Differential Susceptibility to Particulate Matter-Induced Cardiac Remodeling and Senescence: A Comparative Study in Young and Aged Mice
Abstract
:1. Introduction
2. Materials and Methods
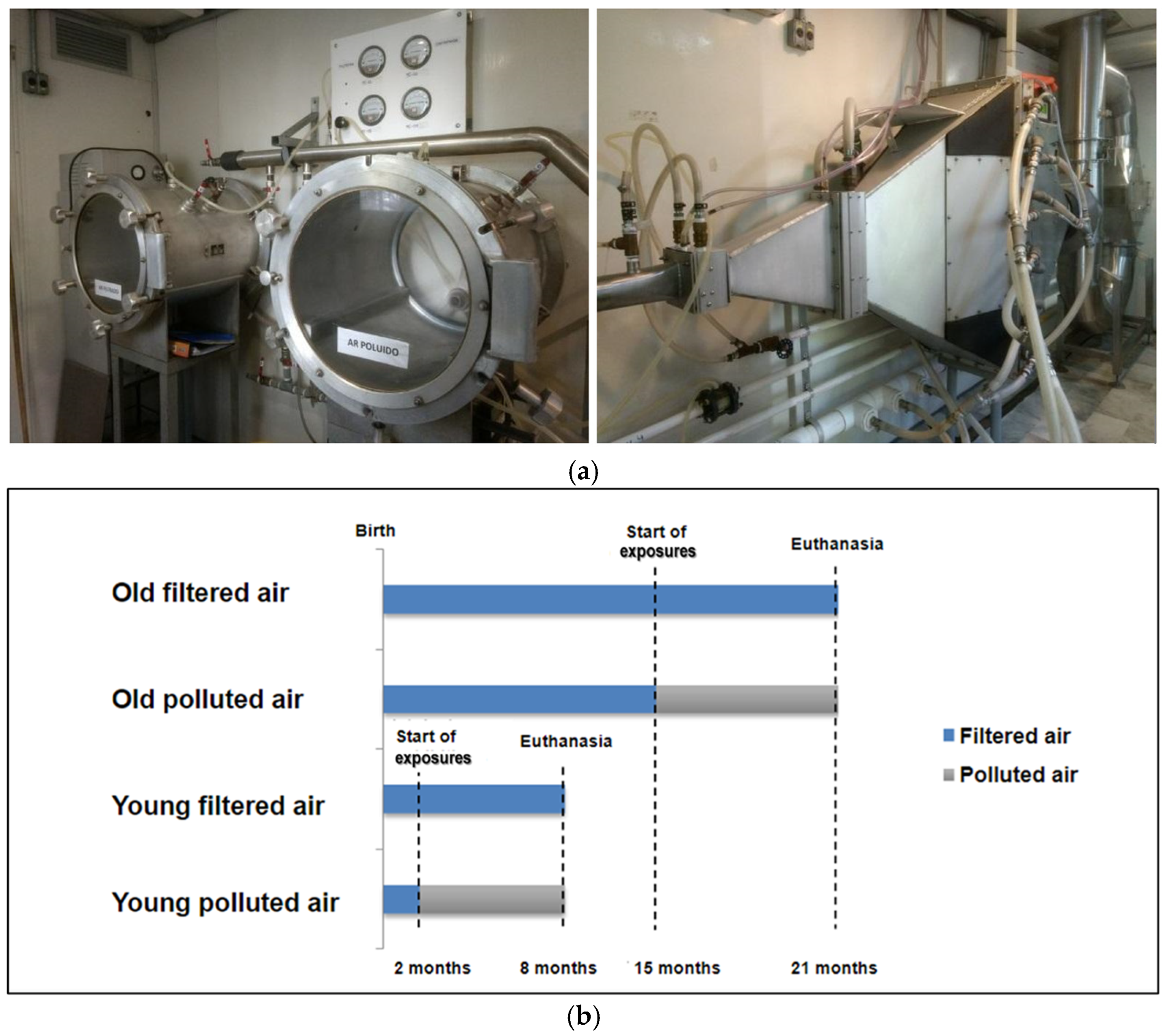
2.1. Animals and Experimental Protocol
2.2. Exposure to Air Pollution
2.3. Exposure Characterization
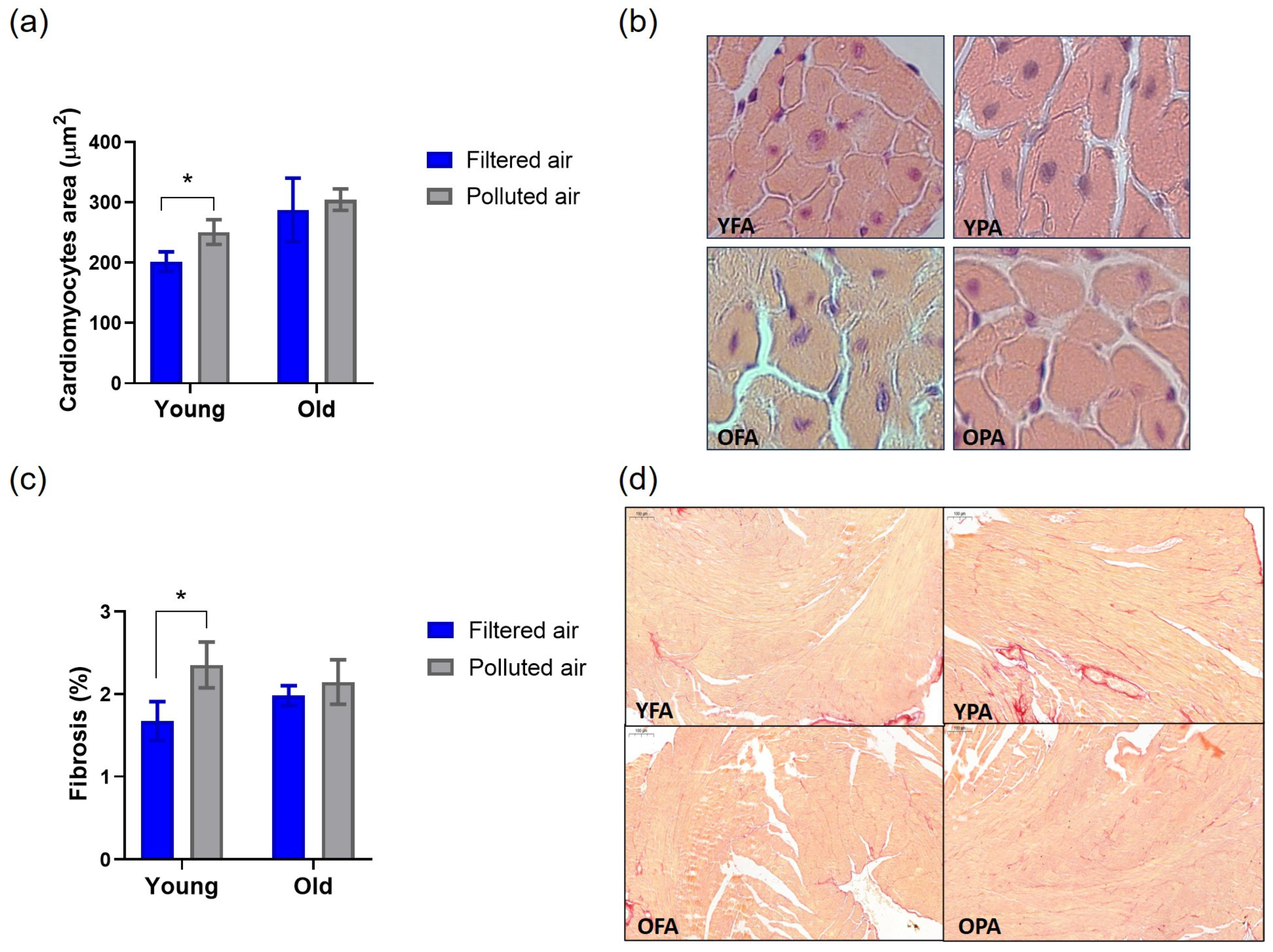
2.4. Histological Analysis
2.5. Lipofuscin Measurements
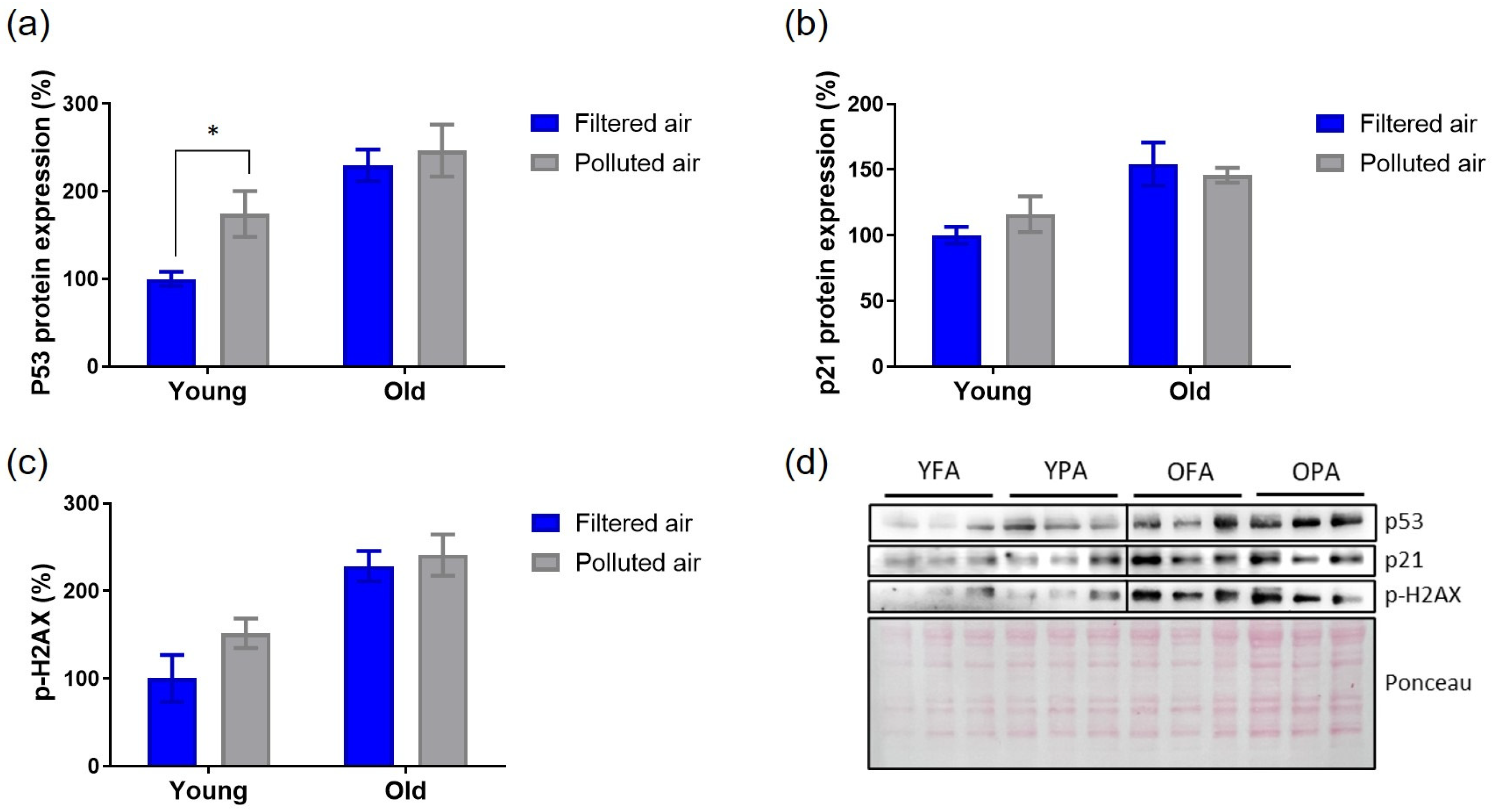
2.6. Analysis of Protein Expression of Cellular Senescence Markers
2.7. Statistical Analysis
3. Results
3.1. Experimental Exposure
3.2. Chronic PM Exposure Did Not Affect Body Weight and Cardiac Weight Ratio
3.3. Chronic PM Exposure Affects Cardiac Remodeling in Young Mice
3.4. Cardiac Senescence Parameters Are Partially Triggered by Chronic Exposure to PM in Young Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1 (accessed on 9 December 2024).
- World Health Organization (WHO). Available online: https://www.who.int/teams/environment-climate-change-and-health/air-quality-and-health/health-impacts/types-of-pollutants (accessed on 9 December 2024).
- Krewski, D.; Rainham, D. Ambient air pollution and population health: Overview. J. Toxicol. Environ. Health Part A 2007, 70, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Pryor, J.T.; Cowley, L.O.; Simonds, S.E. The Physiological Effects of Air Pollution: Particulate Matter, Physiology and Disease. Front. Public Health 2022, 10, 882569. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D. Cardiovascular effects of air pollution. Clin. Sci. 2008, 115, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Akinaga, L.; Lichtenfels, A.; Oliveira, R.; Caldini, E.; Dolhnikoff, M.; Silva, L.; Bueno, H.; Pereira, L.; Saldiva, P.; Garcia, M. Effects of Chronic Exposure to Air Pollution from Sao Paulo City on Coronary of Swiss Mice, from Birth to Adulthood. Toxicol. Pathol. 2009, 37, 306–314. [Google Scholar] [CrossRef]
- Damaceno-Rodrigues, N.R.; Veras, M.M.; Negri, E.M.; Zanchi, A.C.; Rhoden, C.R.; Saldiva, P.H.; Dolhnikoff, M.; Caldini, E.G. Effect of pre- and postnatal exposure to urban air pollution on myocardial lipid peroxidation levels in adult mice. Inhal. Toxicol. 2009, 21, 1129–1137. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Vishwakarma, D.; Dubey, P.; Reddy, S.Y. Air Pollution and the Heart: Updated Evidence from Meta-analysis Studies. Curr. Cardiol. Rep. 2022, 24, 1811–1835. [Google Scholar] [CrossRef]
- Krittanawong, C.; Qadeer, Y.K.; Hayes, R.B.; Wang, Z.; Virani, S.; Thurston, G.D.; Lavie, C.J. PM2.5 and Cardiovascular Health Risks. Curr. Probl. Cardiol. 2023, 48, 101670. [Google Scholar] [CrossRef]
- Abdul-Rahman, T.; Roy, P.; Bliss, Z.S.B.; Mohammad, A.; Corriero, A.C.; Patel, N.T.; Wireko, A.A.; Shaikh, R.; Faith, O.E.; Arevalo-Rios, E.C.E.; et al. The impact of air quality on cardiovascular health: A state of the art review. Curr. Probl. Cardiol. 2024, 49, 102174. [Google Scholar] [CrossRef]
- Feng, S.; Huang, F.; Zhang, Y.; Feng, Y.; Zhang, Y.; Cao, Y.; Wang, X. The pathophysiological and molecular mechanisms of atmospheric PM2.5 affecting cardiovascular health: A review. Ecotoxicol. Environ. Saf. 2023, 249, 114444. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Zhou, Q.; Wu, C.; Shabani, P.; Huang, S.; Yu, X.; Singh, A.P.; Guseh, J.S.; Li, H.; Lerchenmüller, C.; et al. Cardiac aging: From hallmarks to therapeutic opportunities. Cardiovasc. Res. 2024, cvae124. [Google Scholar] [CrossRef]
- Kuntic, M.; Kuntic, I.; Hahad, O.; Lelieveld, J.; Münzel, T.; Daiber, A. Impact of air pollution on cardiovascular aging. Mech. Ageing Dev. 2023, 214, 111857. [Google Scholar] [CrossRef]
- Yang, X.; Sreejayan, N.; Ren, J. Views from within and beyond: Narratives of cardiac contractile dysfunction under senescence. Endocrine 2005, 26, 127–137. [Google Scholar] [CrossRef]
- Hua, Y.; Robinson, T.J.; Cao, Y.; Shi, G.P.; Ren, J.; Nair, S. Cathepsin K knockout alleviates aging-induced cardiac dysfunction. Aging Cell 2015, 14, 345–351. [Google Scholar] [CrossRef]
- Nakou, E.S.; Parthenakis, F.I.; Kallergis, E.M.; Marketou, M.E.; Nakos, K.S.; Vardas, P.E. Healthy aging and myocardium: A complicated process with various effects in cardiac structure and physiology. Int. J. Cardiol. 2016, 209, 167–175. [Google Scholar] [CrossRef]
- Terman, A.; Brunk, U.T. The aging myocardium: Roles of mitochondrial damage and lysosomal degradation. Heart Lung Circ. 2005, 14, 107–114. [Google Scholar] [CrossRef]
- Kakimoto, Y.; Okada, C.; Kawabe, N.; Sasaki, A.; Tsukamoto, H.; Nagao, R.; Osawa, M. Myocardial lipofuscin accumulation in ageing and sudden cardiac death. Sci. Rep. 2019, 9, 3304. [Google Scholar] [CrossRef]
- Li, W.W.; Wang, H.J.; Tan, Y.Z.; Wang, Y.L.; Yu, S.N.; Li, Z.H. Reducing lipofuscin accumulation and cardiomyocytic senescence of aging heart by enhancing autophagy. Exp. Cell Res. 2021, 403, 112585. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fernández, B.; Gredilla, R. Mitochondria and oxidative stress in heart aging. Age 2016, 38, 225–238. [Google Scholar] [CrossRef]
- Bernardes de Jesus, B.; Blasco, M.A. Assessing cell and organ senescence biomarkers. Circ. Res. 2012, 111, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Marín-Aguilar, F.; Lechuga-Vieco, A.V.; Alcocer-Gómez, E.; Castejón-Vega, B.; Lucas, J.; Garrido, C.; Peralta-Garcia, A.; Pérez-Pulido, A.J.; Varela-López, A.; Quiles, J.L.; et al. NLRP3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging Cell 2020, 19, e13050. [Google Scholar] [CrossRef] [PubMed]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chiao, Y.A.; Clark, R.; Flynn, E.R.; Yabluchanskiy, A.; Ghasemi, O.; Zouein, F.; Lindsey, M.L.; Jin, Y.F. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc. Res. 2015, 106, 421–431. [Google Scholar] [CrossRef]
- Yeh, J.K.; Wang, C.Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, B.; Fu, B.; Shang, C.; Feng, H.; Chen, T.; Jiang, Y. PM2.5 Induces Cardiomyoblast Senescence via AhR-Mediated Oxidative Stress. Antioxidants 2024, 13, 786. [Google Scholar] [CrossRef]
- Cheng, Z.; Ito, S.; Nishio, N.; Thanasegaran, S.; Fang, H.; Isobe, K. Characteristics of cardiac aging in C57BL/6 mice. Exp. Gerontol. 2013, 48, 341–348. [Google Scholar] [CrossRef]
- Wold, L.E.; Ying, Z.; Hutchinson, K.R.; Velten, M.; Gorr, M.W.; Velten, C.; Youtz, D.J.; Wang, A.; Lucchesi, P.A.; Sun, Q.; et al. Cardiovascular remodeling in response to long-term exposure to fine particulate matter air pollution. Circ. Heart Fail. 2012, 5, 452–461. [Google Scholar] [CrossRef]
- Sioutas, C.; Koutrakis, P.; Burton, R.M. A technique to expose animals to concentrated fine ambient aerosols. Environ. Health Perspect. 1995, 103, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Benevenuto, S.G.M.; Tomasini, P.P.; Yariwake, V.Y.; de Oliveira Alves, N.; Rahmeier, F.L.; da Cruz Fernandes, M.; Moura, D.J.; Nascimento Saldiva, P.H.; Veras, M.M. Concentrated ambient fine particulate matter (PM2.5) exposure induces brain damage in pre and postnatal exposed mice. Neurotoxicology 2020, 79, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Yariwake, V.Y.; Torres, J.I.; Dos Santos, A.R.P.; Freitas, S.C.F.; De Angelis, K.; Farhat, S.C.L.; Câmara, N.O.S.; Veras, M.M. Chronic exposure to PM2.5 aggravates SLE manifestations in lupus-prone mice. Part. Fibre Toxicol. 2021, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Waked, D.; Rodrigues, A.C.B.; Silva, T.M.; Yariwake, V.Y.; Farhat, S.C.L.; Veras, M.M. Effect of chronic exposure to fine particulate matter on cardiac tissue of NZBWF1 mice. Int. J. Exp. Pathol. 2023, 104, 177–187. [Google Scholar] [CrossRef]
- Andrade, S.J. Investigação Sobre a Composição Química e Avaliação da Mutagenicidade do Material Particulado Atmosférico sob a Influência da Fuligem da Queima de Cana-De-Açúcar. Ph.D. Thesis, Instituto de Química/UNESP, Araraquara-Sao Paulo, Brazil, 2004. [Google Scholar]
- de Castro, K.R.; Almeida, G.H.D.R.; Matsuda, M.; de Paula Vieira, R.; Martins, M.G.; Rici, R.E.G.; Saldiva, P.H.N.; Veras, M.M. Exposure to urban ambient particles (PM2.5) before pregnancy affects the expression of endometrial receptive markers to embryo implantation in mice: Preliminary results. Tissue Cell 2024, 88, 102368. [Google Scholar] [CrossRef]
- Takano, A.P.C.; de André, C.D.S.; de Almeida, R.; Waked, D.; Veras, M.M.; Saldiva, P.H.N. Association of pulmonary black carbon accumulation with cardiac fibrosis in residents of Sao Paulo, Brazil. Environ. Res. 2024, 248, 118380. [Google Scholar] [CrossRef]
- Tohma, H.; Hepworth, A.R.; Shavlakadze, T.; Grounds, M.D.; Arthur, P.G. Quantification of ceroid and lipofuscin in skeletal muscle. J. Histochem. Cytochem. 2011, 59, 769–779. [Google Scholar] [CrossRef]
- Takano, A.P.C.; Senger, N.; Munhoz, C.D.; Barreto-Chaves, M.L.M. AT1 receptor blockage impairs NF-κB activation mediated by thyroid hormone in cardiomyocytes. Pflügers Arch. Eur. J. Physiol. 2018, 470, 549–558. [Google Scholar] [CrossRef]
- Andrade, M.D.; de Miranda, R.M.; Fornaro, A.; Kerr, A.; Oyama, B.; de Andre, P.A.; Saldiva, P. Vehicle emissions and PM2.5 mass concentrations in six Brazilian cities. Air Qual. Atmos. Health 2012, 5, 79–88. [Google Scholar] [CrossRef]
- Andrade, M.F.; Kumar, P.; de Freitas, E.D.; Ynoue, R.Y.; Martins, J.; Martins, L.D.; Nogueira, T.; Perez-Martinez, P.; de Miranda, R.M.; Albuquerque, T.; et al. Air quality in the megacity of São Paulo: Evolution over the last 30 years and future perspectives. Atmos. Environ. 2017, 159, 66–82. [Google Scholar] [CrossRef]
- Jia, Y.Y.; Wang, Q.; Liu, T. Toxicity Research of PM2.5 Compositions In Vitro. Int. J. Environ. Res. Public Health 2017, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Maglione, G.A.; Kurtz, M.L.; Orona, N.S.; Astort, F.; Brites, F.; Morales, C.; Berra, A.; Tasat, D.R. Changes in extrapulmonary organs and serum enzyme biomarkers after chronic exposure to Buenos Aires air pollution. Environ. Sci. Pollut. Res. 2020, 27, 14529–14542. [Google Scholar] [CrossRef] [PubMed]
- Marchini, T.; Magnani, N.; Garces, M.; Kelly, J.; Paz, M.; Caceres, L.; Calabro, V.; Lasagni Vitar, R.; Caltana, L.; Contin, M.; et al. Chronic exposure to polluted urban air aggravates myocardial infarction by impaired cardiac mitochondrial function and dynamics. Environ. Pollut. 2022, 295, 118677. [Google Scholar] [CrossRef]
- Qin, G.; Xia, J.; Zhang, Y.; Guo, L.; Chen, R.; Sang, N. Ambient fine particulate matter exposure induces reversible cardiac dysfunction and fibrosis in juvenile and older female mice. Part. Fibre Toxicol. 2018, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, L.C.D.; Cline, M.; Woodward, N.; Pakbin, P.; Sioutas, C.; Morgan, T.E.; Finch, C.E.; Forman, H.J.; Davies, K.J.A. Aging attenuates redox adaptive homeostasis and proteostasis in female mice exposed to traffic-derived nanoparticles (‘vehicular smog’). Free Radic. Biol. Med. 2018, 121, 86–97. [Google Scholar] [CrossRef]
- Pomatto, L.C.D.; Davies, K.J.A. The role of declining adaptive homeostasis in ageing. J. Physiol. 2017, 595, 7275–7309. [Google Scholar] [CrossRef]
- Zhai, P.; Sadoshima, J. Cardiomyocyte senescence and the potential therapeutic role of senolytics in the heart. J. Cardiovasc. Aging 2024, 4, 18. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Aguilar, M.; Thorin, E.; Ferbeyre, G.; Nattel, S. The role of cellular senescence in cardiac disease: Basic biology and clinical relevance. Nat. Rev. Cardiol. 2022, 19, 250–264. [Google Scholar] [CrossRef]
- Kumar, A.; Bano, D.; Ehninger, D. Cellular senescence in vivo: From cells to tissues to pathologies. Mech. Ageing Dev. 2020, 190, 111308. [Google Scholar] [CrossRef]
- Chen, M.S.; Lee, R.T.; Garbern, J.C. Senescence mechanisms and targets in the heart. Cardiovasc. Res. 2022, 118, 1173–1187. [Google Scholar] [CrossRef]
- Terman, A.; Brunk, U.T. Lipofuscin. Int. J. Biochem. Cell Biol. 2004, 36, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Shi, Y.C.; Lin, S.; He, H.F. Particulate matter 2.5 accelerates aging: Exploring cellular senescence and age-related diseases. Ecotoxicol. Environ. Saf. 2024, 284, 116920. [Google Scholar] [CrossRef]
- Gu, X.; Wen, J.; Wang, M.; Jian, G.; Zheng, G.; Wang, S. Numerical investigation of unsteady particle deposition in a realistic human nasal cavity during inhalation. Exp. Comput. Multiph. Flow 2019, 1, 39–50. [Google Scholar] [CrossRef]
- Amjadimanesh, H.; Faramarzi, M.; Sadrizadeh, S.; Abouali, O. Micro-particle deposition in maxillary sinus for various sizes of opening in a virtual endoscopic surgery. Exp. Comput. Multiph. Flow 2023, 5, 262–271. [Google Scholar] [CrossRef]



| Element | % | SD1 |
|---|---|---|
| C | 55.8707 | 6.8503 |
| S | 27.4882 | 1.6269 |
| Mg | 11.7078 | 9.4825 |
| Ba | 1.6794 | 0.5529 |
| Cl | 0.9622 | 0.2746 |
| P | 0.9093 | 0.4804 |
| Ca | 0.5581 | 0.1705 |
| Fe | 0.4050 | 0.1363 |
| K | 0.2156 | 0.0514 |
| Si | 0.0808 | 0.1452 |
| Zn | 0.0390 | 0.0192 |
| W | 0.0335 | 0.0654 |
| Cu | 0.0320 | 0.0473 |
| Ti | 0.0061 | 0.0113 |
| Cr | 0.0052 | 0.0002 |
| Rb | 0.0046 | 0.0033 |
| V | 0.0013 | 0.0002 |
| Sr | 0.0006 | 0.0011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waked, D.; Guedes, G.H.R.; Macedo, R.; Saldiva, P.H.N.; Veras, M.M.; Takano, A.P.C. Differential Susceptibility to Particulate Matter-Induced Cardiac Remodeling and Senescence: A Comparative Study in Young and Aged Mice. Atmosphere 2025, 16, 109. https://doi.org/10.3390/atmos16010109
Waked D, Guedes GHR, Macedo R, Saldiva PHN, Veras MM, Takano APC. Differential Susceptibility to Particulate Matter-Induced Cardiac Remodeling and Senescence: A Comparative Study in Young and Aged Mice. Atmosphere. 2025; 16(1):109. https://doi.org/10.3390/atmos16010109
Chicago/Turabian StyleWaked, Dunia, Gabriel Henrique Rodella Guedes, Raissa Macedo, Paulo Hilário Nascimento Saldiva, Mariana Matera Veras, and Ana Paula Cremasco Takano. 2025. "Differential Susceptibility to Particulate Matter-Induced Cardiac Remodeling and Senescence: A Comparative Study in Young and Aged Mice" Atmosphere 16, no. 1: 109. https://doi.org/10.3390/atmos16010109
APA StyleWaked, D., Guedes, G. H. R., Macedo, R., Saldiva, P. H. N., Veras, M. M., & Takano, A. P. C. (2025). Differential Susceptibility to Particulate Matter-Induced Cardiac Remodeling and Senescence: A Comparative Study in Young and Aged Mice. Atmosphere, 16(1), 109. https://doi.org/10.3390/atmos16010109









