Review of the Mechanisms of Liquid-Phase Transformation of Atmospheric Phenolic Compounds: Implications for Air Quality and Environmental Health
Abstract
:1. Introduction
2. Direct Conversion of Phenol
3. Reactive Species Involved
3.1. Reactive Nitrogen Species (RNS)
3.1.1. Types and Sources of Atmospheric RNS
- 1.
- Photosensitive reactions have two response mechanisms:
- (1)
- In the presence of light, PhCs are excited to the singlet state (1P*), which is quickly transformed to the triplet state (3P*). Phenol in the triplet state conducts electron transfer with H2O to produce a radical anion with reducing characteristics, which then combines with dissolved oxygen (DO) to produce superoxide anion (O2·−) [29].
Subsequently, the formation of O2·− which converts NO2 or NO to NO2− [30],- (2)
- Light-excited photosensitizers generate a reducing radical intermediate, which interacts immediately with NO2 to yield HONO/NO2−:
- 2.
- The two methods of producing nitrite ions under non-photosensitive condition are as follows:
- (1)
- Under aerobic conditions, some organic compounds containing ·OH form alkyl peroxyl radicals (RO2·), superoxide radicals (O2·−), or their conjugate acids (HO2·), which react directly with nitrogen-containing oxides in the air to produce secondary products such as NO2− [31].
- (2)
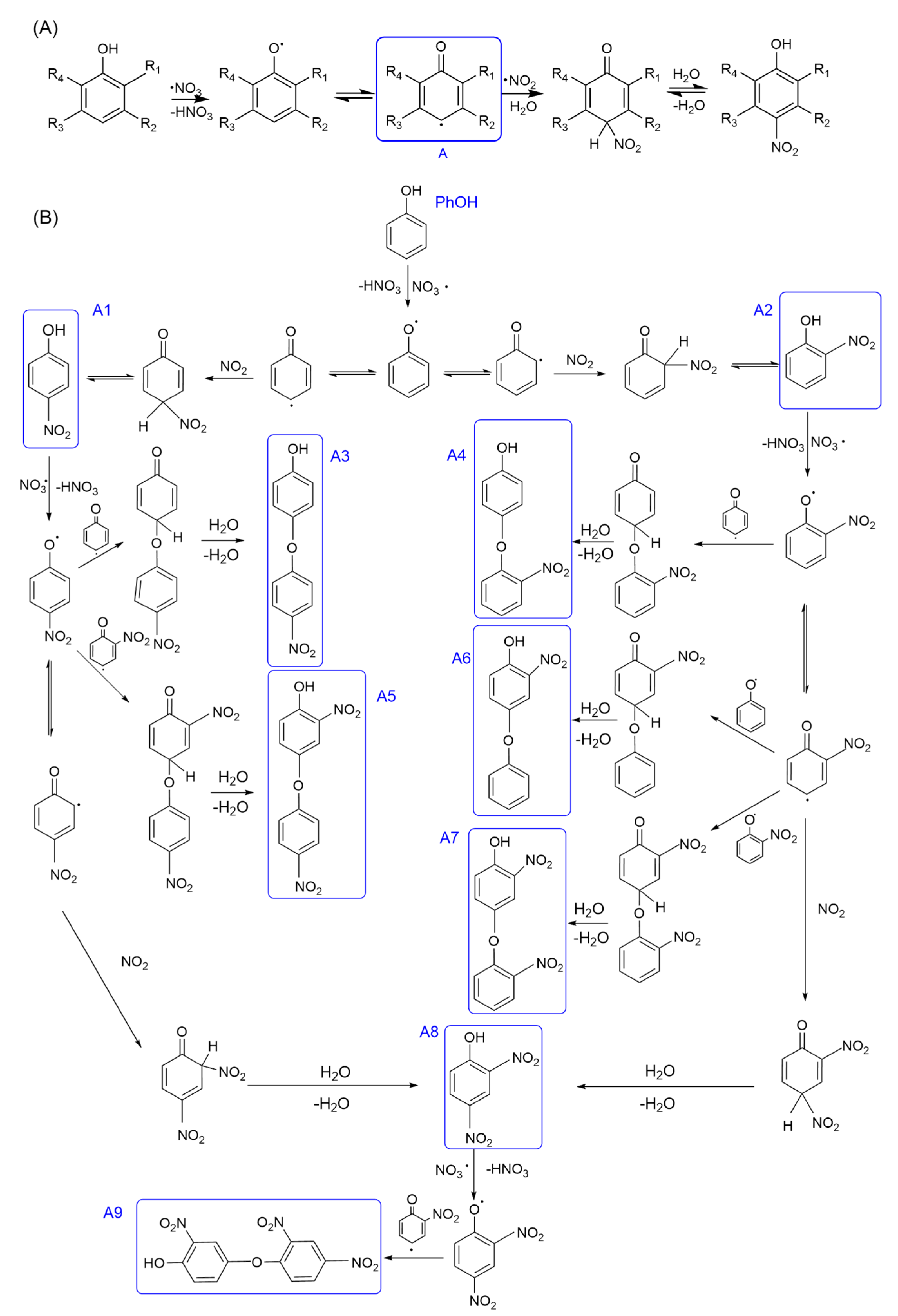
3.1.2. Nitration Mechanism of PhCs
3.1.3. Reaction Pathways and Products
3.2. Hydroxyl Radicals (·OH)
3.2.1. The Generation Pathway of ·OH in the Atmosphere
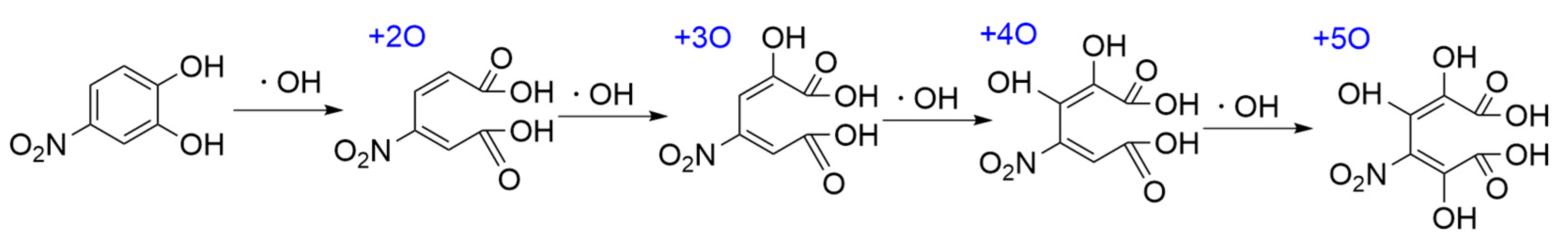
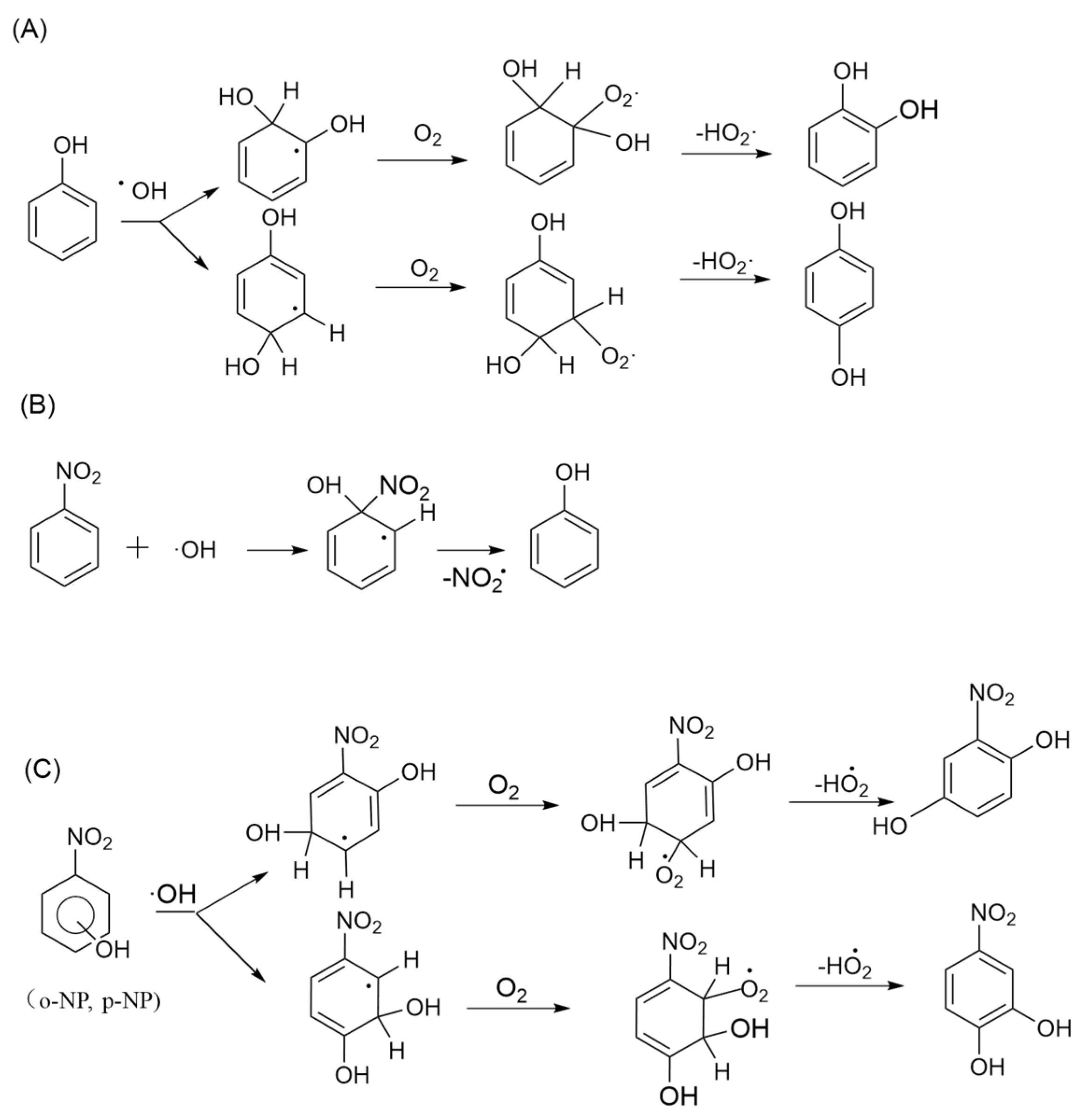
3.2.2. PhCs Oxidation Mechanism Involving ·OH
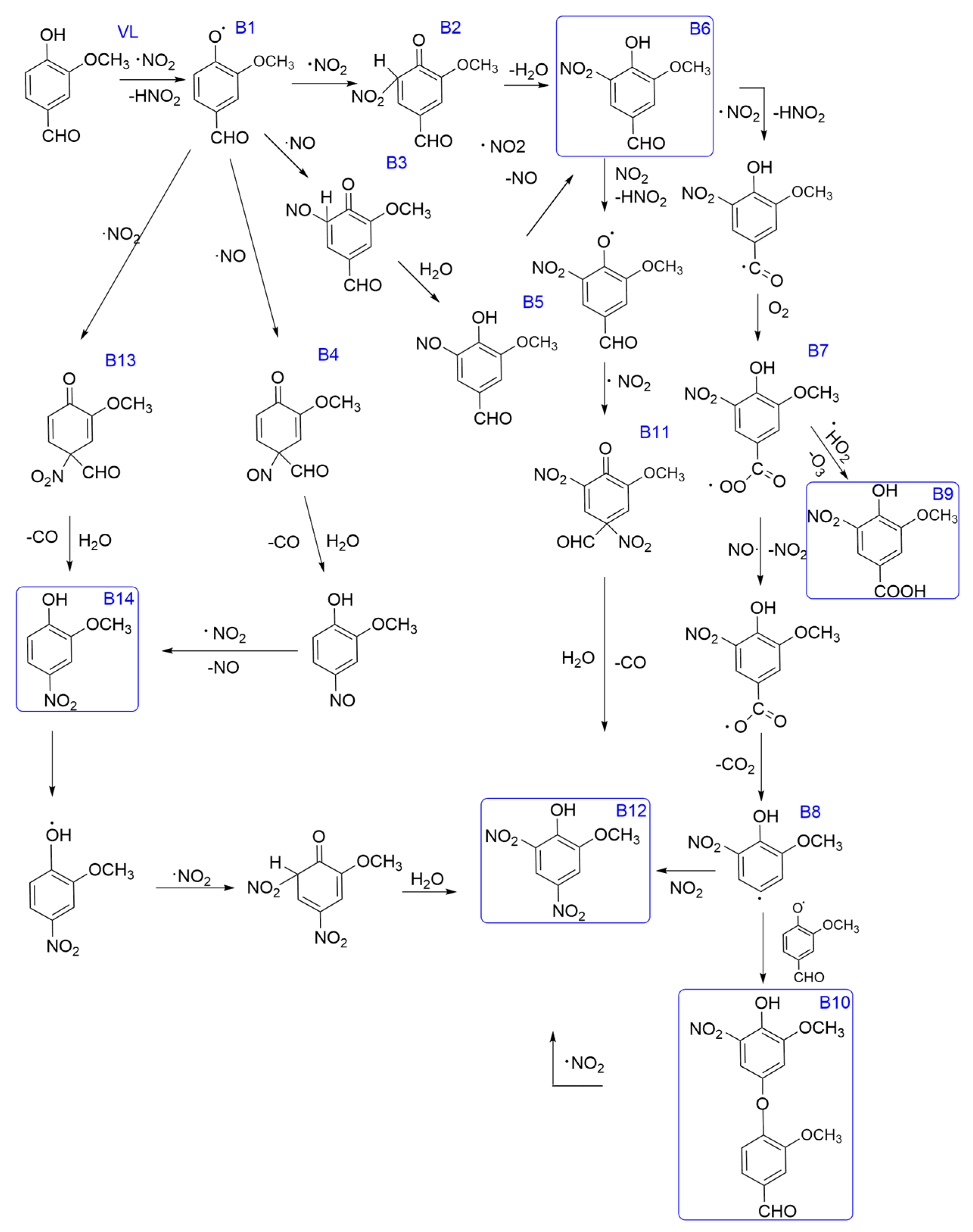
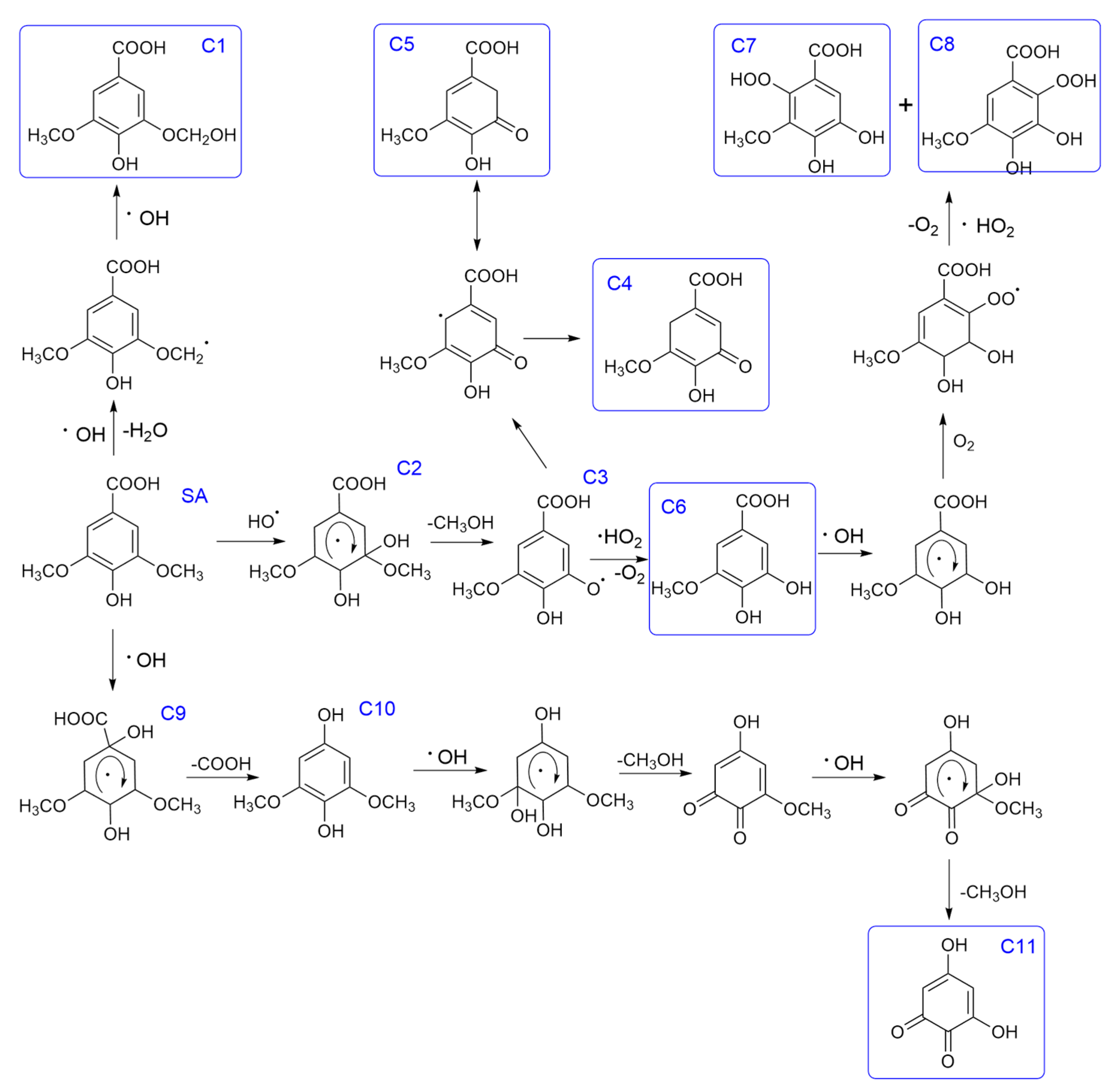
3.2.3. The Oxidation Pathways of Syringate by HO·
3.3. Triplet State Organic Matter (3C*)
3.3.1. Generation of Triplet Compounds
3.3.2. Reaction Mechanism of PhCs and 3C*
3.3.3. Specific Reaction Processes and Products of Phenol with 3C*
4. Factors Affecting the Liquid-Phase Conversion of PhCs in the Atmosphere
4.1. Effect of pH on the Transformation of PhCs
4.2. Effect of Reactant Concentration on the Conversion of PhCs
4.3. Effect of Transition Metal Ions on the Transformation of PhCs
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oak, Y.J.; Park, R.J.; Jo, D.S.; Hodzic, A.; Jimenez, J.L.; Campuzano-Jost, P.; Nault, B.A.; Kim, H.; Kim, H.; Ha, E.S. Evaluation of Secondary Organic Aerosol (SOA) Simulations for Seoul, Korea. J. Adv. Model. Earth Syst. 2022, 14, e2021MS002760. [Google Scholar] [CrossRef]
- Mabato, B.R.G.; Lyu, Y.; Ji, Y.; Li, Y.J.; Huang, D.D.; Li, X.; Nah, T.; Lam, C.H.; Chan, C.K. Aqueous secondary organic aerosol formation from the direct photosensitized oxidation of vanillin in the absence and presence of ammonium nitrate. Atmos. Chem. Phys. 2022, 22, 273–293. [Google Scholar] [CrossRef]
- Akherati, A.; He, Y.; Coggon, M.M.; Koss, A.R.; Hodshire, A.L.; Sekimoto, K.; Warneke, C.; de Gouw, J.; Yee, L.; Seinfeld, J.H. Oxygenated Aromatic Compounds are Important Precursors of Secondary Organic Aerosol in Biomass-Burning Emissions. Environ. Sci. Technol. 2020, 54, 8568–8579. [Google Scholar] [CrossRef]
- Chow, K.S.; Huang, X.H.H.; Yu, J.Z. Quantification of nitroaromatic compounds in at-mospheric fine particulate matter in Hong Kong over 3 years: Field measurement evidence for secondary formation derived from biomass burning emissions. Environ. Chem. 2016, 13, 665–673. [Google Scholar] [CrossRef]
- Mohr, C.; Lopez-Hilfiker, F.D.; Zotter, P.; Prévôt, A.S.H.; Xu, L.; Ng, N.L.; Herndon, S.C.; Williams, L.R.; Franklin, J.P.; Zahniser, M.S.; et al. Contribution of nitrated phenols to wood burning brown carbon light absorption in Detling, United Kingdom during winter time. Environ. Sci. Technol. 2013, 47, 6316–6324. [Google Scholar] [CrossRef]
- Nakao, S.; Clark, C.; Tang, P.; Sato, K.; Cocker, D., III. Secondary organic aerosol forma-tion from phenolic compounds in the absence of NOx. Atmos. Chem. Phys. 2011, 11, 10649–10660. [Google Scholar] [CrossRef]
- Allen, S.K.; Allen, C.W. Phenol concentrations in air and rain water samples col-lected near a wood preserving facility. Bull. Environ. Contam. Toxicol. 1997, 59, 702–707. [Google Scholar] [CrossRef]
- Eldering, A.; Cass, G.R. Source-oriented model for air pollutant effects on visibility. J. Geophys. Res. Atmos. 1996, 101, 19343–19369. [Google Scholar] [CrossRef]
- Cheng, Y.; He, K.-B.; Engling, G.; Weber, R.; Liu, J.-M.; Du, Z.-Y.; Dong, S.-P. Brown and black carbon in Beijing aerosol: Implications for the effects of brown coating on light absorption by black carbon. Sci. Total Environ. 2017, 599–600, 1047–1055. [Google Scholar] [CrossRef]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. One-year study of nitro-organic compounds and their relation to wood burning in PM10 aerosol from a rural site in Belgium. Atmos. Environ. 2005, 81, 561–568. [Google Scholar]
- Desyaterik, Y.; Sun, Y.; Shen, X.; Lee, T.; Wang, X.; Wang, T.; Collett, J.L. Speciation of “brown” carbon in cloud water impacted by agricultural biomass burning in eastern China. J. Geophys. Res. Atmos. 2013, 118, 7389–7399. [Google Scholar] [CrossRef]
- Andreae, M.O.; Gelencsér, A. Black carbon or brown carbon? The nature of light-absorbing carbonaceous aerosols. Atmos. Chem. Phys. 2006, 6, 3131–3148. [Google Scholar] [CrossRef]
- Feng, Y.; Ramanathan, V.; Kotamarthi, V.R. Brown carbon: A significant atmospheric absorber of solar radiation? Atmos. Chem. Phys. 2013, 13, 8607–8621. [Google Scholar] [CrossRef]
- Wang, X.; Gu, R.; Wang, L.; Xu, W.; Zhang, Y.; Chen, B.; Li, W.; Xue, L.; Chen, J.; Wang, W. Emissions of fine particulate nitrated phenols from the burning of five common types of biomass. Environ. Pollut. 2017, 230, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Lu, C.; Li, R.; Zhang, J.; Dong, S.; Yang, L.; Xue, L.; Chen, J.; Wang, W. Nitrated phenols and the phenolic precursors in the atmosphere in urban Jinan, China. Sci. Total Environ. 2020, 714, 136760. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, N.; Vinogradova, E.; Chaplygin, V.; Mandzhieva, S.; Kumar, P.; Rajput, V.D.; Minkina, T.; Seth, C.S.; Burachevskaya, M.; Lysenko, D.; et al. Phenolic Compounds of the Medicinal Plants in an Anthropogenically Transformed Environment. Molecules 2023, 28, 6322. [Google Scholar] [CrossRef]
- Kazamias, G.; Roulia, M.; Kapsimali, I.; Chassapis, K. Innovative biocatalytic production of soil substrate from green waste compost as a sustainable peat substitute. J. Environ. Manag. 2017, 203, 670–678. [Google Scholar] [CrossRef]
- Chaparro-Hernández, I.; Rodríguez-Ramírez, J.; Barriada-Bernal, L.G.; Méndez-Lagunas, L. Tree ferns (Cyatheaceae) as a source of phenolic compounds—A review. J. Herb. Med. 2022, 35, 100587. [Google Scholar] [CrossRef]
- Pereira, E.; Cadavez, V.; Barros, L.; Encina-Zelada, C.; Stojković, D.; Sokovic, M.; Calhelha, R.C.; Gonzales-Barron, U.; Ferreira, I.C.F.R. Chenopodium quinoa Willd. (quinoa) grains: A good source of phenolic compounds. Food Res. Int. 2020, 137, 109574. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Citrus reticulata Blanco peels as a source of antioxidant and anti-proliferative phenolic compounds. Ind. Crops Prod. 2018, 111, 141–148. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, H.; Xia, D.; Li, X.; Qu, B. Formation of free radicals by direct photolysis of halogenated phenols (HPs) and effects of DOM: A case study on monobromophenols. J. Hazard. Mater. 2020, 391, 122220. [Google Scholar] [CrossRef]
- Davde, V.V.M.; Claudio, M.D.B.; Mirco, L.A.; Pelizzetti, E. New Processes in theEnvironmental Chemistry of Nitrite 2. The Role of Hydrogen Peroxide. Environ. Sci. Technol. 2003, 37, 4635–4641. [Google Scholar]
- Martin, P.A.I.; Rodriguez, P.D.; Garcia, C.A.; Len, C.; Miguel, G.; Munoz, B.M.J.; Luque, R. Photocatalytic Production of Vanillin over CeOx and ZrO2 Modified Biomass-Templated Titania. Ind. Eng. Chem. Res. 2020, 59, 17085–17093. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, B.; Liu, X.; Luo, Z.; Xing, R.; Li, Y.; Xiong, R.; Li, G.; Cheng, H.; Lu, Q.; et al. Variabilities in Primary N-Containing Aromatic Compound Emissions from Residential Solid Fuel Combustion and Implications for Source Tracers. Environ. Sci. Technol. 2022, 56, 13622–13633. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, E.; Rubasinghege, G. Mechanistic Study on Iron Solubility in Atmospheric Mineral Dust Aerosol: Roles of Titanium, Dissolved Oxygen, and Solar Flux in Solutions Containing Different Acid Anions. ACS Earth Space Chem. 2019, 4, 101–111. [Google Scholar] [CrossRef]
- Kroflic, A.; Anders, J.; Drventic, I.; Mettke, P.; Boge, O.; Mutzel, A.; Kleffmann, J.; Herrmann, H. Guaiacol Nitration in a Simulated Atmospheric Aerosol with an Emphasis on Atmospheric Nitrophenol Formation Mechanisms. ACS Earth Space Chem. 2021, 5, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Zhang, Q.; Lu, X.; Li, K.; Chen, H.; Chen, J.; Yang, X.; Ma, Y.; Ma, J.; Huang, C. Nitrite-Mediated Photooxidation of Vanillin in the Atmospheric Aqueous Phase. Environ. Sci. Technol. 2019, 53, 14253–14263. [Google Scholar] [CrossRef]
- Wang, Y.; Jorga, S.; Abbatt, J. Nitration of Phenols by Reaction with Aqueous Nitrite: A Pathway for the Formation of Atmospheric Brown Carbon. ACS Earth Space Chem. 2023, 7, 632–641. [Google Scholar] [CrossRef]
- Smith, J.D.; Sio, V.; Yu, L.; Zhang, Q.; Anastasio, C. Secondary Organic Aerosol Production from Aqueous Reactions of Atmospheric Phenols with an Organic Triplet Excited State. Environ. Sci. Technol. 2014, 48, 1049–1057. [Google Scholar] [CrossRef]
- Chen, L.; Kong, L.; Tong, S.; Yang, K.; Jin, S.; Wang, C.; Xia, L.; Wang, L. Aqueous phase oxidation of bisulfite influenced by nitrate and its photolysis. Sci. Total Environ. 2021, 785, 147345. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Liao, Y.; Ji, H.; Guo, Y. Study of methylation of nitrogen-containing compounds in the gas phase. J. Mass Spectrom. 2007, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Kristijan, V.D.; Martin, S.; Kroflic, A.; Irena, G. Nighttime aqueous-phase formation of nitrocatechols in the atmospheric condensed phase. Environ. Sci. Technol. Let. 2018, 52, 9722–9730. [Google Scholar]
- Barbara, B.; Bolzacchini, S.M.; Marco, O.A.; Rindone, B. The NO3 Radical-Mediated Liquid Phase Nitration of Phenols with Nitrogen Dioxide. Environ. Sci. Technol. 2000, 34, 2224–2230. [Google Scholar]
- Carena, L.; Zoppi, B.; Sordello, F.; Fabbri, D.; Minella, M.; Minero, C. Phototransformation of Vanillin in Artificial Snow by Direct Photolysis and Mediated by Nitrite. Environ. Sci. Technol. 2023, 57, 8785–8795. [Google Scholar] [CrossRef] [PubMed]
- Kroflic, A.; Grilc, M.; Grgic, I. Unraveling Pathways of Guaiacol Nitration in Atmospheric Waters: Nitrite, A Source of Reactive Nitronium Ion in the Atmosphere. Environ. Sci. Technol. 2015, 49, 9150–9158. [Google Scholar] [CrossRef]
- Zhao, Z.; Le, C.; Xu, Q.; Peng, W.; Jiang, H.; Lin, Y.H.; Cocker, D.R.; Zhang, H. Compositional Evolution of Secondary Organic Aerosol as Temperature and Relative Humidity Cycle in Atmospherically Relevant Ranges. ACS Earth Space Chem. 2019, 3, 2549–2558. [Google Scholar] [CrossRef]
- Mayorga, R.J.; Zhao, Z.; Zhang, H. Formation of secondary organic aerosol from nitrate radical oxidation of phenolic VOCs: Implications for nitration mechanisms and brown carbon formation. Atmos. Environ. 2021, 244, 117910. [Google Scholar] [CrossRef]
- Yu, L.; Smith, J.; Laskin, A.; George, K.M.; Anastasio, C.; Laskin, J.; Dillner, A.M.; Zhang, Q. Molecular transformations of phenolic SOA during photochemical aging in the aqueous phase: Competition among oligomerization, functionalization, and fragmentation. Atmos. Chem. Phys. 2016, 16, 4511–4527. [Google Scholar] [CrossRef]
- Robert, G.C.; Andrew, W.D. The Mechanism of Nitration of Phenol and 4-Methylphenol by Nitrogen Dioxide in Solution. Tetrahedron Lett. 1994, 35, 63734376. [Google Scholar]
- Biberger, T.; Hess, S.N.; Leutzsch, M.; Furstner, A. Hydrogenative Cycloisomerization and Sigmatropic Rearrangement Reactions of Cationic Ruthenium Carbenes Formed by Catalytic Alkyne gem-Hydrogenation. Angew. Chem. Int. Ed. 2022, 61, e202113827. [Google Scholar] [CrossRef]
- De Vrieze, J.E.; Thybaut, J.W.; Saeys, M. Role of Keto–Enol Tautomerization in the Copper-Catalyzed Hydrogenation of Ketones. ACS Catal. 2019, 9, 3831–3839. [Google Scholar] [CrossRef]
- Yan, J.; Cheo, H.W.; Teo, W.K.; Shi, X.; Wu, H.; Idres, S.B.; Deng, L.-W.; Wu, J. A Radical Smiles Rearrangement Promoted by Neutral Eosin Y as a Direct Hydrogen Atom Transfer Photocatalyst. J. Am. Chem. Soc. 2020, 142, 11357–11362. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, W.; Tian, L.; Wang, Y.; Li, F.; Huang, D.D.; Zhang, R.; Go Mabato, B.R.; Huang, R.-J.; Chen, Q.; et al. Decay Kinetics and Absorption Changes of Methoxyphenols and Nitrophenols during Nitrate-Mediated Aqueous Photochemical Oxidation at 254 and 313 nm. ACS Earth Space Chem. 2022, 6, 1115–1125. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Wen, M.; Tang, Z.; Long, B.; Yu, X.; Zhao, C.; Wang, W. Correction: Effects of water, ammonia and formic acid on HO2 + Cl reactions under atmospheric conditions: Competition between a stepwise route and one elementary step. RSC Adv. 2019, 9, 22987. [Google Scholar] [CrossRef] [PubMed]
- El Gehani, A.A.M.A.; Maashi, H.A.; Harnedy, J.; Morrill, L.C. Electrochemical generation and utilization of alkoxy radicals. Chem. Commun. 2023, 59, 3655–3664. [Google Scholar] [CrossRef]
- Mizugaki, T.; Ebitani, K.; Kaneda, K. Catalysis by polymer-bound Rh 6 carbonyl clusters Selective hydrogenation of carbonyl compounds in the presence of CO and H2O. Appl. Surf. Sci. 1997, 121, 360–365. [Google Scholar] [CrossRef]
- Li, Y.J.; Huang, D.D.; Cheung, H.Y.; Lee, A.K.Y.; Chan, C.K. Aqueous-phase photochemical oxidation and direct photolysis of vanillin–a model compound of methoxy phenols from biomass burning. Atmos. Chem. Phys. 2014, 14, 2871–2885. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Liu, H.; Wu, Y.; Dong, W. Discrepant oxidation behavior of ferric ion and hydroxyl radical on syringic acid and vanillic acid in atmospheric Fenton-like system. Chemosphere 2022, 287, 132022. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Lakey, P.S.J.; Rivera-Rios, J.C.; Keutsch, F.N.; Shiraiwa, M. Aqueous-Phase Decomposition of Isoprene Hydroxy Hydroperoxide and Hydroxyl Radical Formation by Fenton-like Reactions with Iron Ions. J. Phys. Chem. A 2020, 124, 5230–5236. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, Q.; Wang, H.; Cai, D.; Ma, Y.; Li, L.; Li, K.; Lu, X.; Chen, H.; Yang, X.; et al. Photochemical Aging of Cuaiacol by Fe(III)–Oxalate Complexes in Atmospheric Aqueous Phase. Environ. Sci. Technol. 2018, 53, 127–136. [Google Scholar] [CrossRef]
- Hems, R.F.; Abbatt, J.P.D. Aqueous Phase Photo-oxidation of Brown Carbon Nitrophenols: Reaction Kinetics, Mechanism, and Evolution of Light Absorption. ACS Earth Space Chem. 2018, 2, 225–234. [Google Scholar] [CrossRef]
- Chen, B.; Yang, C.; Goh, N.K. Photolysis pathway of nitroaromatic compounds in aqueous solutions in the UV/H2O2 process. J. Environ. Sci. 2006, 18, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.R.; How, K.C.-Y.; Lee, Y.-P. Hydrogen Abstraction of Acetic Acid by Hydrogen Atom to Form Carboxymethyl Radical ·CH2C(O)OH in Solid para-Hydrogen and Its Implication in Astrochemistry. ACS Earth Space Chem. 2021, 5, 106–117. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, H.; Hu, H.; Zhou, Y.; Li, J.; Jin, L. Novel insight into pyrolysis behaviors of lignin using in-situ pyrolysis-double ionization time-of-flight mass spectrometry combined with electron paramagnetic resonance spectroscopy. Bioresour. Technol. 2020, 312, 123555. [Google Scholar] [CrossRef] [PubMed]
- Teng, A.P.; Crounse, J.D.; Wennberg, P.O. Isoprene Peroxy Radical Dynamics. Isoprene Peroxy Radical Dynamics. J. Am. Chem. Soc. 2017, 139, 5367–5377. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guzman, C.; Niedek, C.; Tran, T.; Zhang, Q.; Anastasio, C. Kinetics and Mass Yields of Aqueous Secondary Organic Aerosol from Highly Substituted Phenols Reacting with a Triplet Excited State. Environ. Sci. Technol. 2021, 55, 5772–5781. [Google Scholar] [CrossRef]
- Arciva, S.; Ma, L.; Mavis, C.; Guzman, C.; Anastasio, C. Formation and Loss of Light Absorbance by Phenolic Aqueous SOA by OH and an Organic Triplet Excited State. Atmos. Chem. Phys. 2023, 24, 4473–4485. [Google Scholar] [CrossRef]
- Chen, Y.; Li, N.; Li, X.; Tao, Y.; Luo, S.; Zhao, Z.; Ma, S.; Huang, H.; Chen, Y.; Ye, Z.; et al. Secondary organic aerosol formation from 3C⁎-initiated oxidation of 4-ethylguaiacol in atmospheric aqueous-phase. Sci. Total Environ. 2020, 723, 137953. [Google Scholar] [CrossRef]
- Silvio, C.; Bruno, H.; Jakob, W. Oxidation of Phenols by Triplet Aromatic Ketones in Aqueous Solution. J. Phys. Chem. A. 2000, 104, 1226–1232. [Google Scholar]
- Yu, L.; Smith, J.; Laskin, A.; Anastasio, C.; Laskin, J.; Zhang, Q. Chemical characterization of SOA formed from aqueous-phase reactions of phenols with the triplet excited state of carbonyl and hydroxyl radical. Atmos. Chem. Phys. 2014, 14, 13801–13816. [Google Scholar] [CrossRef]
- Yang, J.; Au, W.C.; Law, H.; Lam, C.H.; Nah, T. Formation and evolution of brown carbon during aqueous-phase nitrate-mediated photooxidation of guaiacol and 5-nitroguaiacol. Atmos. Environ. 2021, 254, 118401. [Google Scholar] [CrossRef]
- Barsotti, F.; Bartels-Rausch, T.; De Laurentiis, E.; Ammann, M.; Brigante, M.; Mailhot, G.; Maurino, V.; Minero, C.; Vione, D. Photochemical Formation of Nitrite and Nitrous Acid (HONO) upon Irradiation of Nitrophenols in Aqueous Solution and in Viscous Secondary Organic Aerosol Proxy. Environ. Sci. Technol. 2017, 51, 7486–7495. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.; Haymann, L.; Chun, H.L.; Chun, H.L.; Theodora, N. pH affects the aqueous-phase nitrate-mediated photooxidation of phenolic compounds: Implications for brown carbon formation and evolution. Environ. Sci.-Proc. Impacts 2022, 25, 176–189. [Google Scholar] [CrossRef]
- Vidovic, K.; Kroflic, A.; Sala, M.; Grgic, I. Aqueous-Phase Brown Carbon Formation from Aromatic Precursors under Sunlight Conditions. Atmosphere 2020, 11, 131. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, T.; Huang, W.; Fang, D.; Zhang, X.; Zhang, L.; Huo, P.; Xiao, K.; Zhang, Y.; Zhang, Y. The complexation between transition metals and water-soluble organic compounds (WSOC) and its effect on reactive oxygen species (ROS) formation. Atmos. Environ. 2022, 287, 119247. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Sha, T.; Liu, Y. Significant Promotion of Light Absorption Ability and Formation of Triplet Organics and Reactive Oxygen Species in Atmospheric HULIS by Fe(III) Ions. Environ. Sci. Technol. 2022, 56, 16652–16664. [Google Scholar] [CrossRef]
- Lavi, A.; Lin, P.; Bhaduri, B.; Carmieli, R.; Laskin, A.; Rudich, Y. Characterization of Light-Absorbing Oligomers from Reactions of Phenolic Compounds and Fe(III). ACS Earth Space Chem. 2017, 1, 637–646. [Google Scholar] [CrossRef]
- Ito, A. Atmospheric Processing of Combustion Aerosols as a Source of Bioavailable Iron. Environ. Sci. Technol. Lett. 2015, 2, 70–75. [Google Scholar] [CrossRef]
- Weller, C.; Horn, S.; Herrmann, H. Effects of Fe(III)-concentration, speciation, excitation-wavelength and light intensity on the quantum yield of iron(III)-oxalato complex photolysis. J. Photochem. Photobiol. A Chem. 2013, 255, 41–49. [Google Scholar] [CrossRef]
- Thomas, D.A.; Coggon, M.M.; Lignell, H.; Schilling, K.A.; Zhang, X.; Schwantes, R.H.; Flagan, R.C.; Seinfeld, J.H.; Beauchamp, J.L. Real-Time Studies of Iron Oxalate-Mediated Oxidation of Glycolaldehyde as a Model for Photochemical Aging of Aqueous Tropospheric Aerosols. Environ. Sci. Technol. 2016, 50, 12241–12249. [Google Scholar] [CrossRef]
- Yan, R.; Yang, W.; You, D.; Yang, H.; Han, C. Photoinduced evolution of optical properties and compositions of methoxyphenols by Fe(III)-carboxylates complexes in atmospheric aqueous phase. Chemosphere 2022, 295, 133860. [Google Scholar] [CrossRef]
- Zuo, Y.; Hoigne, J. Formation of Hydrogen Peroxide and Depletion of Oxalic Acid in Atmospheric Water by Photolysis of Iron(III)-Oxalato Complexes. Envlron. Sci. Technol. 1992, 26, 1014–1022. [Google Scholar] [CrossRef]
- Ling, J.; Zheng, S.; Sheng, F.; Wu, H.; Chen, Z.; Gu, C.; Jin, X. Effect of common inorganic anions on iron-catalyzed secondary brown carbon formation from guaiacol. Sci. Total Environ. 2021, 770, 145206. [Google Scholar] [CrossRef] [PubMed]
- Slikboer, S.; Grandy, L.; Blair, S.L.; Nizkorodov, S.A.; Smith, R.W.; Al-Abadleh, H.A. Formation of Light Absorbing Soluble Secondary Organics and Insoluble Polymeric Particles from the Dark Reaction of Catechol and Guaiacol with Fe(III). Environ. Sci. Technol. 2015, 49, 7793–7801. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Kinney, H.; Anastasio, C. Aqueous benzene-diols react with an organic triplet excited state and hydroxyl radical to form secondary organic aerosol. Phys. Chem. Chem. 2015, 17, 10227–10237. [Google Scholar] [CrossRef] [PubMed]


| Compound | CAS | Molecular Weight (g/mol) | Molecular Formula | Structural Formula | Melting Point | Boiling Point | pKa | Solubility (g/L) |
|---|---|---|---|---|---|---|---|---|
| Vanillin (VL) | 121-33-5 | 152.15 | C8H8O3 | 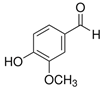 | 81–83 °C | 170 °C | 7.40 (25 °C) | 10 (25 °C) |
| Guaiacol (GUA) | 90-05-1 | 124.14 | C7H8O2 | 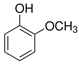 | 26–29 °C | 205 °C | 9.98 (25 °C) | 17 (15 °C) |
| Catechol (CAT) | 120-80-9 | 110.11 | C6H6O2 |  | 100–103 °C | 245 °C | 9.85 (20 °C) | 430 (20 °C) |
| Phenol (PhOH) | 108-95-2 | 94.11 | C6H6O |  | 40–43 °C | 182 °C | 9.89 (20 °C) | 9 (20 °C) |
| Syringic acid (SA) | 530-57-4 | 198.17 | C9H10O5 | 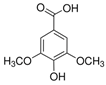 | 205–109 °C | 193 °C | 4.33 (25 °C) | 5.7 (25 °C) |
| 4-Nitrocatechol (4NC) | 3316-09-4 | 155.11 | C6H5NO4 |  | 173–177 °C | 289 °C | 6.84 (25 °C) | soluble |
| 5-Nitroguaiacol (5NG) | 636-93-1 | 169.13 | C7H7NO4 | 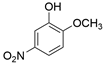 | 103–107 °C | 110–112 °C | 8.31 (25 °C) | soluble |
| Compound | PhOH | VL | GUA | CAT | SA | |
|---|---|---|---|---|---|---|
| ·OH | Experimental conditions | 293 K pH = 5 | 298 K pH = 5 | 293 K pH = 5 | 293 K pH = 5.5 | 313 K pH = 5.5 |
| k (M−1 s−1) | 1.5 ± 0.5 × 1010 | 4 × 108 | 1.6 ± 0.5 × 1010 | 6.9 ± 2.4 × 107 | 1.66 × 1010 | |
| Reference | [75] | [25] | [75] | [75] | [48] | |
| NO2· | Experimental conditions | 298 K pH = 5 | - | 298 K pH = 4.5 | 313 K pH = 5 | - |
| k (M−1 s−1) | 2.7 ± 0.04 × 108 | - | 4.01 ± 0.04 × 109 | 1.9 × 109 | - | |
| Reference | [22] | - | [35] | [28] | - | |
| 3C* | Experimental conditions | 293 K pH = 5 | - | 293 K pH = 5 | 293 K pH = 5 | 293 K pH = 2 |
| k (M−1 s−1) | 1.3 ± 0.9 × 108 | - | 2.5 ± 0.6 × 109 | 5.8 ± 2.0 × 108 | 1.1 ± 0.3 × 1010 | |
| Reference | [29] | - | [75] | [75] | [56] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, X.; Cai, M.; Chen, K. Review of the Mechanisms of Liquid-Phase Transformation of Atmospheric Phenolic Compounds: Implications for Air Quality and Environmental Health. Atmosphere 2024, 15, 1040. https://doi.org/10.3390/atmos15091040
Yang Y, Li X, Cai M, Chen K. Review of the Mechanisms of Liquid-Phase Transformation of Atmospheric Phenolic Compounds: Implications for Air Quality and Environmental Health. Atmosphere. 2024; 15(9):1040. https://doi.org/10.3390/atmos15091040
Chicago/Turabian StyleYang, Yuyan, Xingru Li, Min Cai, and Kaitao Chen. 2024. "Review of the Mechanisms of Liquid-Phase Transformation of Atmospheric Phenolic Compounds: Implications for Air Quality and Environmental Health" Atmosphere 15, no. 9: 1040. https://doi.org/10.3390/atmos15091040
APA StyleYang, Y., Li, X., Cai, M., & Chen, K. (2024). Review of the Mechanisms of Liquid-Phase Transformation of Atmospheric Phenolic Compounds: Implications for Air Quality and Environmental Health. Atmosphere, 15(9), 1040. https://doi.org/10.3390/atmos15091040








