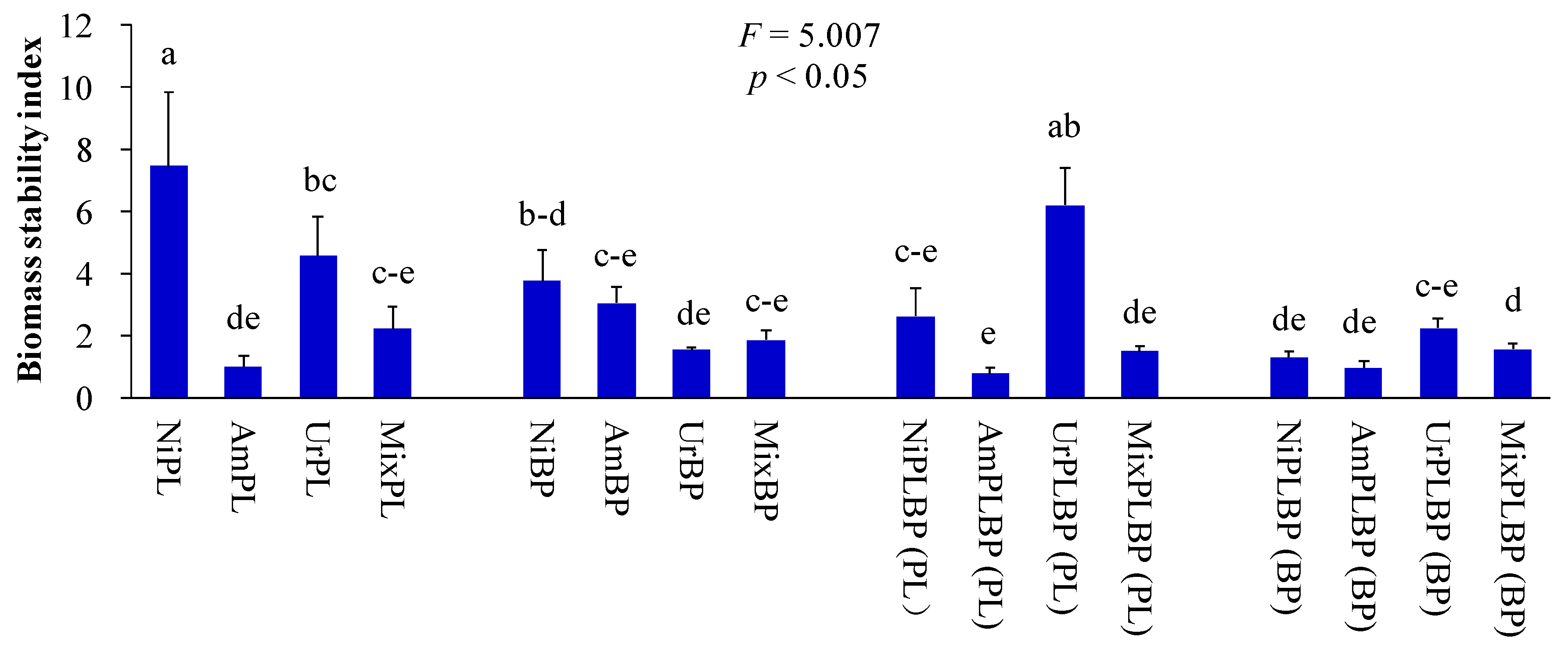
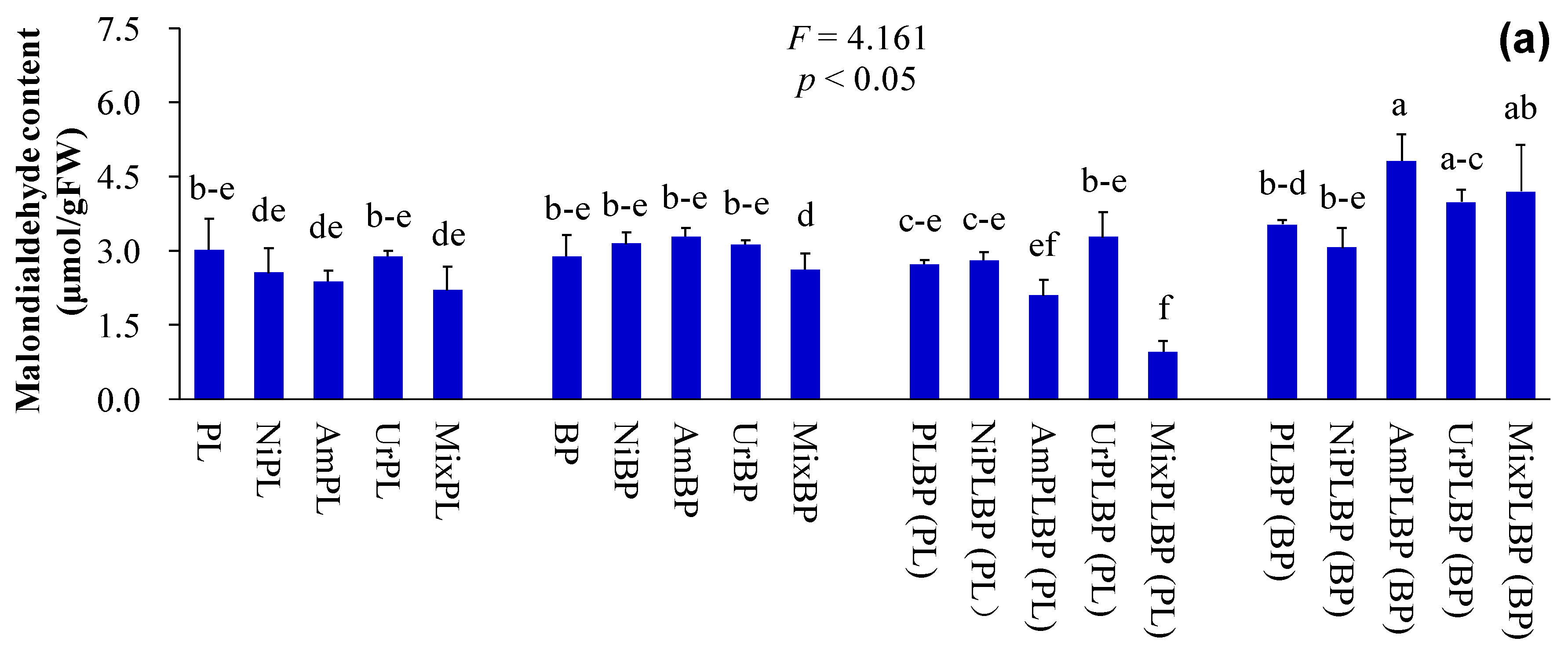
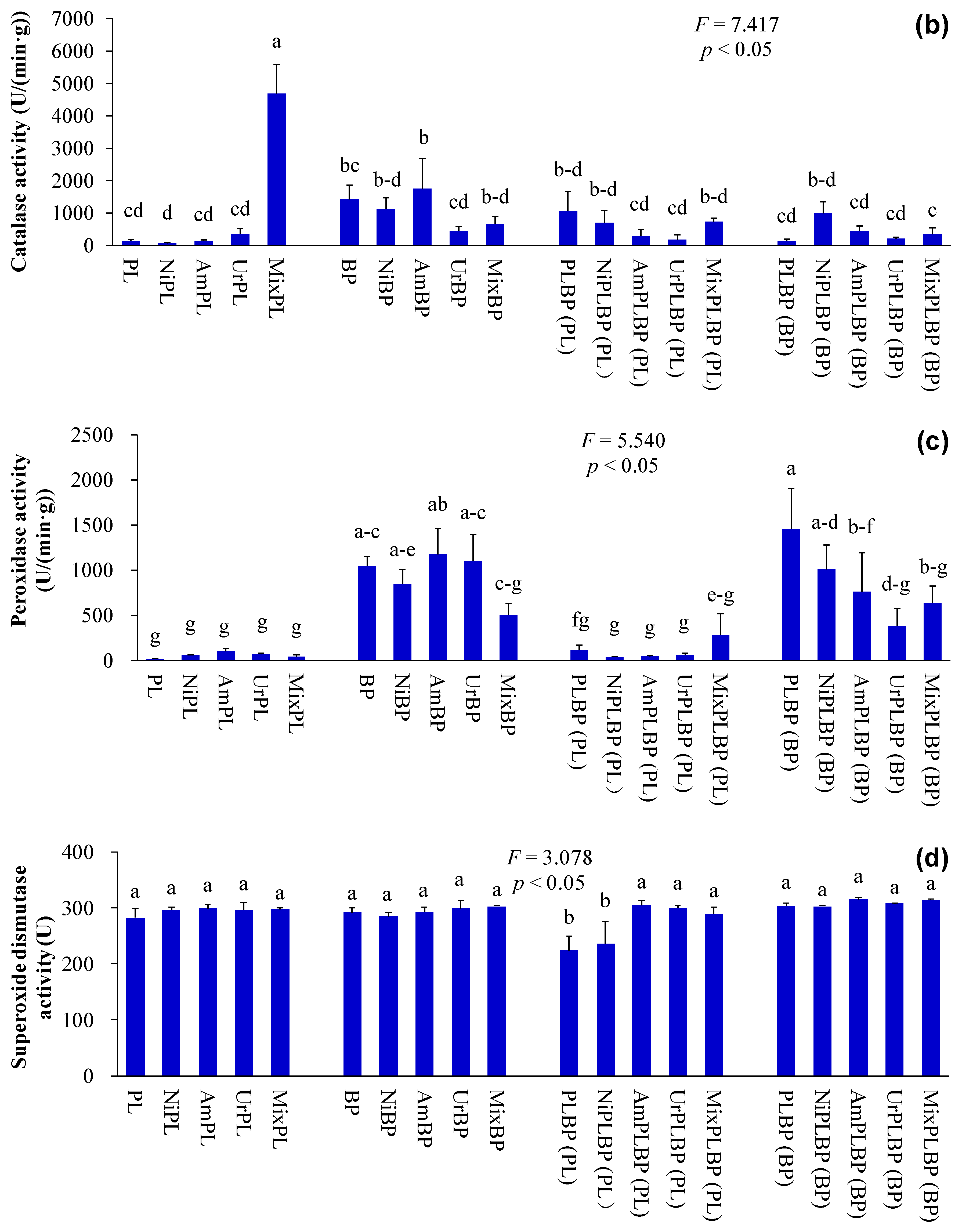
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IPs | invasive plants |
| N | nitrogen |
| PL | monocultural P. laciniata |
| NiPL | monocultural P. laciniata treated with nitrate |
| AmPL | monocultural P. laciniata treated with ammonium |
| UrPL | monocultural P. laciniata treated with urea |
| MixPL | monocultural P. laciniata treated with mixed N |
| BP | monocultural B. pilosa |
| NiBP | monocultural B. pilosa treated with nitrate |
| AmBP | monocultural B. pilosa treated with ammonium |
| UrBP | monocultural B. pilosa treated with urea |
| MixBP | monocultural B. pilosa treated with mixed N |
| PLBP(PL) | co-cultivated P. laciniata |
| NiPLBP(PL) | co-cultivated P. laciniata treated with nitrate |
| AmPLBP(PL) | co-cultivated P. laciniata treated with ammonium |
| UrPLBP(PL) | co-cultivated P. laciniata treated with urea |
| MixPLBP(PL) | co-cultivated P. laciniata treated with mixed N |
| PLBP(BP) | co-cultivated B. pilosa |
| NiPLBP(BP) | co-cultivated B. pilosa treated with nitrate |
| AmPLBP(BP) | co-cultivated B. pilosa treated with ammonium |
| UrPLBP(BP) | co-cultivated B. pilosa treated with urea |
| Mix AmPLBP(BP) | co-cultivated B. pilosa treated with mixed N |
References
- Beshai, R.A.; Truong, D.A.; Henry, A.K.; Sorte, C.J.B. Biotic resistance or invasional meltdown? Diversity reduces invasibility but not exotic dominance in southern California epibenthic communities. Biol. Invasions 2023, 25, 533–549. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, A.; Kohli, R.K.; Singh, H.P.; Batish, D.R. Bidens pilosa (Asteraceae) invasion reshapes the pattern of plant communities and edaphic properties across the north-western Himalayan landscape. Ecol. Inform. 2023, 77, 102281. [Google Scholar] [CrossRef]
- Savage, C.; Savage, K.; Keller, K.R. Effect of Carpobrotus edulis invasion history on plant communities. West. N. Am. Nat. 2023, 83, 484–497. [Google Scholar] [CrossRef]
- Ettinger, C.L.; LaForgia, M.L. Invasive plant species interact with drought to shift key functions and families in the native rhizosphere. Plant Soil 2024, 494, 567–588. [Google Scholar] [CrossRef]
- Yan, X.L.; Liu, Q.R.; Shou, H.Y.; Zeng, X.F.; Zhang, Y.; Chen, L.; Liu, Y.; Ma, H.Y.; Qi, S.Y.; Ma, J.S. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodivers. Sci. 2014, 22, 667–676. [Google Scholar]
- Wang, C.Y.; Liu, J.; Xiao, H.G.; Zhou, J.W.; Du, D.L. Floristic characteristics of alien invasive seed plant species in China. An. Acad. Bras. Ciênc. 2016, 88, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.J.; Png, G.K.; Jo, I.; McGrannachan, C.; Allen, K.; Peltzer, D.A.; D’Antonio, C.; Dickie, I.A.; French, K.; Leishman, M.R.; et al. Managing multi-species plant invasions when interactions influence their impact. Front. Ecol. Environ. 2023, 21, 370–379. [Google Scholar] [CrossRef]
- Guo, X.; Ma, J.Y.; Liu, L.L.; Li, M.Y.; Wang, H.; Sun, Y.K.; Wang, T.; Wang, K.L.; Meyerson, L.A. Effects of salt stress on interspecific competition between an invasive alien plant Oenothera biennis and three native species. Front. Plant Sci. 2023, 14, 1144511. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Dar, M.; Ahmad, M.; Singh, R.; Kumar Kohli, R.; Singh, H.P.; Batish, D.R. Invasive plants alter soil properties and nutrient dynamics: A case study of Anthemis cotula invasion in Kashmir Himalaya. Catena 2023, 226, 107069. [Google Scholar] [CrossRef]
- Minden, V.; Verhoeven, K.; Olde Venterink, H. Adaptive plasticity and fitness costs of endangered, nonendangered, and invasive plants in response to variation in nitrogen and phosphorus availabilities. Ecol. Evol. 2023, 13, e10075. [Google Scholar] [CrossRef]
- Khatri, K.; Negi, B.; Bargali, K.; Bargali, S.S. Trait variability in co-occurring invasive and native plant species in road side population of Kumaun Himalaya. Braz. J. Bot. 2022, 45, 1099–1110. [Google Scholar] [CrossRef]
- Petruzzellis, F.; Tordoni, E.; Tomasella, M.; Savi, T.; Tonet, V.; Palandrani, C.; Castello, M.; Nardini, A.; Bacaro, G. Functional differentiation of invasive and native plants along a leaf efficiency/safety trade-off. Environ. Exp. Bot. 2021, 188, 104518. [Google Scholar] [CrossRef]
- Kumar, M.; Garkoti, S.C. Functional traits, growth patterns, and litter dynamics of invasive alien and co-occurring native shrub species of chir pine forest in the central Himalaya, India. Plant Ecol. 2021, 222, 723–735. [Google Scholar] [CrossRef]
- Hibit, J.; Daehler, C.C. Plant functional, biogeographical and phylogenetic diversity are related to native and non-native plant abundance in invaded Hawaiian forests. Biol. Invasions 2024, 26, 705–717. [Google Scholar] [CrossRef]
- Grotkopp, E.; Erskine-Ogden, J.; Rejmánek, M. Assessing potential invasiveness of woody horticultural plant species using seedling growth rate traits. J. Appl. Ecol. 2010, 47, 1320–1328. [Google Scholar] [CrossRef]
- Yu, Y.L.; Cheng, H.Y.; Wang, S.; Wei, M.; Wang, C.Y.; Du, D.L. Drought may be beneficial to the competitive advantage of Amaranthus spinosus. J. Plant Ecol. 2022, 15, 494–508. [Google Scholar] [CrossRef]
- Lenda, M.; Steudel, B.; Skórka, P.; Zagrodzka, Z.B.; Moroń, D.; Bączek-Kwinta, R.; Janowiak, F.; Baran, A.; Possingham, H.P.; Knops, J.M.H. Multiple invasive species affect germination, growth, and photosynthesis of native weeds and crops in experiments. Sci. Rep. 2023, 13, 22146. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.X.; Le Roux, J.J.; Jiang, Z.Y.; Sun, F.; Peng, C.L.; Li, W.H. Soil nitrogen dynamics and competition during plant invasion: Insights from Mikania micrantha invasions in China. New Phytol. 2021, 229, 3440–3452. [Google Scholar] [CrossRef]
- Francis, C.A.; Beman, J.M.; Kuypers, M.M.M. New processes and players in the nitrogen cycle: The microbial ecology of anaerobic and archaeal ammonia oxidation. ISME J. 2007, 1, 19–27. [Google Scholar] [CrossRef]
- van den Elzen, E.; van den Berg, L.J.L.; van der Weijden, B.; Fritz, C.; Sheppard, L.J.; Lamers, L.P.M. Effects of airborne ammonium and nitrate pollution strongly differ in peat bogs, but symbiotic nitrogen fixation remains unaffected. Sci. Total Environ. 2018, 610–611, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.Y.; Jiang, S.; Li, B.; Pan, X.Y. Higher resource capture ability and utilization efficiency facilitate the successful invasion of exotic plant? A case study of Alternanthera philoxeroides. Am. J. Plant Sci. 2013, 4, 1839–1845. [Google Scholar] [CrossRef]
- Osunkoya, O.O.; Bayliss, D.; Panetta, F.D.; Vivian-Smith, G. Leaf trait co-ordination in relation to construction cost, carbon gain and resource-use efficiency in exotic invasive and native woody vine species. Ann. Bot. 2010, 106, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Lei, Y.B.; Wang, R.F.; Callaway, R.M.; Alfonso, V.B.; Inderjit; Li, Y.P.; Zheng, Y.L. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc. Natl. Acad. Sci. USA 2009, 106, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.W.; Zhang, X.Y.; Liang, J.F.; Gao, J.Q.; Xu, X.L.; Yu, F.H. High nitrogen uptake and utilization contribute to the dominance of invasive Spartina alterniflora over native Phragmites australis. Biol. Fertil. Soils 2021, 57, 1007–1013. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Kamutando, C.N.; Vikram, S.; Kamgan-Nkuekam, G.; Makhalanyane, T.P.; Greve, M.; Le Roux, J.J.; Richardson, D.M.; Cowan, D.; Valverde, A. Soil nutritional status and biogeography influence rhizosphere microbial communities associated with the invasive tree Acacia dealbata. Sci. Rep. 2017, 7, 6472. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.W.; Yang, M.Z.; Chen, Y.J.; Chen, L.M.; Zhang, D.Z.; Mei, L.; Shi, Y.T.; Zhang, H.B. Changes in non-symbiotic nitrogen-fixing bacteria inhabiting rhizosphere soils of an invasive plant Ageratina adenophora. Appl. Soil Ecol. 2012, 54, 32–38. [Google Scholar] [CrossRef]
- Li, J.; He, J.Z.; Liu, M.; Yan, Z.Q.; Xu, X.L.; Kuzyakov, Y. Invasive plant competitivity is mediated by nitrogen use strategies and rhizosphere microbiome. Soil Biol. Biochem. 2024, 192, 109361. [Google Scholar] [CrossRef]
- Chen, H.Y.; Huang, S.Z. Composition and supply of inorganic and organic nitrogen species in dry and wet atmospheric deposition: Use of organic nitrogen composition to calculate the Ocean’s external nitrogen flux from the atmosphere. Cont. Shelf Res. 2021, 213, 104316. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Zhang, F.; Zhang, X.; Xu, W.; Liu, X.; Li, Y.; Wang, Z.; Xie, Y. Enhanced nitrous oxide emissions caused by atmospheric nitrogen deposition in agroecosystems over China. Environ. Sci. Pollut. Res. 2021, 28, 15350–15360. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, L.; Zhang, F.; Zhang, X.; Xu, W.; Liu, X.; Wang, Z.; Xie, Y. Soil Nitrous Oxide Emissions by Atmospheric Nitrogen Deposition over Global Agricultural Systems. Environ. Sci. Technol. 2021, 55, 4420–4429. [Google Scholar] [CrossRef] [PubMed]
- Dentener, F.; Drevet, J.; Lamarque, J.F.; Bey, I.; Eickhout, B.; Fiore, A.M.; Hauglustaine, D.; Horowitz, L.W.; Krol, M.; Kulshrestha, U.C.; et al. Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Glob. Biogeochem. Cycles 2006, 20, GB4003. [Google Scholar] [CrossRef]
- Fu, Y.D.; Xu, W.; Wen, Z.; Han, M.J.; Sun, J.H.; Tang, A.H.; Liu, X. Enhanced atmospheric nitrogen deposition at a rural site in northwest China from 2011 to 2018. Atmos. Res. 2020, 245, 105071. [Google Scholar] [CrossRef]
- Zhu, J.X.; Chen, Z.; Wang, Q.F.; Xu, L.; He, N.P.; Jia, Y.L.; Zhang, Q.Y.; Yu, G.R. Potential transition in the effects of atmospheric nitrogen deposition in China. Environ. Pollut. 2020, 258, 113739. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, L.; Liu, X.J. A database of atmospheric nitrogen concentration and deposition from the nationwide monitoring network in China. Sci. Data 2019, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.A.; Braswell, B.H.; Sulzman, J.; Lamarque, J.F. Nitrogen deposition onto the United States and Western Europe: Synthesis of observations and models. Ecol. Appl. 2005, 15, 38–57. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Zhang, X.; Dobermann, A.; Heffer, P.; Zhou, F. Global nitrogen deposition inputs to cropland at national scale from 1961 to 2020. Sci. Data 2023, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Ussher, S.J.; Fitzsimons, M.F.; Atkinson, S.; Woodward, E.M.S.; Yang, M.; Bell, T.G. Inorganic nitrogen and phosphorus in Western European aerosol and the significance of dry deposition flux into stratified shelf waters. Atmos. Environ. 2021, 261, 118391. [Google Scholar] [CrossRef]
- Ren, G.Q.; Yang, B.; Cui, M.M.; Dai, Z.C.; Xiang, Y.; Zhang, H.Y.; Li, G.L.; Li, J.; Javed, Q.; Du, D.L. Warming and elevated nitrogen deposition accelerate the invasion process of Solidago canadensis L. Ecol. Process. 2022, 11, 62. [Google Scholar] [CrossRef]
- Ding, W.L.; Xu, W.Z.; Gao, Z.J.; Xu, B.C. Effects of water and nitrogen on growth and relative competitive ability of introduced versus native C-4 grass species in the semi-arid Loess Plateau of China. J. Arid Land 2021, 13, 730–743. [Google Scholar] [CrossRef]
- Zhong, S.S.; Xu, Z.L.; Yu, Y.L.; Liu, J.; Wang, Y.Y.; Guo, E.; Wang, C.Y. Rhus typhina decreased soil nitrogen contents and peroxidase activity following the addition of nitrogen. Int. J. Environ. Sci. Technol. 2023, 111, 17–22. [Google Scholar] [CrossRef]
- Sparrius, L.B.; Kooijman, A.M. Invasiveness of Campylopus introflexus in drift sands depends on nitrogen deposition and soil organic matter. Appl. Veg. Sci. 2011, 14, 221–229. [Google Scholar] [CrossRef]
- Mircea, D.M.; Calone, R.; Estrelles, E.; Soriano, P.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Responses of different invasive and non-invasive ornamental plants to water stress during seed germination and vegetative growth. Sci. Rep. 2023, 13, 13281. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.L.; He, Q.S.; Xie, R.Q.; Hou, J.H.; Shi, C.L.; Li, J.M.; Yu, F.H. Interactive effects of nutrient availability, fluctuating supply, and plant parasitism on the post-invasion success of Bidens pilosa. Biol. Invasions 2021, 23, 3035–3046. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Zhong, S.S.; Xu, Z.L.; Xu, Z.Y.; Zhu, M.W.; Wei, Y.Q.; Wang, C.Y.; Du, D.L. Do the leaves of multiple invasive plants decompose more easily than a native plant’s under nitrogen deposition with different forms? Nitrogen 2024, 5, 202–218. [Google Scholar] [CrossRef]
- Zhejiang Provincial Bureau of Statistics. Zhenjiang Statistical Yearbook 2022; China Statistics Press: Beijing, China, 2022.
- Luo, X.S.; Liu, X.J.; Pan, Y.P.; Wen, Z.; Xu, W.; Zhang, L.; Kou, C.L.; Lv, J.L.; Goulding, K. Atmospheric reactive nitrogen concentration and deposition trends from 2011 to 2018 at an urban site in north China. Atmos. Environ. 2020, 224, 117298. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, L.; Liu, X.J.; Li, W.Q.; Lu, S.H.; Zheng, L.X.; Bai, Z.C.; Cai, G.Y.; Zhang, F.S. Atmospheric organic nitrogen deposition in China. Atmos. Environ. 2012, 49, 422. [Google Scholar] [CrossRef]
- Cornell, S.E. Atmospheric nitrogen deposition: Revisiting the question of the importance of the organic component. Environ. Pollut. 2011, 159, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Cornell, S.E.; Jickells, T.D.; Cape, J.N.; Rowland, A.P.; Duce, R.A. Organic nitrogen deposition on land and coastal environments: A review of methods and data. Atmos. Environ. 2003, 37, 2173–2191. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.D.; Zhang, H.S.; Jiang, K.; Zhou, J.W.; Wang, C.Y. Erigeron canadensis affects the taxonomic and functional diversity of plant communities in two climate zones in the North of China. Ecol. Res. 2019, 34, 535–547. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cheng, H.Y.; Wu, B.D.; Jiang, K.; Wang, S.; Wei, M.; Du, D.L. The functional diversity of native ecosystems increases during the major invasion by the invasive alien species, Conyza canadensis. Ecol. Eng. 2021, 159, 106093. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, B.D.; Jiang, K.; Zhou, J.W. Differences in functional traits between invasive and native Amaranthus species under simulated acid deposition with a gradient of pH levels. Acta Oecol. 2018, 89, 32–37. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Zhong, S.S.; Xu, Z.L.; Liu, J.; Xu, Z.Y.; Zhu, M.W.; Wang, C.Y.; Du, D.L. Is the invasive plant Amaranthus spinosus L. more competitive than the native Plant A. tricolor L. when exposed to acid deposition with different sulfur-nitrogen ratios? Atmosphere 2024, 15, 29. [Google Scholar] [CrossRef]
- Ordonez, A.; Wright, I.J.; Olff, H. Functional differences between native and alien species: A global-scale comparison. Funct. Ecol. 2010, 24, 1353–1361. [Google Scholar] [CrossRef]
- Maire, V.; Gross, N.; Boerger, L.; Proulx, R.; Wirth, C.; Pontes, L.d.S.; Soussana, J.-F.; Louault, F. Habitat filtering and niche differentiation jointly explain species relative abundance within grassland communities along fertility and disturbance gradients. New Phytol. 2012, 196, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.; Boerger, L.; Duncan, R.P.; Hulme, P.E. Functional differences between alien and native species: Do biotic interactions determine the functional structure of highly invaded grasslands? Funct. Ecol. 2013, 27, 1262–1272. [Google Scholar] [CrossRef]
- Silveira, S.D.; Guimaraes, M. The enemy within: Consequences of the invasive bullfrog on native anuran populations. Biol. Invasions 2021, 23, 373–378. [Google Scholar] [CrossRef]
- Pinto-Ledezma, J.N.; Villalobos, F.; Reich, P.B.; Catford, J.A.; Larkin, D.J.; Cavender-Bares, J. Testing Darwin’s naturalization conundrum based on taxonomic, phylogenetic, and functional dimensions of vascular plants. Ecol. Monogr. 2020, 90, e01420. [Google Scholar] [CrossRef]
- Thuiller, W.; Gallien, L.; Boulangeat, I.; De Bello, F.; Münkemüller, T.; Roquet, C.; Lavergne, S. Resolving Darwin’s naturalization conundrum: A quest for evidence. Divers. Distrib. 2010, 16, 461–475. [Google Scholar] [CrossRef]
- Fan, S.Y.; Yang, Q.; Li, S.P.; Fristoe, T.S.; Cadotte, M.W.; Essl, F.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; et al. A latitudinal gradient in Darwin’s naturalization conundrum at the global scale for flowering plants. Nat. Commun. 2023, 14, 6244. [Google Scholar] [CrossRef]
- Omer, A.; Fristoe, T.; Yang, Q.; Razanajatovo, M.; Weigelt, P.; Kreft, H.; Dawson, W.; Dullinger, S.; Essl, F.; Pergl, J.; et al. The role of phylogenetic relatedness on alien plant success depends on the stage of invasion. Nat. Plants 2022, 8, 906–914. [Google Scholar] [CrossRef]
- Richards, C.L.; Bossdorf, O.; Muth, N.Z.; Gurevitch, J.; Pigliucci, M. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 2006, 9, 981–993. [Google Scholar] [CrossRef]
- Funk, J.L. Differences in plasticity between invasive and native plants from a low resource environment. J. Ecol. 2008, 96, 1162–1173. [Google Scholar] [CrossRef]
- Matzek, V. Trait values, not trait plasticity, best explain invasive species’ performance in a changing environment. PLoS ONE 2012, 7, e48821. [Google Scholar] [CrossRef] [PubMed]
- Amanullah; Marwat, K.B.; Shah, P.; Maula, N.; Arifullah, S. Nitrogen levels and its time of application influence leaf area, height and biomass of maize planted at low and high density. Pak. J. Bot. 2009, 41, 761–768. [Google Scholar]
- Cheng, H.Y.; Wei, M.; Wang, S.; Wu, B.D.; Wang, C.Y. Atmospheric N deposition alleviates the unfavorable effects of drought on wheat growth. Braz. J. Bot. 2020, 43, 229–238. [Google Scholar] [CrossRef]
- Bai, T.; Liu, Y.Y.; Muhammad, I.; Yang, X.; Yin, X.J.; Bai, L.; Wang, Y.J. Mixed nitrogen form addition facilitates the growth adaptation of legume plant to heavy metal contamination in degraded mining areas. Glob. Ecol. Conserv. 2020, 24, e01387. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Peñuelas, J.L.; Nicolás-Peragón, J.L.; Benito, L.F.; Domínguez-Lerena, S. Is nitrogen fertilization in the nursery a suitable tool for enhancing the performance of Mediterranean oak plantations? New For. 2013, 44, 733–751. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Xu, H.; Fan, Z.W.; Hou, Y.P. Effects of Rhus typhina invasion into young Pinus thunbergii forests on soil chemical properties. Ecol. Environ. Sci. 2013, 22, 1119–1123. [Google Scholar]
- Hou, Y.P.; Liu, L.; Chu, H.; Ma, S.J.; Zhao, D.; Liang, R.R. Effects of exotic plant Rhus typhina invasion on soil properties in different forest types. Acta Ecol. Sin. 2015, 35, 5324–5330. [Google Scholar]
- Huangfu, C.H.; Li, H.Y.; Chen, X.W.; Liu, H.M.; Wang, H.; Yang, D.L. Response of an invasive plant, Flaveria bidentis, to nitrogen addition: A test of form-preference uptake. Biol. Invasions 2016, 18, 3365–3380. [Google Scholar] [CrossRef]
- Chen, S.L.; Chen, B.; Wang, S.Q.; Sun, L.G.; Shi, H.; Liu, Z.H.; Wang, Q.Y.; Li, H.; Zhu, T.T.; Li, D.H.; et al. Spatiotemporal variations of atmospheric nitrogen deposition in China during 2008–2020. Atmos. Environ. 2023, 315, 120120. [Google Scholar] [CrossRef]
- Dong, J.; Li, B.; Li, Y.; Zhou, R.; Gan, C.; Zhao, Y.; Liu, R.; Yang, Y.; Wang, T.; Liao, H. Atmospheric ammonia in China: Long-term spatiotemporal variation, urban-rural gradient, and influencing factors. Sci. Total Environ. 2023, 883, 163733. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xing, C.; Li, Q.; Wang, S.; Hu, Q.; Zhu, Y.; Liu, T.; Zhang, C.; Liu, C. Long-term spatiotemporal variations of ammonia in the Yangtze River Delta region of China and its driving factors. J. Environ. Sci. 2025, 150, 202–217. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Burns, D.A.; Driscoll, C.T.; Frey, S.D.; Lovett, G.M.; Watmough, S.A. Decreased atmospheric nitrogen deposition in eastern North America: Predicted responses of forest ecosystems. Environ. Pollut. 2019, 244, 560–574. [Google Scholar] [CrossRef]
- Conrad-Rooney, E.; Gewirtzman, J.; Pappas, Y.; Pasquarella, V.J.; Hutyra, L.R.; Templer, P.H. Atmospheric wet deposition in urban and suburban sites across the United States. Atmos. Environ. 2023, 305, 119783. [Google Scholar] [CrossRef]
- Johansen, A.M.; Duncan, C.; Reddy, A.; Swain, N.; Sorey, M.; Nieber, A.; Agren, J.; Lenington, M.; Bolstad, D.; Samora, B.; et al. Precipitation chemistry and deposition at a high-elevation site in the Pacific Northwest United States (1989–2015). Atmos. Environ. 2019, 212, 221–230. [Google Scholar] [CrossRef]
- Wang, S.; Wei, M.; Cheng, H.Y.; Wu, B.D.; Du, D.L.; Wang, C.Y. Indigenous plant species and invasive alien species tend to diverge functionally under heavy metal pollution and drought stress. Ecotox. Environ. Safe. 2020, 205, 111160. [Google Scholar] [CrossRef]
- Jiang, K.; Wu, B.D.; Wang, C.Y.; Ran, Q. Ecotoxicological effects of metals with different concentrations and types on the morphological and physiological performance of wheat. Ecotox. Environ. Safe. 2019, 167, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Rybiński, W.; Garczyński, S. Influence of laser light on leaf area and parameters of photosynthetic activity in DH lines of spring barley (Hordeum vulgare L.). Int. Agrophys. 2004, 18, 261–267. [Google Scholar]
- Xia, T.T.; Miao, Y.X.; Wu, D.L.; Shao, H.; Khosla, R.; Mi, G.H. Active optical sensing of spring maize for in-season diagnosis of nitrogen status based on nitrogen nutrition index. Remote Sens. 2016, 8, 605. [Google Scholar] [CrossRef]
- Huang, S.S.; Sun, L.Q.; Hu, X.; Wang, Y.H.; Zhang, Y.J.; Nevo, E.; Peng, J.H.; Sun, D.F. Associations of canopy leaf traits with SNP markers in durum wheat (Triticum turgidum L. durum (Desf.)). PLoS ONE 2018, 13, e0206226. [Google Scholar] [CrossRef] [PubMed]
- Anwar, J.; Subhani, G.M.; Hussain, M.; Ahmad, J.; Hussain, M.; Munir, M. Drought tolerance indices and their correlation with yield in exotic wheat genotypes. Pak. J. Bot. 2011, 43, 1527–1530. [Google Scholar]
- Farshadfar, E.; Elyasi, P. Screening quantitative indicators of drought tolerance in bread wheat (Triticum aestivum L.) landraces. Eur. J. Exp. Biol. 2012, 2, 577–584. [Google Scholar]
- Sánchez-Reinoso, A.D.; Ligarreto-Moreno, G.; Restrepo-Díaz, H. Evaluation of drought indices to identify tolerant genotypes in common bean bush (Phaseolus vulgaris L.). J. Integr. Agric. 2020, 19, 99–107. [Google Scholar] [CrossRef]
- Darkwa, K.; Ambachew, D.; Mohammed, H.; Asfaw, A.; Blair, M.W. Evaluation of common bean (Phaseolus vulgaris L.) genotypes for drought stress adaptation in Ethiopia. Crop J. 2016, 4, 367–376. [Google Scholar] [CrossRef]
- Niu, H.B.; Liu, W.X.; Wan, F.H.; Liu, B. An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: Altered soil microbial communities facilitate the invader and inhibit natives. Plant Soil 2007, 294, 73–85. [Google Scholar] [CrossRef]
- Ding, W.; Wang, R.; Yuan, Y.; Liang, X.; Liu, J. Effects of nitrogen deposition on growth and relationship of Robinia pseudoacacia and Quercus acutissima seedlings. Dendrobiology 2012, 67, 3–13. [Google Scholar]
- Yuan, Y.F.; Guo, W.H.; Ding, W.J.; Du, N.; Luo, Y.J.; Liu, J.; Xu, F.; Wang, R.Q. Competitive interaction between the exotic plant Rhus typhina L. and the native tree Quercus acutissima Carr. in Northern China under different soil N:P ratios. Plant Soil 2013, 372, 389–400. [Google Scholar] [CrossRef]
- Li, L. Experimental Guidance of Plant Physiology Module, 1st ed.; Science Press: Beijing, China, 2009. [Google Scholar]
- Zhang, J.E. Experimental Methods and Techniques Commonly Used in Ecology; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, Q.X.; Tian, Y.; Meng, F.J. Physiological and proteomic analyses of the drought stress response in Amygdalus Mira (Koehne) Yü et Lu roots. BMC Plant Biol. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, Y.; Liu, Y.; Zhong, S.; Zhang, H.; Xu, Z.; Xu, Z.; Du, D.; Wang, C. Does Atmospheric Nitrogen Deposition Confer a Competitive Advantage to Invasive Bidens pilosa L. over Native Pterocypsela laciniata (Houtt.) Shih? Atmosphere 2024, 15, 825. https://doi.org/10.3390/atmos15070825
Li C, Li Y, Liu Y, Zhong S, Zhang H, Xu Z, Xu Z, Du D, Wang C. Does Atmospheric Nitrogen Deposition Confer a Competitive Advantage to Invasive Bidens pilosa L. over Native Pterocypsela laciniata (Houtt.) Shih? Atmosphere. 2024; 15(7):825. https://doi.org/10.3390/atmos15070825
Chicago/Turabian StyleLi, Chuang, Yue Li, Yingsheng Liu, Shanshan Zhong, Huanshi Zhang, Zhelun Xu, Zhongyi Xu, Daolin Du, and Congyan Wang. 2024. "Does Atmospheric Nitrogen Deposition Confer a Competitive Advantage to Invasive Bidens pilosa L. over Native Pterocypsela laciniata (Houtt.) Shih?" Atmosphere 15, no. 7: 825. https://doi.org/10.3390/atmos15070825





