Oxidation of Aminoacetaldehyde Initiated by the OH Radical: A Theoretical Mechanistic and Kinetic Study
Abstract
:1. Introduction
2. Methods
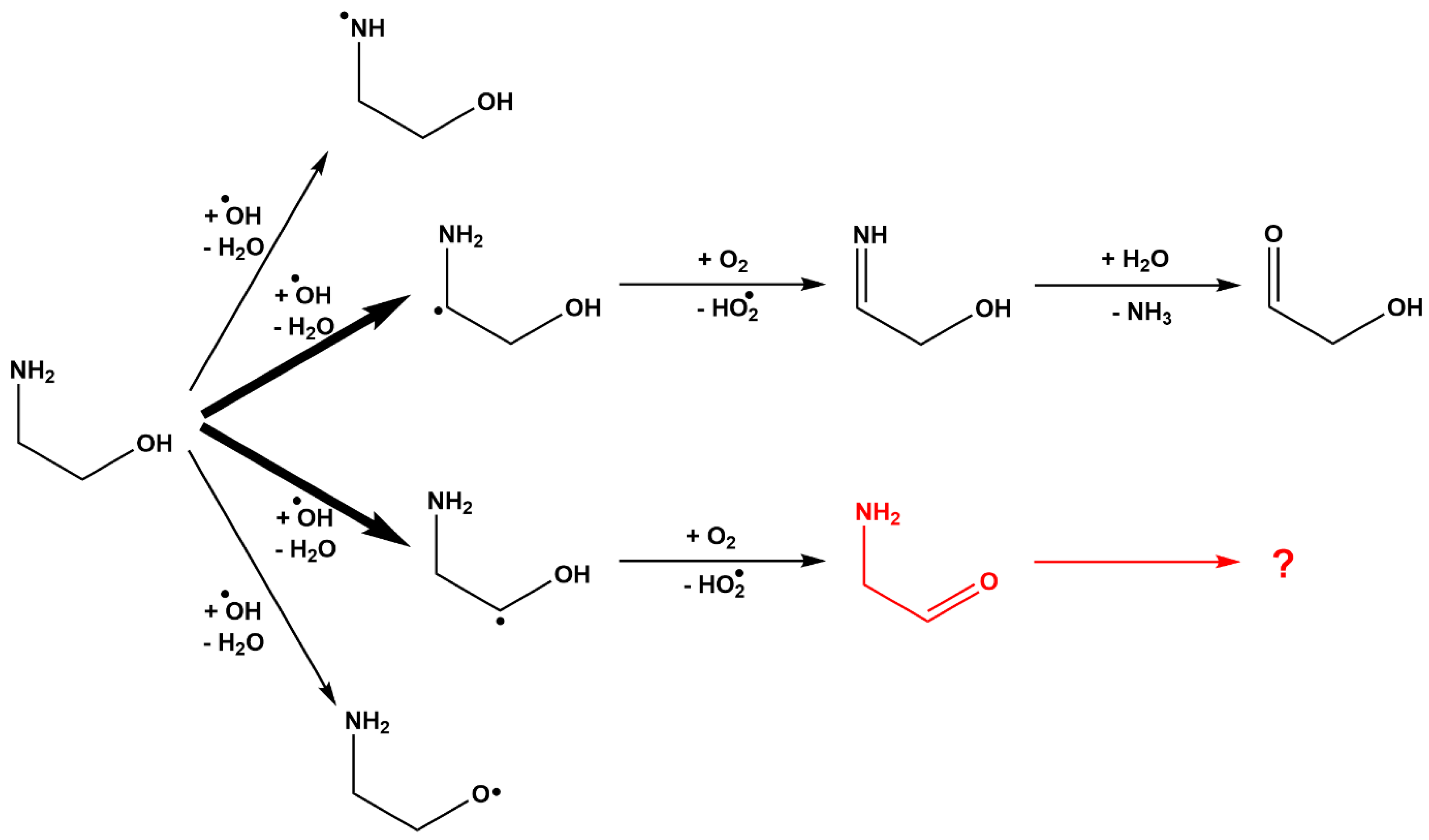
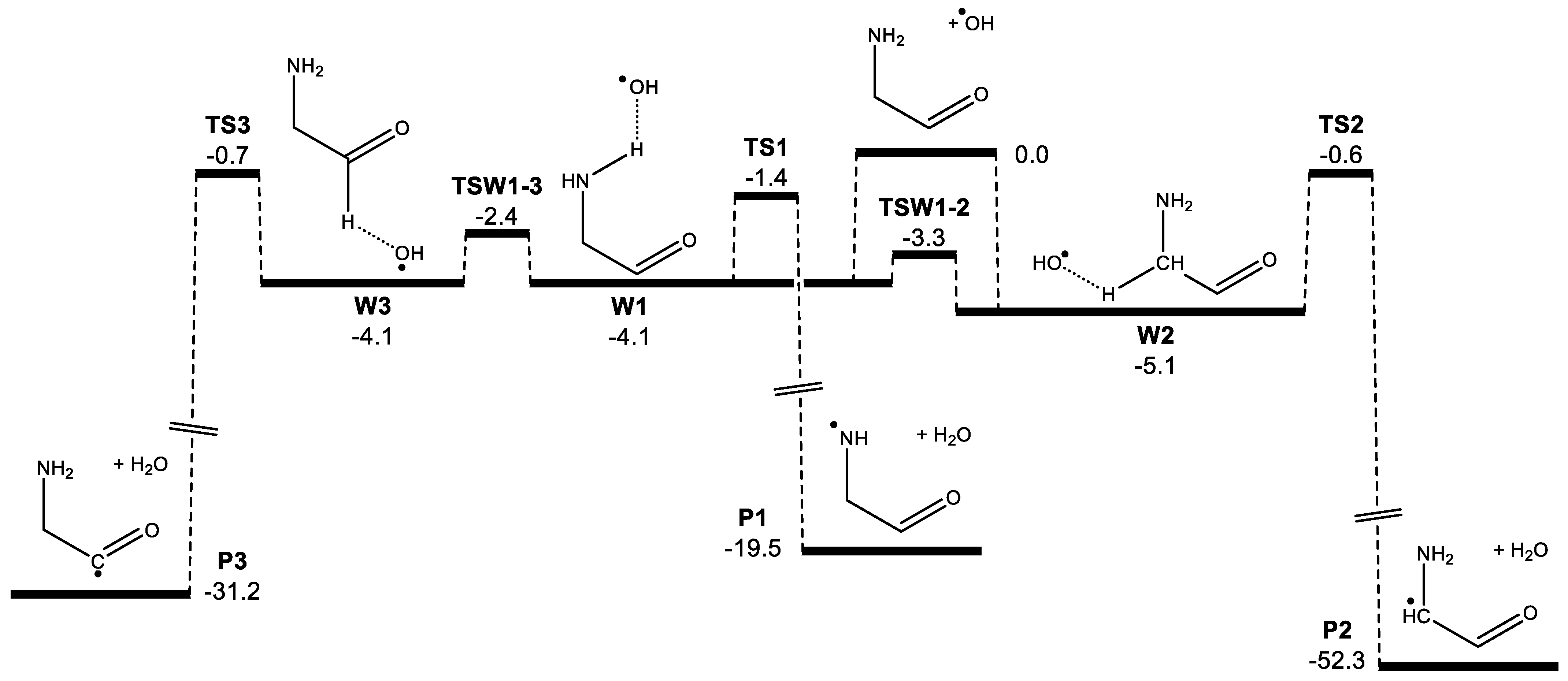
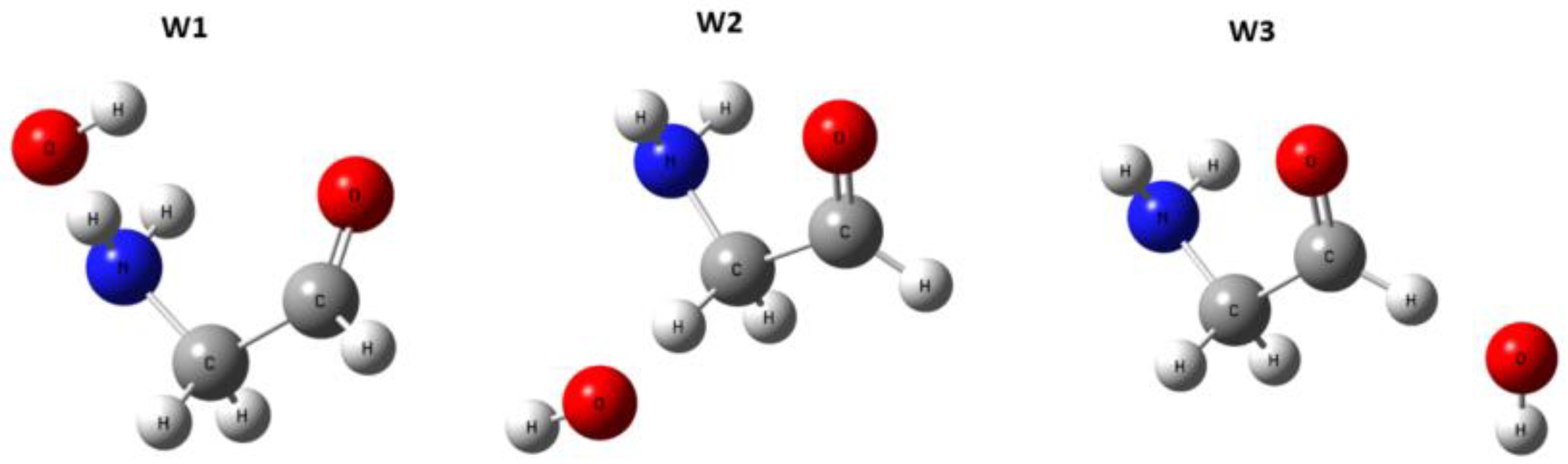
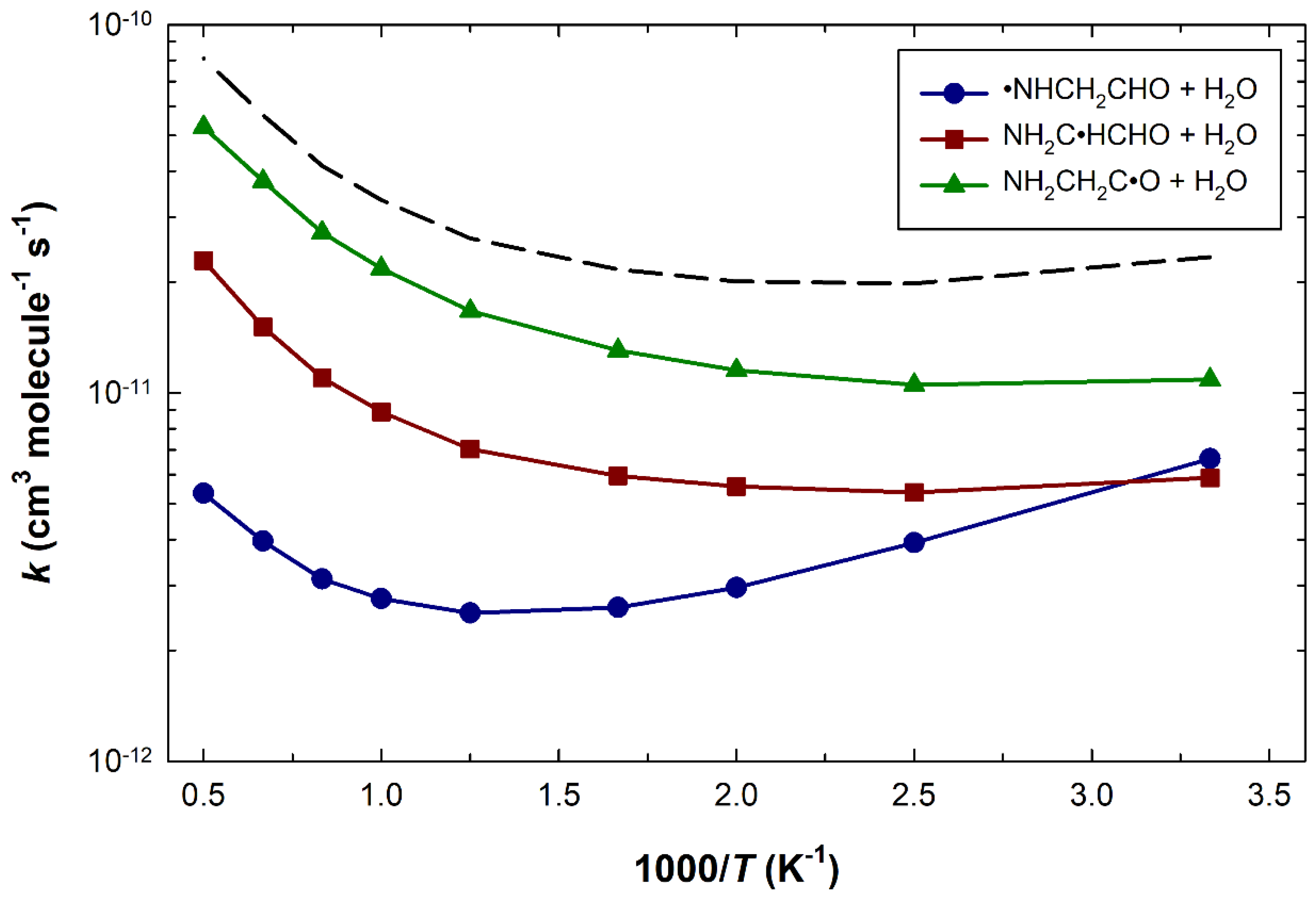
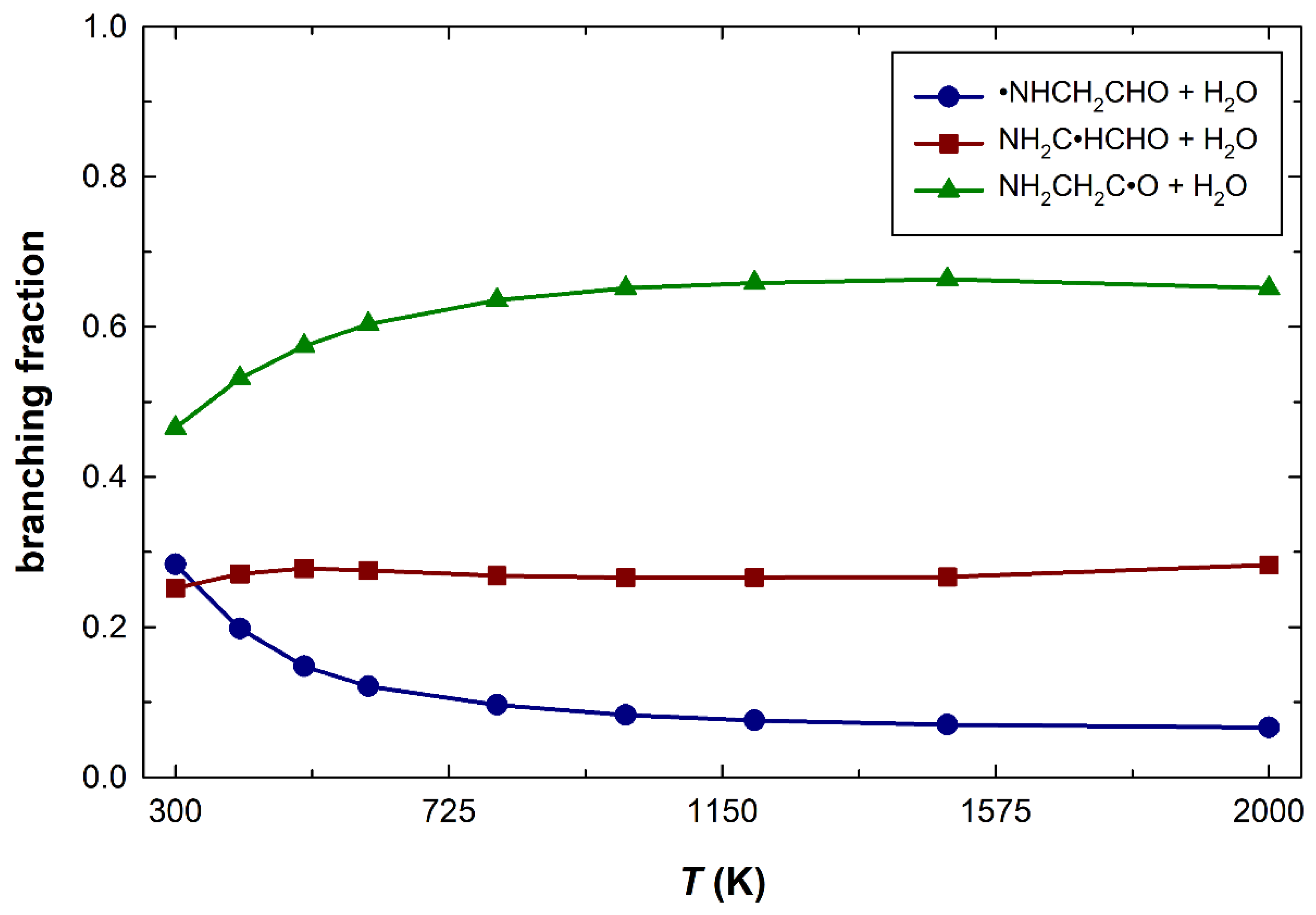
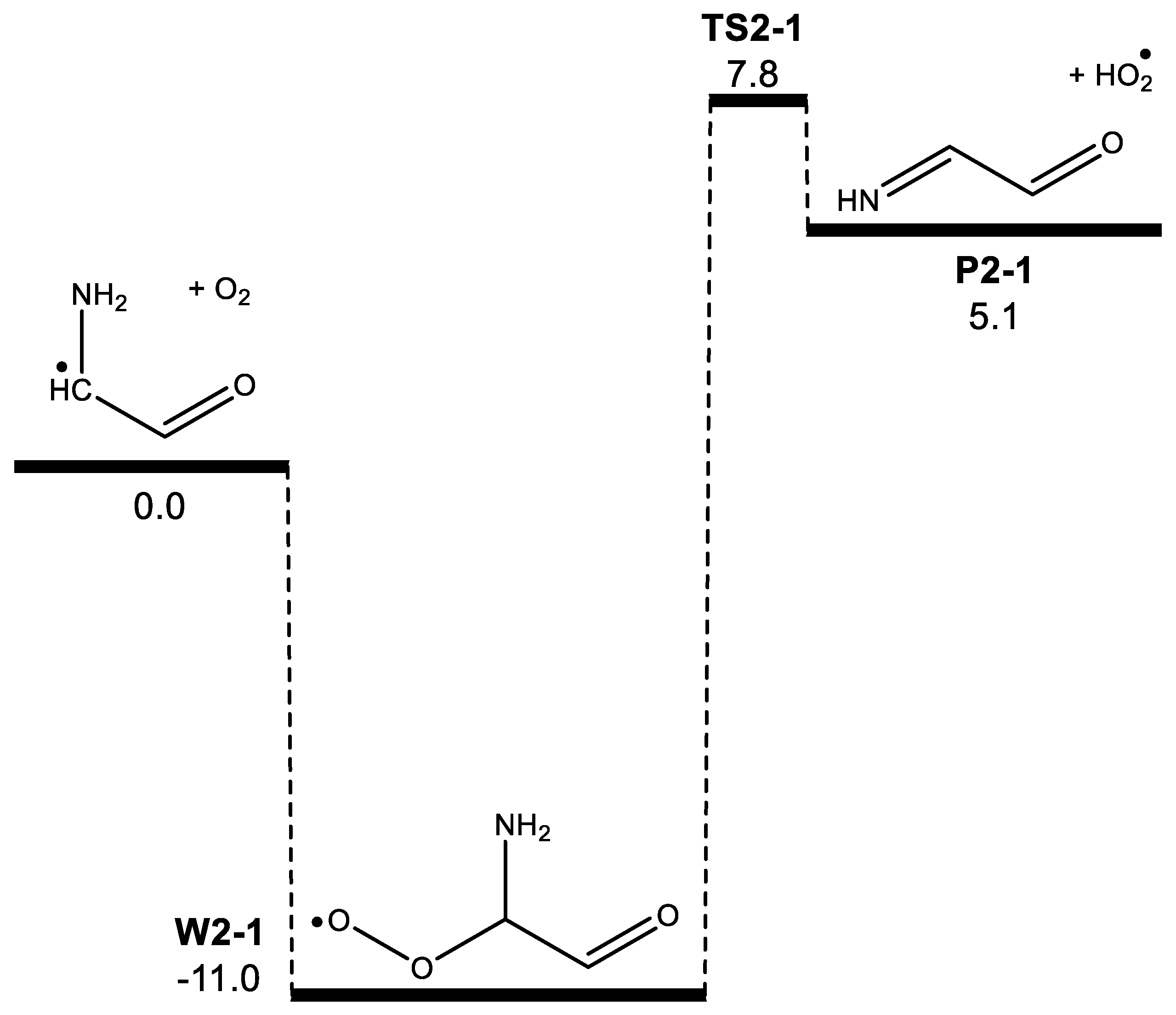
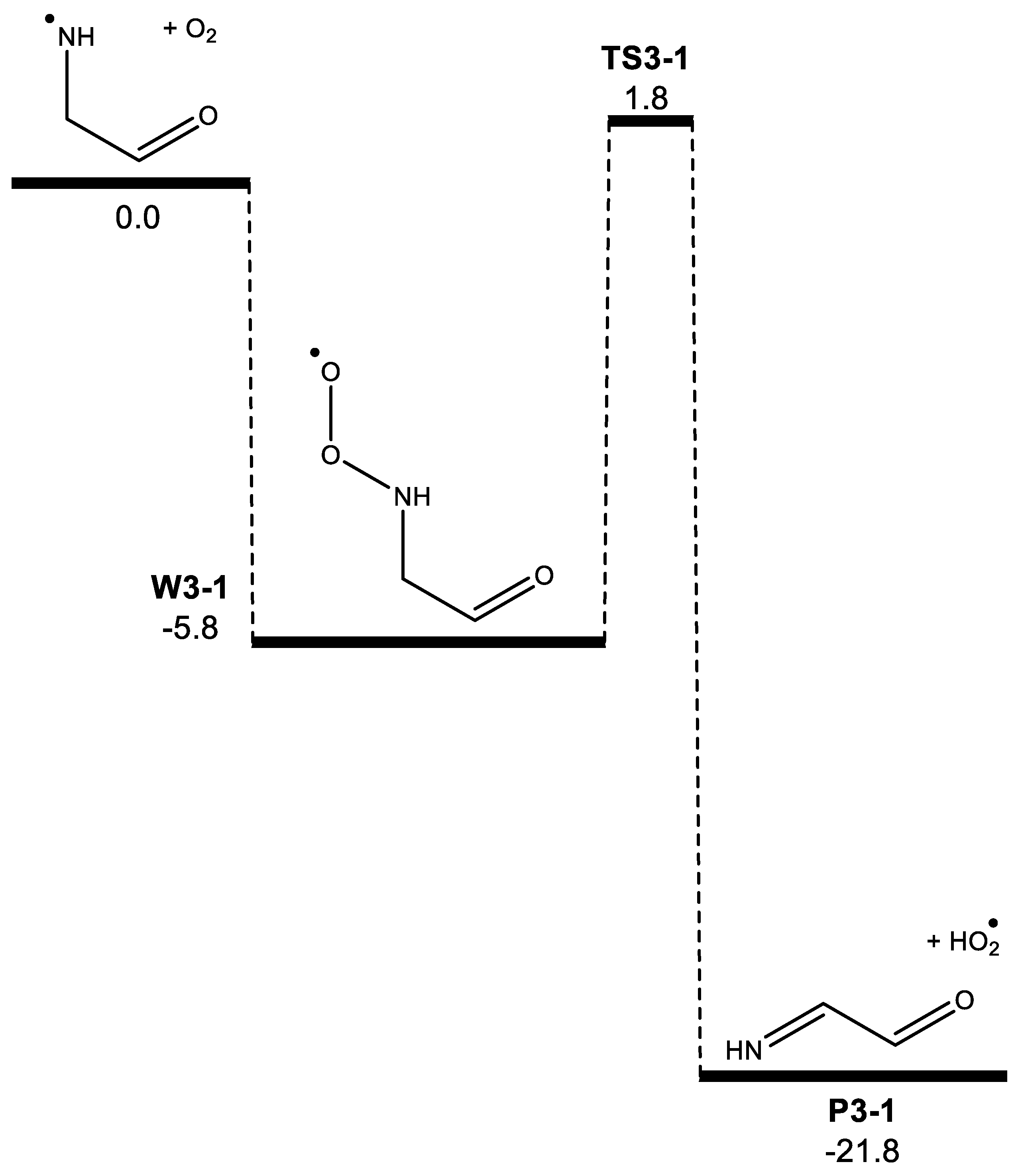
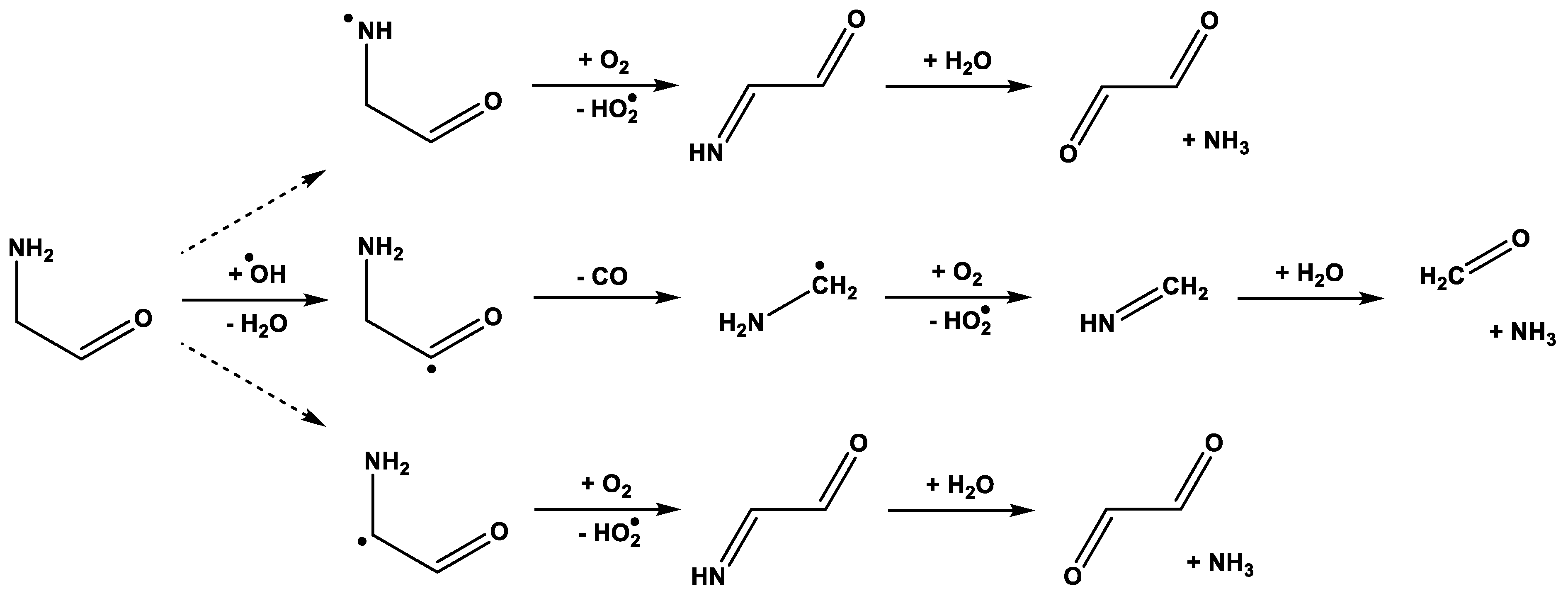
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veawab, A.; Tontiwachwuthikul, P.; Chakma, A. Corrosion behavior of carbon steel in the CO2 absorption process using aqueous amine solutions. Ind. Eng. Chem. Res. 1999, 38, 3917–3924. [Google Scholar]
- Puxty, G.; Rowland, R.; Allport, A.; Yang, Q.; Bown, M.; Burns, R.; Maeder, M.; Attalla, M. Carbon dioxide postcombustion capture: A novel screening study of the carbon dioxide absorption performance of 76 amines. Environ. Sci. Technol. 2009, 43, 6427–6433. [Google Scholar]
- Rubin, E.S.; Mantripragada, H.; Marks, A.; Versteeg, P.; Kitchin, J. The outlook for improved carbon capture technology. Prog. Energy Combust. Sci. 2012, 38, 630–671. [Google Scholar]
- Nielsen, C.J.; D’Anna, B.; Dye, C.; Graus, M.; Karl, M.; King, S.; Maguto, M.M.; Müller, M.; Schmidbauer, N.; Stenstrøm, Y. Atmospheric chemistry of 2-aminoethanol (MEA). Energy Procedia 2011, 4, 2245–2252. [Google Scholar]
- Veltman, K.; Singh, B.; Hertwich, E.G. Human and environmental impact assessment of postcombustion CO2 capture focusing on emissions from amine-based scrubbing solvents to air. Environ. Sci. Technol. 2010, 44, 1496–1502. [Google Scholar] [PubMed]
- Karl, M.; Wright, R.F.; Berglen, T.F.; Denby, B. Worst case scenario study to assess the environmental impact of amine emissions from a CO2 capture plant. Int. J. Greenh. Gas Control 2011, 5, 439–447. [Google Scholar]
- Reynolds, A.J.; Verheyen, T.V.; Adeloju, S.B.; Meuleman, E.; Feron, P. Towards commercial scale postcombustion capture of CO2 with monoethanolamine solvent: Key considerations for solvent management and environmental impacts. Environ. Sci. Technol. 2012, 46, 3643–3654. [Google Scholar] [PubMed]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar]
- Sharma, S.D.; Azzi, M. A critical review of existing strategies for emission control in the monoethanolamine-based carbon capture process and some recommendations for improved strategies. Fuel 2014, 121, 178–188. [Google Scholar]
- Pitts, J.N., Jr.; Grosjean, D.; Van Cauwenberghe, K.; Schmid, J.P.; Fitz, D.R. Photooxidation of aliphatic amines under simulated atmospheric conditions: Formation of nitrosamines, nitramines, amides, and photochemical oxidant. Environ. Sci. Technol. 1978, 12, 946–953. [Google Scholar]
- Xie, H.-B.; Li, C.; He, N.; Wang, C.; Zhang, S.; Chen, J. Atmospheric chemical reactions of monoethanolamine initiated by OH radical: Mechanistic and kinetic study. Environ. Sci. Technol. 2014, 48, 1700–1706. [Google Scholar] [PubMed]
- Borduas, N.; Abbatt, J.P.; Murphy, J.G. Gas phase oxidation of monoethanolamine (MEA) with OH radical and ozone: Kinetics, products, and particles. Environ. Sci. Technol. 2013, 47, 6377–6383. [Google Scholar] [PubMed]
- Onel, L.; Blitz, M.; Seakins, P. Direct Determination of the Rate Coefficient for the Reaction of OH Radicals with Monoethanol Amine (MEA) from 296 to 510 K. J. Phys. Chem. Lett. 2012, 3, 853–856. [Google Scholar]
- Karl, M.; Dye, C.; Schmidbauer, N.; Wisthaler, A.; Mikoviny, T.; d’Anna, B.; Müller, M.; Borrás, E.; Clemente, E.; Muñoz, A. Study of OH-initiated degradation of 2-aminoethanol. Atmos. Chem. Phys. 2012, 12, 1881–1901. [Google Scholar]
- da Silva, G. Atmospheric chemistry of 2-aminoethanol (MEA): Reaction of the NH2•CHCH2OH radical with O2. J. Phys. Chem. A 2012, 116, 10980–10986. [Google Scholar] [PubMed]
- da Silva, G.; Bozzelli, J.W. Role of the a-hydroxyethylperoxy radical in the reactions of acetaldehyde and vinyl alcohol with HO2. Chem. Phys. Lett. 2009, 483, 25–29. [Google Scholar]
- Bunkan, A.J.C.; Reijrink, N.G.; Mikoviny, T.; Muller, M.; Nielsen, C.J.; Zhu, L.; Wisthaler, A. Atmospheric chemistry of N-methylmethanimine (CH3N=CH2): A theoretical and experimental study. J. Phys. Chem. A 2022, 126, 3247–3264. [Google Scholar]
- Zhu, L.; Schade, G.W.; Nielsen, C.J. Real-time monitoring of emissions from monoethanolamine-based industrial scale carbon capture facilities. Environ. Sci. Technol. 2013, 47, 14306–14314. [Google Scholar]
- Venkata Nadh, R.; Syama Sundar, B.; Radhakrishnamurti, P.S. Kinetics of ruthenium(III) catalyzed and uncatalyzed oxidation of monoethanolamine by n-bromosuccinimide. Russ. J. Phys. Chem. A 2016, 90, 1760–1765. [Google Scholar]
- Nomoto, S.; Takasaki, M.; Sakata, N.; Harada, K. Flame-induced oxidation reaction of aliphatic amines in an aqueous solution. Tetrahed. Lett. 1983, 24, 3357–3360. [Google Scholar]
- Nomoto, S.; Shimoyama, A.; Shiraishi, S.; Sahara, D. Under-flame oxidation of amines and amino acids in an aqueous solution. Biosci. Biotechnol. Biochem. 1996, 60, 1851–1855. [Google Scholar]
- Karl, M.; Svendby, T.; Walker, S.-E.; Velken, A.S.; Castell, N.; Solberg, S. Modelling atsmopheric oxidation of 2-aminoethanol (MEA) emitted from post-combustion capture using WRF-Chem. Sci. Total Environ. 2015, 527–528, 185–202. [Google Scholar]
- Mestrom, L.; Bracco, P.; Hanefeld, U. Amino aldehydes revisited. Eur. J. Org. Chem. 2017, 7019, 7019–7025. [Google Scholar]
- Garrod, R.T. A three-phase chemical model of hot cores: The formation of glycine. Astrophys. J. 2013, 765, 60. [Google Scholar]
- Redondo, P.; Sanz-Novo, M.; Largo, A.; Barrientos, C. Amino acetaldehyde conformers: Structure and spectroscopic properties. Mon. Not. R. Astron. Soc. 2020, 492, 1827–1833. [Google Scholar]
- Simmie, J.M. C2H5NO isomers: From acetamide to 1,2-oxazetidine and beyond. J. Phys. Chem. A 2022, 126, 924–939. [Google Scholar] [PubMed]
- Marks, J.H.; Wang, J.; Kleimeier, N.F.; Turner, A.M.; Eckhardt, A.E.; Kaiser, R.I. Prebiotic synthesis and isomerization in interstellar analog ice: Glycinal, acetamide, and their enol tautomers. Angew. Chem. Int. Ed. 2023, 62, e202218645. [Google Scholar]
- da Silva, G. G3X-K theory: A composite theoretical method for thermochemical kinetics. Chem. Phys. Lett. 2013, 558, 109–113. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Barker, J.; Nguyen, T.; Stanton, J.; Aieta, C.; Ceotto, M.; Gabas, F.; Kumar, T.; Li, C.; Lohr, L.; Maranzana, A. MultiWell-2016 Software Suite; University of Michigan: Ann Arbor, MI, USA, 2016. [Google Scholar]
- Smith, G.; Golden, D. Application of RRKM theory to the reactions OH + NO2 + N2 → HONO2 + N2 (1) and ClO + NO2 + N2 → ClONO2 + N2 (2); a modified gorin model transition state. Int. J. Chem. Kinet. 1978, 10, 489–501. [Google Scholar]
- da Silva, G. Reaction of methacrolein with the hydroxyl radical in air: Incorporation of secondary O2 addition into the MACR + OH master equation. J. Phys. Chem. A 2012, 116, 5317–5324. [Google Scholar] [PubMed]
- Ren, Z.; da Silva, G. Atmospheric oxidation of piperazine initiated by OH: A theoretical kinetics investigation. ACS Earth Space Chem. 2019, 3, 2510–2516. [Google Scholar]
- Sherwood, T.K.; Reid, R.C. The Properties of Gases and Liquids: Their Estimation and Correlation; McGraw-Hill: New York, NY, USA, 1958. [Google Scholar]
- Ly, T.; Kirk, B.B.; Hettiarachchi, P.I.; Poad, B.L.J.; Trevitt, A.J.; da Silva, G.; Blanksby, S.J. Reactions of simple and peptidic alpha-carboxylate radical anions with dioxygen in the gas phase. Phys. Chem. Chem. Phys. 2011, 13, 16314–16323. [Google Scholar]
- da Silva, G.; Kirk, B.B.; Lloyd, C.; Trevitt, A.J.; Blanksby, S.J. Concerted HO2 elimination from α-aminoalkylperoxyl free radicals: Experimental and theoretical evidence from the gas-phase NH2•CHCO2− + O2 reaction. J. Phys. Chem. Lett. 2012, 3, 805–811. [Google Scholar]
- Dash, M.R.; Ali, M.A. Can a single ammonia and water molecule enhance the formation of methanimine under tropospheric conditions? Kinetics of CH2NH2 + O2 (+NH3/H2O). Front. Chem. 2023, 11, 1243235. [Google Scholar] [CrossRef]
- Leroy, G.; Dewispelaere, J.-P.; Benkadour, H.; Riffi Temsamani, D.; Wilante, C. Theoretical investigation of some evidences of the captodative effect. Bull. Soc. Chim. Belg. 1994, 103, 367–378. [Google Scholar]
- Barnes, I.; Solignac, G.; Mellouki, A.; Becker, K.H. Aspects of the atmospheric chemistry of amides. ChemPhysChem 2010, 11, 3844–3857. [Google Scholar]
- Borduas, N.; da Silva, G.; Murphy, J.G.; Abbatt, J.P.D. Experimental and theoretical understanding of the gas phase oxidation of atmospheric amides with OH radicals: Kinetics, products, and mechanisms. J. Phys. Chem. A 2015, 119, 4298–4308. [Google Scholar] [PubMed]
- Borduas, N.; Abbatt, J.P.D.; Murphy, J.G.; So, S.; da Silva, G. Gas-phase mechanisms of the reactions of reduced organic nitrogen compounds with OH radicals. Environ. Sci. Technol. 2016, 50, 11723–11734. [Google Scholar]
- Alam, M.A.; Ren, Z.; da Silva, G. Nitramine and nitrosamine formation is a minor pathway in the atmospheric oxidation of methylamine: A theoretical kinetic study of the CH3NH + O2 reaction. Int. J. Chem. Kinet. 2019, 51, 723–728. [Google Scholar]
- Yizhen, T.; Nielsen, M.; Jorgen, C. Do primary nitrosamines form and exist in the gas phase? A computational study of CH3NHNO and (CH3)2NNO. Phys. Chem. Chem. Phys. 2012, 14, 16365–16370. [Google Scholar]
- da Silva, G. Formation of nitrosamines and alkyldiazohydroxides in the gas phase: The CH3NH + NO reaction revisited. Environ. Sci. Technol. 2013, 47, 7766–7772. [Google Scholar] [PubMed]
- Liu, C.; Ma, F.; Elm, J.; Fu, Z.; Tang, W.; Chen, J.; Xie, H.-B. Mechanism and predictive model development of reaction rate constants for N-center radicals with O2. Chemosphere 2019, 237, 124411. [Google Scholar] [PubMed]
- Nguyen, L.T.; Mai, T.V.-T.; Vien, H.D.; Nguyen, T.T.; Huynh, L.K. Ab initio kinetics of the CH3NH + NO2 reaction: Formation of nitramines and N-alkyl nitroxides. Phys. Chem. Chem. Phys. 2023, 25, 31936–31947. [Google Scholar] [PubMed]


| Morse potential parameters | |||
| Vibrational frequency of cleaving bond (cm−1) | 152.07 | ||
| Dissociation energy (kcal mol−1) | 4.14 | ||
| Centre of mass distance between fragments (Å) | 2.5 | ||
| Capture rate (cm3 molecule−1 s−1) | 7.81 × 10−11 | ||
| Fitted Gorin parameters | |||
| η | 0.9964 (300)/0.9971 (600)/0.9973 (800)/0.9974 (1000) 0.9975 (1200)/0.9976 (1500)/0.9976 (2000) | ||
| Γ a | 0.0598 (300)/0.0540 (600)/0.0521 (800)/0.0509 (1000) 0.0501 (1200)/0.0494 (1500)/0.0493 (2000) | ||
| Moments of inertia (amu Å2) | |||
| adiabatic external rotor [2D] b | 303.5779 (300)/247.9012 (600)/225.6456 (800)/208.5839 (1000)/194.6673 (1200) 177.445(1500)/154.00 (2000) | ||
| A | Ea | |
|---|---|---|
| (cm3 molecule−1 s−1 or s−1) | (cal mol−1) | |
| NH2CH2CHO + OH → NHCH2CHO + H2O | 2.93 × 10−12 | −252 |
| NH2CH2CHO + OH → NH2CHCHO + H2O | 1.64 × 10−11 | 848 |
| NH2CH2CHO + OH → NH2CH2CO + H2O | 4.32 × 10−11 | 1060 |
| NHCH2CHO + O2 → NHCHCHO + HO2 | 4.46 × 10−14 | 3060 |
| NH2CHCHO + O2 → NHCHCHO + HO2 | 3.50 × 10−12 | 4990 |
| NH2CH2CO → CH2NH2 + CO | 3.84 × 1016 | 11,200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, A.; da Silva, G. Oxidation of Aminoacetaldehyde Initiated by the OH Radical: A Theoretical Mechanistic and Kinetic Study. Atmosphere 2024, 15, 1011. https://doi.org/10.3390/atmos15081011
Alam A, da Silva G. Oxidation of Aminoacetaldehyde Initiated by the OH Radical: A Theoretical Mechanistic and Kinetic Study. Atmosphere. 2024; 15(8):1011. https://doi.org/10.3390/atmos15081011
Chicago/Turabian StyleAlam, Ashraful, and Gabriel da Silva. 2024. "Oxidation of Aminoacetaldehyde Initiated by the OH Radical: A Theoretical Mechanistic and Kinetic Study" Atmosphere 15, no. 8: 1011. https://doi.org/10.3390/atmos15081011
APA StyleAlam, A., & da Silva, G. (2024). Oxidation of Aminoacetaldehyde Initiated by the OH Radical: A Theoretical Mechanistic and Kinetic Study. Atmosphere, 15(8), 1011. https://doi.org/10.3390/atmos15081011








