Influence of Field Sampling Methods on Measuring Volatile Organic Compounds in a Swine Facility Using SUMMA Canisters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Sampling Method
2.3. Analysis Method
2.3.1. Standard Curve Creation
2.3.2. Instrument Analysis
3. Results and Discussion
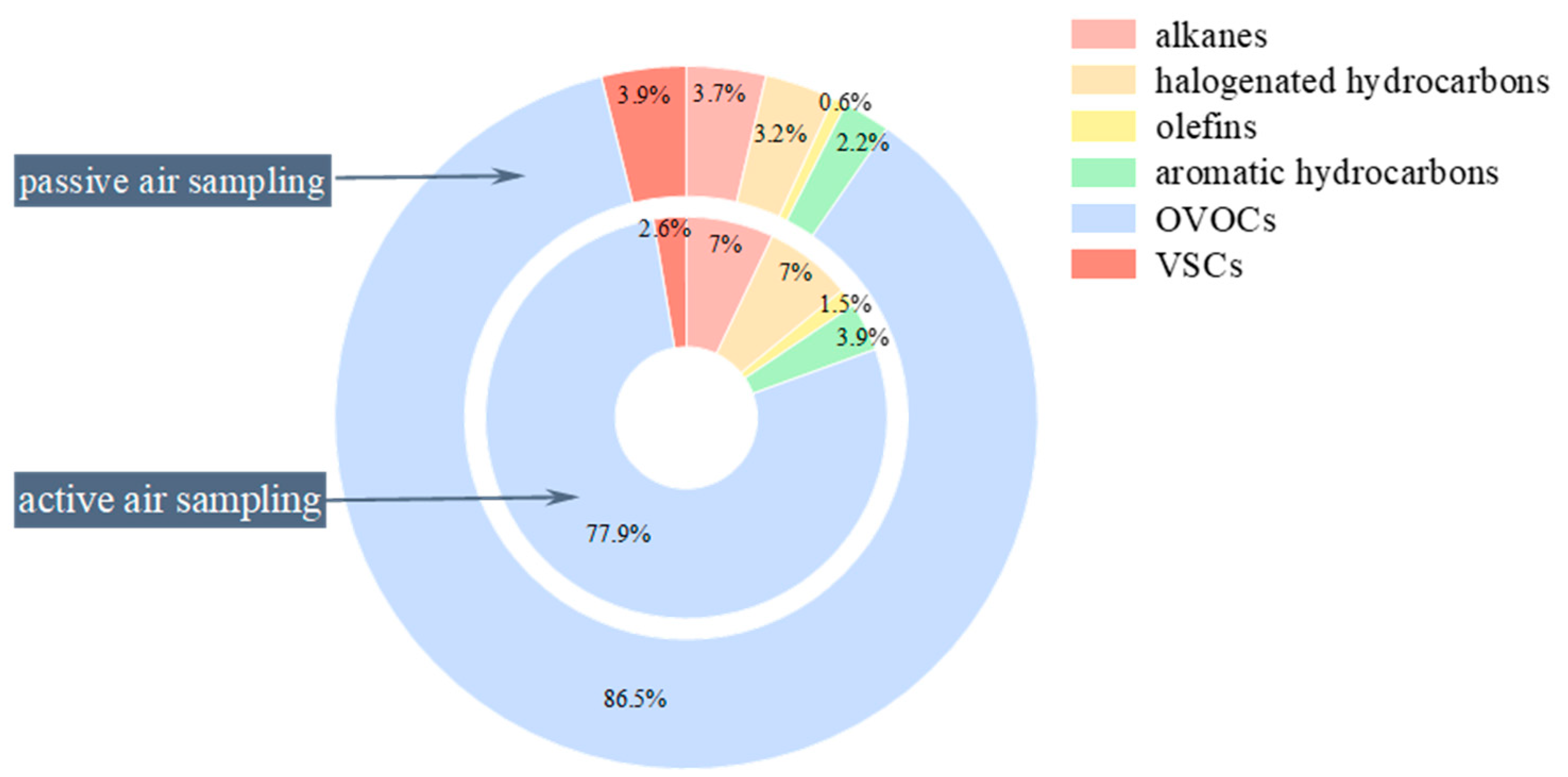
3.1. Main Compositions of VOCs at Swine House
3.2. Odorous VOC Emissions
3.3. The Influence of Storage Time on VOC Concentrations
3.3.1. Compounds Determined by FID
3.3.2. Compounds Determined by MS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Number | Standard Compound | RT | Ion1 | Ion2 | Ion3 | CAS NO. | R2 | RSD |
|---|---|---|---|---|---|---|---|---|
| 1 | Ethylene | 12.456 | 28 | 27 | 26 | 74-85-1 | 0.999 | 0.052 |
| 2 | Acetylene | 12.710 | 26 | 25 | 24 | 74-86-2 | 0.999 | 0.053 |
| 3 | Ethane | 13.864 | 28 | 27 | 29 | 74-84-0 | 0.999 | 0.046 |
| 4 | Propylene | 21.325 | 41 | 42 | 39 | 115-07-1 | 0.999 | 0.051 |
| 5 | Propane | 21.987 | 44 | 43 | 39 | 74-98-6 | 0.999 | 0.050 |
| 6 | Bromochloromethane (is) | 28.744 | 130 | 128 | 93 | 74-97-5 | - | 0.090 |
| 7 | Dichlorodifluoromethane | 10.620 | 85 | 87 | 101 | 75-71-8 | 0.999 | 0.010 |
| 8 | Methyl chloride | 11.817 | 50 | 52 | - | 74-87-3 | 0.999 | 0.020 |
| 9 | Isobutane | 12.761 | 43 | 42 | 41 | 75-28-5 | 0.999 | 0.049 |
| 10 | 1,2-Dichlorotetrafluoroethane | 12.637 | 85 | 135 | 137 | 76-14-2 | 0.999 | 0.012 |
| 11 | Vinyl chloride | 13.439 | 62 | 64 | 63 | 75-01-4 | 0.998 | 0.021 |
| 12 | 1-Butylene | 14.179 | 41 | 39 | 56 | 106-98-9 | 0.997 | 0.004 |
| 13 | 1,3-Butadiene | 14.386 | 54 | 53 | 39 | 106-99-0 | 0.998 | 0.021 |
| 14 | n-Butane | 14.749 | 58 | 42 | 41 | 106-97-8 | 0.999 | 0.007 |
| 15 | trans-2-Butene | 15.388 | 56 | 41 | 50 | 624-64-6 | 0.997 | 0.036 |
| 16 | Bromomethane | 16.009 | 94 | 96 | 93 | 74-83-9 | 0.998 | 0.008 |
| 17 | cis-2-Butene | 16.344 | 56 | 55 | 41 | 590-18-1 | 0.997 | 0.026 |
| 18 | Chloroethane | 16.995 | 64 | 66 | 49 | 75-00-3 | 0.999 | 0.029 |
| 19 | Ethyl alcohol | 17.505 | 45 | 46 | - | 64-17-5 | 0.987 | 0.075 |
| 20 | Acrolein | 19.147 | 56 | 55 | 38 | 107-02-8 | 0.985 | 0.013 |
| 21 | Isopentane | 19.937 | 57 | 43 | 42 | 78-78-4 | 0.997 | 0.004 |
| 22 | Acetone | 19.649 | 43 | 58 | - | 67-64-1 | 0.999 | 0.103 |
| 23 | Trichlorofluoromethane | 20.504 | 101 | 103 | - | 75-69-4 | 0.999 | 0.007 |
| 24 | 1-Pentene | 20.981 | 42 | 55 | 70 | 109-67-1 | 0.996 | 0.040 |
| 25 | Propan-2-ol | 20.571 | 45 | 43 | - | 67-63-0 | 0.990 | 0.015 |
| 26 | n-Pentane | 21.821 | 43 | 42 | 41 | 109-66-0 | 0.997 | 0.019 |
| 27 | 2-Methyl-1,3-Butadiene | 22.181 | 55 | 70 | 39 | 78-79-5 | 0.996 | 0.077 |
| 28 | trans-2-Pentene | 22.338 | 67 | 53 | 39 | 646-04-8 | 0.997 | 0.041 |
| 29 | Vinylidene chloride | 22.766 | 61 | 96 | 98 | 75-35-4 | 0.999 | 0.055 |
| 30 | cis-2-Pentene | 22.920 | 55 | 70 | 42 | 627-20-3 | 0.996 | 0.078 |
| 31 | Dichloromethane | 23.105 | 49 | 86 | 84 | 75-09-2 | 0.999 | 0.029 |
| 32 | Carbon disulfide | 24.170 | 76 | 78 | 77 | 75-15-0 | 0.998 | 0.083 |
| 33 | 1,1,2-Trichlorotrifluoroethane | 23.921 | 151 | 101 | - | 76-13-1 | 0.999 | 0.017 |
| 34 | 2,2-Dimethyl butane | 24.460 | 57 | 71 | 43 | 75-83-2 | 0.997 | 0.006 |
| 35 | trans-Dichloroethylene | 25.812 | 96 | 98 | 61 | 156-60-5 | 0.997 | 0.080 |
| 36 | 1,1-Dichloroethane | 26.337 | 63 | 65 | 98 | 75-34-3 | 0.999 | 0.018 |
| 37 | Methyl tert-Butyl Ether | 26.360 | 73 | 57 | - | 1634-04-4 | 0.995 | 0.078 |
| 38 | Cyclopentane | 26.551 | 42 | 55 | 70 | 287-92-3 | 0.999 | 0.072 |
| 39 | 2,3-Dimethylbutane | 26.535 | 42 | 43 | 71 | 79-29-8 | 0.998 | 0.066 |
| 40 | 2-Methylpentane | 26.735 | 43 | 71 | 55 | 107-83-5 | 0.998 | 0.003 |
| 41 | Ethenyl ethanoate | 26.512 | 43 | 86 | - | 108-05-4 | 0.995 | 0.027 |
| 42 | 2-Butanone | 27.138 | 43 | 72 | 57 | 78-93-3 | 0.995 | 0.057 |
| 43 | 3-Methylpentane | 27.715 | 57 | 56 | 41 | 96-14-0 | 0.995 | 0.036 |
| 44 | 1-Hexene | 28.032 | 56 | 41 | 84 | 592-41-6 | 0.997 | 0.029 |
| 45 | 1,2-cis-Dichloroethene | 28.336 | 96 | 98 | 61 | 156-59-2 | 0.996 | 0.096 |
| 46 | n-Hexane | 28.750 | 57 | 41 | 86 | 110-54-3 | 0.998 | 0.008 |
| 47 | Ethyl acetate | 28.563 | 43 | 61 | 45 | 141-78-6 | 0.996 | 0.093 |
| 48 | Trichloromethane | 28.992 | 83 | 85 | - | 67-66-3 | 0.999 | 0.016 |
| 49 | Tetrahydrofuran | 29.855 | 40 | 71 | 72 | 109-99-9 | 0.996 | 0.116 |
| 50 | Methylcyclopentane | 30.699 | 57 | 43 | 85 | 96-37-7 | 0.998 | 0.066 |
| 51 | 2,4-Dimethylpentane | 30.665 | 56 | 69 | 41 | 108-08-7 | 0.997 | 0.100 |
| 52 | 1,2-Dichloroethane | 30.641 | 62 | 64 | 49 | 107-06-2 | 0.999 | 0.141 |
| 53 | 1,1,1-Trichloroethane | 31.233 | 97 | 61 | 117 | 71-55-6 | 0.999 | 0.073 |
| 54 | 1,4-Difluorobenzene (is) | 32.690 | 114 | 63 | 88 | 540-36-3 | - | 0.177 |
| 55 | Benzene | 32.210 | 78 | 77 | 52 | 71-43-2 | 1.000 | 0.141 |
| 56 | Carbontetrachloride | 32.528 | 117 | 119 | 121 | 56-23-5 | 0.999 | 0.114 |
| 57 | Cyclohexane | 32.814 | 56 | 69 | 84 | 110-82-7 | 1.000 | 0.093 |
| 58 | 2-Methylhexane | 32.813 | 43 | 57 | 85 | 591-76-4 | 0.999 | 0.078 |
| 59 | 2,3-Dimethylpentane | 33.070 | 56 | 43 | 71 | 565-59-3 | 1.000 | 0.065 |
| 60 | 3-Methylhexane | 33.341 | 57 | 43 | 70 | 589-34-4 | 1.000 | 0.069 |
| 61 | 1,2-Dichloropropane | 33.821 | 63 | 62 | 76 | 78-87-5 | 1.000 | 0.067 |
| 62 | 2,2,4-Trimethylpentane | 34.231 | 57 | 56 | 41 | 540-84-1 | 0.999 | 0.085 |
| 63 | 1,4-Dioxane | 34.108 | 88 | 58 | - | 123-91-1 | 0.998 | 0.084 |
| 64 | Bromodichloromethane | 34.190 | 83 | 129 | 47 | 75-27-4 | 0.999 | 0.061 |
| 65 | Trichloroethylene | 34.257 | 130 | 132 | 95 | 79-01-6 | 0.999 | 0.086 |
| 66 | Methyl methacrylate | 34.268 | 69 | 41 | 39 | 80-62-6 | 0.998 | 0.011 |
| 67 | Heptane | 34.580 | 43 | 57 | 71 | 142-82-5 | 0.999 | 0.044 |
| 68 | Methyl isobutyl ketone | 35.756 | 43 | 58 | 85 | 108-10-1 | 0.999 | 0.072 |
| 69 | cis-1,3-Dichloropropylene | 35.863 | 75 | 110 | - | 10061-01-5 | 1.000 | 0.012 |
| 70 | Methylcyclohexane | 36.270 | 83 | 98 | 55 | 108-87-2 | 1.000 | 0.065 |
| 71 | trans-1,3-Dichloropropene | 36.831 | 75 | 110 | 39 | 10061-02-6 | 0.998 | 0.051 |
| 72 | 1,1,2-Trichloroethane | 37.316 | 97 | 83 | 61 | 79-00-5 | 0.999 | 0.088 |
| 73 | 2,3,4-Trimethylpentane | 37.506 | 43 | 71 | 55 | 565-75-3 | 1.000 | 0.090 |
| 74 | Toluene | 37.931 | 57 | 43 | 70 | 108-88-3 | 0.999 | 0.005 |
| 75 | 2-Methylheptane | 37.960 | 91 | 92 | - | 592-27-8 | 0.999 | 0.060 |
| 76 | 2-Hexanone | 38.092 | 58 | 100 | - | 591-78-6 | 0.999 | 0.050 |
| 77 | 3-Methylheptane | 39.529 | 85 | 57 | 43 | 589-81-1 | 1.000 | 0.045 |
| 78 | Chlorodibromomethane | 38.896 | 129 | 127 | - | 124-48-1 | 1.000 | 0.060 |
| 79 | 1,2-Dibromoethane | 39.453 | 107 | 109 | - | 106-93-4 | 0.998 | 0.064 |
| 80 | Octane | 39.529 | 85 | 71 | 57 | 111-65-9 | 1.000 | 0.084 |
| 81 | Tetrachloroethene | 40.302 | 166 | 131 | 94 | 127-18-4 | 0.998 | 0.081 |
| 82 | Chlorobenzene-d5 (is) | 41.652 | 117 | 82 | 119 | 3114-55-4 | - | 0.100 |
| 83 | Chlorobenzene | 41.753 | 112 | 77 | 114 | 108-90-7 | 0.999 | 0.033 |
| 84 | Ethylbenzene | 42.413 | 91 | 106 | - | 100-41-4 | 0.998 | 0.042 |
| 85 | (o + p)-Xylene | 42.761 | 91 | 106 | 104 | 108-38-3 | 0.998 | 0.101 |
| 86 | Tribromomethane | 43.248 | 173 | 171 | 175 | 75-25-2 | 0.999 | 0.063 |
| 87 | Styrene | 43.600 | 104 | 78 | 51 | 100-42-5 | 0.998 | 0.096 |
| 88 | 1,2-Dimethylbenzene | 43.873 | 91 | 106 | 104 | 95-47-6 | 0.998 | 0.071 |
| 89 | 1,1,2,2-Tetrachloroethane | 43.832 | 83 | 95 | 131 | 79-34-5 | 0.999 | 0.043 |
| 90 | n-Nonane | 43.891 | 91 | 106 | - | 111-84-2 | 0.999 | 0.063 |
| 91 | 4-Bromofluorobenzene (is) | 44.941 | 174 | 176 | - | 460-00-4 | - | 0.081 |
| 92 | Cumene | 45.144 | 105 | 120 | - | 98-82-8 | 1.000 | 0.085 |
| 93 | n-Propylbenzene | 46.376 | 91 | 120 | - | 103-65-1 | 1.000 | 0.046 |
| 94 | 3-Ethyltoluene | 46.579 | 105 | 120 | - | 620-14-4 | 1.000 | 0.081 |
| 95 | 4-Ethyltoluene | 46.706 | 105 | 120 | 91 | 622-96-8 | 0.998 | 0.096 |
| 96 | Mesitylene | 46.851 | 105 | 120 | 77 | 108-67-8 | 0.999 | 0.019 |
| 97 | 2-Ethyltoluene | 47.451 | 105 | 120 | 611-14-3 | 1.000 | 0.064 | |
| 98 | 1,2,4-Trimethylbenzene | 47.996 | 105 | 120 | 77 | 95-63-6 | 0.999 | 0.063 |
| 99 | Decane | 47.834 | 105 | 120 | - | 124-18-5 | 0.998 | 0.055 |
| 100 | Benzyl chloride | 48.492 | 91 | 126 | - | 100-44-7 | 0.999 | 0.045 |
| 101 | 1,3-Dichlorbenzene | 48.621 | 146 | 111 | 148 | 541-73-1 | 0.999 | 0.078 |
| 102 | 1,4-Dichlorobenzene | 48.784 | 146 | 111 | 148 | 106-46-7 | 0.999 | 0.071 |
| 103 | 1,2,3-Trimethylbenzene | 49.249 | 105 | 120 | - | 526-73-8 | 0.998 | 0.078 |
| 104 | 1,2-Dichlorobenzene | 49.830 | 146 | 111 | 148 | 95-50-1 | 0.999 | 0.003 |
| 105 | 1,3-Diethylbenzene | 49.957 | 119 | 105 | 134 | 141-93-5 | 0.999 | 0.039 |
| 106 | p-Diethyl benzene | 50.272 | 119 | 105 | 134 | 105-05-5 | 0.999 | 0.057 |
| 107 | n-Hendecane | 51.599 | 57 | 71 | 156 | 1120-21-4 | 0.999 | 0.092 |
| 108 | 1,2,4-Trichlorobenzene | 56.085 | 180 | 145 | 182 | 120-82-1 | 0.998 | 0.052 |
| 109 | Dodecane | 55.760 | 57 | 71 | 120 | 112-40-3 | 0.999 | 0.053 |
| 110 | Naphthalene | 56.730 | 128 | 64 | - | 465-73-6 | 0.995 | 0.046 |
| 111 | Hexachloro-1,3-butadiene | 58.169 | 225 | 190 | 118 | 87-68-3 | 0.999 | 0.051 |
| 112 | Methanethiol | 15.314 | 47 | 48 | 45 | 74-93-1 | 0.994 | 0.029 |
| 113 | Ethyl mercaptan | 21.358 | 62 | 47 | 45 | 75-08-1 | 0.989 | 0.042 |
| 114 | Dimethyl sulfide | 22.571 | 62 | 47 | 45 | 75-18-3 | 0.998 | 0.061 |
| 115 | Methylthioethane | 29.032 | 61 | 76 | 48 | 624-89-5 | 0.999 | 0.042 |
| 116 | Thiophene | 32.513 | 84 | 58 | 45 | 110-02-1 | 1.000 | 0.036 |
| 117 | Diethyl sulfide | 34.141 | 75 | 90 | 61 | 352-93-2 | 0.998 | 0.039 |
| 118 | Dimethyl Disulfide | 36.567 | 94 | 79 | 45 | 624-92-0 | 0.995 | 0.031 |
References
- Wang, Y.C.; Han, M.F.; Jia, T.P.; Hu, X.R.; Zhu, H.Q.; Tong, Z.; Lin, Y.T.; Wang, C.; Liu, D.Z.; Peng, Y.Z.; et al. Emissions, measurement, and control of odor in livestock farms: A review. Sci. Total Environ. 2021, 776, 145735. [Google Scholar] [CrossRef]
- Ubeda, Y.; Lopez-Jimenez, P.A.; Nicolas, J.; Calvet, S. Strategies to control odours in livestock facilities: A critical review. Span. J. Agric. Res. 2013, 11, 1004–1015. [Google Scholar] [CrossRef]
- Bibbiani, C.; Russo, C. Odour emission from intensive livestock production system: Approaches for emission abatement and evaluation of their effectiveness. Large Anim. Rev. 2012, 18, 135–138. [Google Scholar]
- Nimmermark, S. Odour influence on well-being and health with specific focus on animal production emissions. Ann. Agric. Environ. Med. 2004, 11, 163–173. [Google Scholar]
- Schiffman, S.S.; Miller, E.A.; Suggs, M.S.; Graham, B.G. The effect of environmental odors emanating from commercial swine operations on the mood of nearby residents. Brain Res. Bull. 1995, 37, 369–375. [Google Scholar] [CrossRef]
- Valdes Labrada, G.M.; Kumar, S.; Azar, R.; Predicala, B.; Nemati, M. Simultaneous capture of NH3 and H2S using TiO2 and ZnO nanoparticles—laboratory evaluation and application in a livestock facility. J. Environ. Chem. Eng. 2020, 8, 103615. [Google Scholar] [CrossRef]
- Yao, H.Q.; Choi, H.L.; Zhu, K.; Lee, J.H. Key volatile organic compounds emitted from swine nursery house. Atmos. Environ. 2011, 45, 2577–2584. [Google Scholar] [CrossRef]
- Cai, L.; Koziel, J.A.; Zhang, S. Odorous chemical emissions from livestock operations in United States. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, China, 24–26 June 2011; pp. 532–535. [Google Scholar]
- Ni, J.Q.; Robarge, W.P.; Xiao, C.; Heber, A.J. Volatile organic compounds at swine facilities: A critical review. Chemosphere 2012, 89, 769–788. [Google Scholar] [CrossRef]
- Li, J.; Deng, S.; Li, G.; Lu, Z.; Song, H.; Gao, J.; Sun, Z.; Xu, K. VOCs characteristics and their ozone and SOA formation potentials in autumn and winter at Weinan, China. Environ. Res. 2022, 203, 111821. [Google Scholar] [CrossRef]
- Mozaffar, A.; Zhang, Y.-L. Atmospheric Volatile Organic Compounds (VOCs) in China: A Review. Curr. Pollut. Rep. 2020, 6, 250–263. [Google Scholar] [CrossRef]
- Plachá, D.; Raclavská, H.; Kučerová, M.; Kuchařová, J. Volatile fatty acid evolution in biomass mixture composts prepared in open and closed bioreactors. Waste Manag. 2013, 33, 1104–1112. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Bennett, J.L.; Raymer, J.H. Quantification of odors and odorants from swine operations in North Carolina. Agr For. Meteorol. 2001, 108, 213–240. [Google Scholar] [CrossRef]
- Mielcarek, P.; Rzeźnik, W. Odor Emission Factors from Livestock Production. Pol. J. Environ. Stud. 2015, 24, 27–35. [Google Scholar] [CrossRef]
- Hiroyuki, U. The Potential Contribution of VOCs on Ambient Air Odor. Asian J. Atmos. Environ. 2021, 15, 94–101. [Google Scholar] [CrossRef]
- Wang, K.Y.; Li, X.; Ying, Y.F. Research advances in characteristics and reduction technology of VOCs from swine production. Trans. Chin. Soc. Agric. Eng. (Trans. CSAE) 2023, 39, 168–179. [Google Scholar] [CrossRef]
- Jia, H.; Gao, S.; Duan, Y.; Fu, Q.; Che, X.; Xu, H.; Wang, Z.; Cheng, J. Investigation of health risk assessment and odor pollution of volatile organic compounds from industrial activities in the Yangtze River Delta region, China. Ecotoxicol. Environ. Saf. 2021, 208, 111474. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Wan, X.; Zhao, X.; Wu, Y.; Liu, X.; Li, X. Important contributions of alkenes and aromatics to VOCs emissions, chemistry and secondary pollutants formation at an industrial site of central eastern China. Atmos. Environ. 2021, 244, 117927. [Google Scholar] [CrossRef]
- Martel, M.C.; Lemay, S.P.; Predicala, B.Z.; Girard, M.; Belzile, M.; Feddes, J.; Hogue, R.; Godbout, S. Detailed Study of Odor from Pig Buildings to Improve Understanding of Biotrickling Filter Performance. Trans. ASABE 2017, 60, 2151–2162. [Google Scholar] [CrossRef]
- GB 14554-93; Emission Standards for Odor Pollutants. Standardization Administration of China: Beijing, China, 1993.
- Wang, M.; Zeng, L.; Lu, S.; Shao, M.; Liu, X.; Yu, X.; Chen, W.; Yuan, B.; Zhang, Q.; Hu, M.; et al. Development and validation of a cryogen-free automatic gas chromatograph system (GC-MS/FID) for online measurements of volatile organic compounds. Anal. Methods 2014, 6, 9424–9434. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, L.; Koziel, J.A.; Hoff, S.J.; Schmidt, D.R.; Clanton, C.J.; Jacobson, L.D.; Parker, D.B.; Heber, A.J. Field air sampling and simultaneous chemical and sensory analysis of livestock odorants with sorbent tubes and GC–MS/olfactometry. Sens. Actuators B Chem. 2010, 146, 427–432. [Google Scholar] [CrossRef]
- Ras, M.R.; Borrull, F.; Marcé, R.M. Sampling and preconcentration techniques for determination of volatile organic compounds in air samples. Trac Trends Anal. Chem. 2009, 28, 347–361. [Google Scholar] [CrossRef]
- Mochalski, P.; Wzorek, B.; Śliwka, I.; Amann, A. Suitability of different polymer bags for storage of volatile sulphur compounds relevant to breath analysis. J. Chromatogr. B 2009, 877, 189–196. [Google Scholar] [CrossRef]
- Man, Z.; Dai, X.; Rong, L.; Kong, X.; Ying, S.; Xin, Y.; Liu, D. Evaluation of storage bags for odour sampling from intensive pig production measured by proton-transfer-reaction mass-spectrometry. Biosyst. Eng. 2020, 189, 48–59. [Google Scholar] [CrossRef]
- Hamon, L.; Andres, Y.; Dumont, E. Aerial pollutants in swine buildings: A review of their characterization and methods to reduce them. Environ. Sci. Technol. 2012, 46, 12287–12301. [Google Scholar] [CrossRef]
- Polvara, E.; Gallego, E.; Invernizzi, M.; Perales, J.F.; Sironi, S. Chemical characterization of odorous emissions: A comparative performance study of different sampling methods. Talanta 2023, 253, 124110. [Google Scholar] [CrossRef]
- Brown, V.M.; Crump, D.R.; Plant, N.T.; Pengelly, I. Evaluation of the stability of a mixture of volatile organic compounds on sorbents for the determination of emissions from indoor materials and products using thermal desorption/gas chromatography/mass spectrometry. J. Chromatogr. A 2014, 1350, 1–9. [Google Scholar] [CrossRef]
- Brown, V.M.; Crump, D.R. An investigation into the performance of a multi-sorbent sampling tube for the measurement of VVOC and VOC emissions from products used indoors. Anal. Methods 2013, 5, 2746–2756. [Google Scholar] [CrossRef]
- Arnts, R.R. Evaluation of adsorbent sampling tube materials and Tenax-TA for analysis of volatile biogenic organic compounds. Atmos. Environ. 2010, 44, 1579–1584. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Z.; Zheng, Y.; Chen, Y.; Yin, F.; Zhang, W.; Dong, H.; Xin, H. Characterization of Volatile Organic Compound (VOC) Emissions from Swine Manure Biogas Digestate Storage. Atmosphere 2019, 10, 411. [Google Scholar] [CrossRef]
- Mariné, S.; Pedrouzo, M.; Maria Marcé, R.; Fonseca, I.; Borrull, F. Comparison between sampling and analytical methods in characterization of pollutants in biogas. Talanta 2012, 100, 145–152. [Google Scholar] [CrossRef]
- Sin, D.W.M.; Wong, Y.C.; Sham, W.C.; Wang, D. Development of an analytical technique and stability evaluation of 143 C3-C12 volatile organic compounds in Summa® canisters by gas chromatography-mass spectrometry. Analyst 2001, 126, 310–321. [Google Scholar] [CrossRef]
- Ochiai, N.; Tsuji, A.; Nakamura, N.; Daishima, S.; Cardin, D.B. Stabilities of 58 volatile organic compounds in fused-silica-lined and SUMMA polished canisters under various humidified conditions. J. Environ. Monit. 2002, 4, 879–889. [Google Scholar] [CrossRef]
- Brymer, D.A.; Ogle, L.D.; Jones, C.J.; Lewis, D.L. Viability of Using SUMMA Polished Canisters for the Collection and Storage of Parts per Billion by Volume Level Volatile Organics. Environ. Sci. Technol. 1996, 30, 188–195. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Determination Of VOCs in Ambient Air Using Specially Prepared Canisters with Subsequent Analysis By Gas Chromatography; U.S. Environmental Protection Agency: Washington, DC, USA, 1999.
- Environmental Protection Agency. Determination of VOCs in Air Collected in Specially Prepared Canisters and Analyzed by Gas Chromatography–Mass Spectrometry (GC-MS); U.S. Environmental Protection Agency: Washington, DC, USA, 2019.
- Cheng, L.; Wei, W.; Zhang, C.; Xu, X.; Sha, K.; Meng, Q.; Jiang, Y.; Cheng, S. Quantitation study on VOC emissions and their reduction potential for coking industry in China: Based on in-situ measurements on treated and untreated plants. Sci. Total Environ. 2022, 836, 155466. [Google Scholar] [CrossRef]
- Dai, L.; Meng, J.; Zhao, X.; Li, Q.; Shi, B.; Wu, M.; Zhang, Q.; Su, G.; Hu, J.; Shu, X. High-spatial-resolution VOCs emission from the petrochemical industries and its differential regional effect on soil in typical economic zones of China. Sci. Total Environ. 2022, 827, 154318. [Google Scholar] [CrossRef]
- Hong, Z.; Li, M.; Wang, H.; Xu, L.; Hong, Y.; Chen, J.; Chen, J.; Zhang, H.; Zhang, Y.; Wu, X.; et al. Characteristics of atmospheric volatile organic compounds (VOCs) at a mountainous forest site and two urban sites in the southeast of China. Sci. Total Environ. 2019, 657, 1491–1500. [Google Scholar] [CrossRef]
- Blunden, J.; Aneja, V.P.; Lonneman, W.A. Characterization of non-methane volatile organic compounds at swine facilities in eastern North Carolina. Atmos. Environ. 2005, 39, 6707–6718. [Google Scholar] [CrossRef]
- de Saint Laumer, J.-Y.; Cicchetti, E.; Merle, P.; Egger, J.; Chaintreau, A. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010, 82, 6457–6462. [Google Scholar] [CrossRef]
- Pacchiarotta, T.; Nevedomskaya, E.; Carrasco-Pancorbo, A.; Deelder, A.M.; Mayboroda, O.A. Evaluation of GC-APCI/MS and GC-FID As a Complementary Platform. J. Biomol. Tech. 2010, 21, 205–213. [Google Scholar]
- Polvara, E.; Morosini, D.; Invernizzi, M.; Sironi, S. Analysis of Odorous VOCs using TD-GC-MS/FID/PFPD: Development and Applications to Real Samples. Chem. Eng. Trans. 2022, 95, 151–156. [Google Scholar]
- Walters, C.C.; Wang, F.C.; Higgins, M.B.; Madincea, M.E. Universal biomarker analysis using GC×GC with dual FID and ToF-MS (EI/FI) detection. Org. Geochem. 2018, 115, 57–66. [Google Scholar] [CrossRef]
- Evans, J.C.; Huckaby, J.L.; Mitroshkov, A.V.; Julya, J.L.; Hayes, J.C.; Edwards, J.A.; Sasaki, L.M. 32-Week Holding-Time Study of SUMMA Polished Canisters and Triple Sorbent Traps Used To Sample Organic Constituents in Radioactive Waste Tank Vapor Headspace. Environ. Sci. Technol. 1998, 32, 3410–3417. [Google Scholar] [CrossRef]
- Zhai, H.; Zhou, J.; Zhao, W.; Du, W.; Wei, N.; Liu, Q.; Wang, S.; Zhang, J.; Cui, W.; Zhang, W.; et al. Development of a UAV-borne sorbent tube sampler and its application on the vertical profile measurement of volatile organic compounds. J. Environ. Sci. 2025, 152, 276–286. [Google Scholar] [CrossRef]
- Seethapathy, S.; Górecki, T.; Li, X. Passive sampling in environmental analysis. J. Chromatogr. A 2008, 1184, 234–253. [Google Scholar] [CrossRef] [PubMed]
- Rumsey, I.C.; Aneja, V.P.; Lonneman, W.A. Characterizing non-methane volatile organic compounds emissions from a swine concentrated animal feeding operation. Atmos. Environ. 2012, 47, 348–357. [Google Scholar] [CrossRef]





| Zone | Trap Temperature (°C) | Preheat (°C) | Transfer (°C) | Precool (°C) | Inject (°C) | Bakeout (°C) | Transfer Line (°C) | |
|---|---|---|---|---|---|---|---|---|
| M1→M2 | M2→M3 | |||||||
| M1 | −40 | 10 | 10 | 120 | ||||
| M2 | −100 | −40 | −40 | 235 | 220 | |||
| M3 | −180 | −185 | 70 | 110 | ||||
| Sample | 20 | |||||||
| GC | 110 | |||||||
| Compound | Concentration (μg m−3) | |
|---|---|---|
| Passive | Active | |
| trimethylamine | 67.44 1 (9.76) 2 | 7.51 (1.32) |
| methyl acetate | 5.36 (0.31) | 4.85 (0.64) |
| 2,3-butanedione | 5.34 (1.02) | 3.23 (0.27) |
| 1-butanol | 5.13 (0.97) | 2.23 (0.46) |
| butyl acetate | 0.89 (0.04) | 0.79 (0.09) |
| benzaldehyde | 1.87 (0.05) | 1.39 (0.12) |
| dimethyl trisulfide | 7.73 (2.13) | 0.58 (0.09) |
| p-cresol | 6.65 (1.04) | 0.87 (0.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Sun, Q.; Yu, L.; Wang, X.; Feng, L.; Wang, K. Influence of Field Sampling Methods on Measuring Volatile Organic Compounds in a Swine Facility Using SUMMA Canisters. Atmosphere 2024, 15, 1021. https://doi.org/10.3390/atmos15091021
Li X, Sun Q, Yu L, Wang X, Feng L, Wang K. Influence of Field Sampling Methods on Measuring Volatile Organic Compounds in a Swine Facility Using SUMMA Canisters. Atmosphere. 2024; 15(9):1021. https://doi.org/10.3390/atmos15091021
Chicago/Turabian StyleLi, Xin, Qinqin Sun, Lei Yu, Xiaoshuai Wang, Li Feng, and Kaiying Wang. 2024. "Influence of Field Sampling Methods on Measuring Volatile Organic Compounds in a Swine Facility Using SUMMA Canisters" Atmosphere 15, no. 9: 1021. https://doi.org/10.3390/atmos15091021
APA StyleLi, X., Sun, Q., Yu, L., Wang, X., Feng, L., & Wang, K. (2024). Influence of Field Sampling Methods on Measuring Volatile Organic Compounds in a Swine Facility Using SUMMA Canisters. Atmosphere, 15(9), 1021. https://doi.org/10.3390/atmos15091021








