Laboratory Efficacy and Disinfection by-Product Formation of a Coagulant/Disinfectant Tablet for Point-of-Use Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Product Description
2.2. Experimental Setup
2.3. CDP Evaluation
2.4. Treatment Performance Trials
2.5. Simulated Storage FCR Experiments
2.6. Disinfection by-Product Formation Tests
2.7. Test Waters
2.8. Analytical Methods
2.9. Statistical Analysis
3. Results
3.1. Bacterial Reductions
3.2. Turbidity Reductions
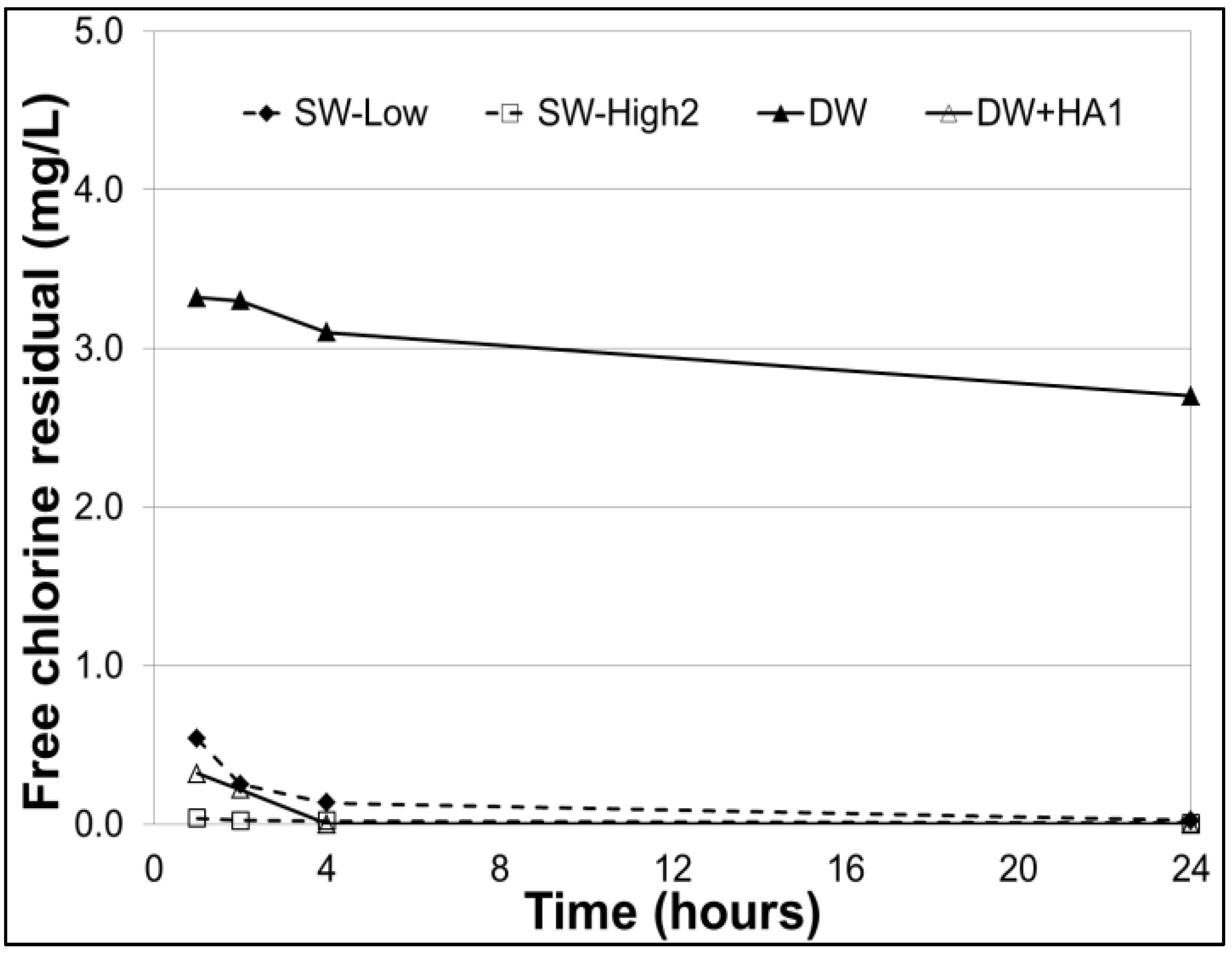
3.3. Free Chlorine Residuals
3.4. Disinfection Byproducts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clasen, T.; Schmidt, W.-P.; Rabie, T.; Roberts, I.; Cairncross, S. Interventions to improve water quality for preventing diarrhoea: Systematic review and meta-analysis. BMJ 2007, 334, 782. [Google Scholar] [CrossRef] [PubMed]
- Onda, K.; LoBuglio, J.; Bartram, J. Global Access to Safe Water: Accounting for Water Quality and the Resulting Impact on MDG Progress. World Health Popul. 2013, 14, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Lantagne, D.S.; Clasen, T.F. Use of Household Water Treatment and Safe Storage Methods in Acute Emergency Response: Case Study Results from Nepal, Indonesia, Kenya, and Haiti. Environ. Sci. Technol. 2012, 46, 11352–11360. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Cavill, S.; Cumming, O.; Jeandron, A. Water, sanitation, and hygiene in emergencies: Summary review and recommendations for further research. Waterlines 2012, 31, 11–29. [Google Scholar] [CrossRef]
- Crump, J.A.; Otieno, P.O.; Slutsker, L.; Keswick, B.H.; Rosen, D.H.; Hoekstra, R.M.; Vulule, J.M.; Luby, S.P. Household based treatment of drinking water with flocculant-disinfectant for preventing diarrhoea in areas with turbid source water in rural western Kenya: Cluster randomised controlled trial. BMJ 2005, 331, 478. [Google Scholar] [CrossRef] [PubMed]
- Chiller, T.M.; Mendoza, C.E.; Lopez, M.B.; Alvarez, M.; Hoekstra, R.M.; Keswick, B.H.; Luby, S.P. Reducing diarrhoea in Guatemalan children: Randomized controlled trial of flocculant-disinfectant for drinking-water. Bull. World Health Organ. 2006, 84, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Doocy, S.; Burnham, G. Point-of-use water treatment and diarrhoea reduction in the emergency context: An effectiveness trial in Liberia. Trop. Med. Int. Health 2006, 11, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Colindres, R.E.; Jain, S.; Bowen, A.; Mintz, E.; Domond, P. After the flood: An evaluation of in-home drinking water treatment with combined flocculent-disinfectant following Tropical Storm Jeanne—Gonaives, Haiti, 2004. J. Water Health 2007, 5, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Marois-Fiset, J.-T.; Shaheed, A.; Brown, J.; Dorea, C.C. Laboratory evaluation of a new coagulant/disinfectant point-of-use water treatment product for emergencies. J. Appl. Microbiol. 2016, 121, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Clasen, T.; Edmondson, P. Sodium dichloroisocyanurate (NaDCC) tablets as an alternative to sodium hypochlorite for the routine treatment of drinking water at the household level. Int. J. Hyg. Environ. Health 2006, 209, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Lantagne, D.S.; Blount, B.C.; Cardinali, F.; Quick, R. Disinfection by-product formation and mitigation strategies in point-of-use chlorination of turbid and non-turbid waters in western Kenya. J. Water Health 2008, 6, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Lantagne, D.S.; Cardinali, F.; Blount, B.C. Disinfection By-Product Formation and Mitigation Strategies in Point-of-Use Chlorination with Sodium Dichloroisocyanurate in Tanzania. Am. J. Trop. Med. Hyg. 2010, 83, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.M.; Plewa, M.J.; Lindell, C.L.; Richardson, S.D.; Mitch, W.A. Comparison of Byproduct Formation in Waters Treated with Chlorine and Iodine: Relevance to Point-of-Use Treatment. Environ. Sci. Technol. 2010, 44, 8446–8452. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Valdivia-Garcia, M.; Weir, P.; Haffey, M. Trihalomethanes formation in point of use surface water disinfection with chlorine or chlorine dioxide tablets. Water Environ. J. 2016, 30, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Marois-Fiset, J.-T.; Carabin, A.; Lavoie, A.; Dorea, C.C. Effects of Temperature and pH on Reduction of Bacteria in a Point-of-Use Drinking Water Treatment Product for Emergency Relief. Appl. Environ. Microbiol. 2013, 79, 2107–2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; ISBN 978-92-4-154815-1. [Google Scholar]
- The Sphere Project. Humanitarian Charter and Minimum Standards in Humanitarian Response, 3rd ed.; The Sphere Project: Rugby, UK, 2011. [Google Scholar]
- WHO. Evaluating Household Water Treatment Options: Health-Based Targets and Microbiological Performance Specifications; World Health Organization: Geneva, Switzerland, 2011; ISBN 978-92-4-154822-9. [Google Scholar]
- Lantagne, D.S. Sodium hypochlorite dosage for household and emergency water treatment. J. Am. Water Works Assoc. 2008, 100, 106–119. [Google Scholar] [CrossRef]
- CDC. Free Chlorine Testing. Available online: http:// http://www.cdc.gov/safewater/chlorine-residual-testing.html (accessed on 30 January 2013).
- USEPA. Comprehensive Disinfectants and Disinfection Byproducts Rules (Stage 1 and Stage 2): Quick Reference Guide; United States Environmental Protection Agency: Washington, DC, USA, 2010.
- Eloidin, O.; Dorea, C.C. Evaluation of semidecentralized emergency drinking water treatment. J. Environ. Sci. Health Part A 2015, 50, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Légaré-Julien, F. Formation des Sous-Produits de Désinfection par Différents Traitements à Base de Chlore Conçus Pour Traiter L’eau Potable à Domicile. Master’s Thesis, Université Laval, Québec, QC, Canada, 2017. [Google Scholar]
- Mercier Shanks, C.; Sérodes, J.-B.; Rodriguez, M.J. Spatio-temporal variability of non-regulated disinfection by-products within a drinking water distribution network. Water Res. 2013, 47, 3231–3243. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.A.; Okoth, G.O.; Slutsker, L.; Ogaja, D.O.; Keswick, B.H.; Luby, S.P. Effect of point-of-use disinfection, flocculation and combined flocculation–disinfection on drinking water quality in western Kenya. J. Appl. Microbiol. 2004, 97, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Souter, P.F.; Cruickshank, G.D.; Tankerville, M.Z.; Keswick, B.H.; Ellis, B.D.; Langworthy, D.E.; Metz, K.A.; Appleby, M.R.; Hamilton, N.; Jones, A.L.; et al. Evaluation of a new water treatment for point-of-use household applications to remove microorganisms and arsenic from drinking water. J. Water Health 2003, 1, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLennan, S.D.; Peterson, L.A.; Rose, J.B. Comparison of Point-of-Use Technologies for Emergency Disinfection of Sewage-Contaminated Drinking Water. Appl. Environ. Microbiol. 2009, 75, 7283–7286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, B.; Helmer, R. Surveillance of Drinking Water Quality in Rural Areas; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Rangel, J.M.; Lopez, B.; Mejia, M.A.; Mendoza, C.; Luby, S. A novel technology to improve drinking water quality: A microbiological evaluation of in-home flocculation and chlorination in rural Guatemala. J. Water Health 2003, 1, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Stauber, C.E.; Elliott, M.A.; Koksal, F.; Ortiz, G.M.; DiGiano, F.A.; Sobsey, M.D. Characterisation of the biosand filter for E. coli reductions from household drinking water under controlled laboratory and field use conditions. Water Sci. Technol. 2006, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Levy, K.; Anderson, L.; Robb, K.A.; Cevallos, W.; Trueba, G.; Eisenberg, J.N.S. Household effectiveness vs. laboratory efficacy of point-of-use chlorination. Water Res. 2014, 54, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, A.L.; Stewart, B.; Hopper, C.; Tobin, E.; Rivera, J.; Mut-Tracy, H.; Stewart, P.; Stewart, C.; Tobin, C.; Goeb, M.; et al. Laboratory efficacy and field effectiveness of hollow fiber membrane microfilters used for household water treatment in Honduras. J. Water Sanit. Hyg. Dev. 2017, 7, 74–84. [Google Scholar] [CrossRef]
- Luoto, J.; Najnin, N.; Mahmud, M.; Albert, J.; Islam, M.S.; Luby, S.; Unicomb, L.; Levine, D.I. What Point-of-Use Water Treatment Products Do Consumers Use? Evidence from a Randomized Controlled Trial among the Urban Poor in Bangladesh. PLoS ONE 2011, 6, e26132. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-0-470-40539-0. [Google Scholar]
- Weber, N.M.; Sawyer, H.R.; Legare, M.E.; Veeramachaneni, D.N.R. Sub-chronic Exposure to Dibromoacetic Acid, a Water Disinfection By-product, Does Not Affect Gametogenic Potential in Mice. Toxicol. Sci. 2006, 89, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Florentin, A.; Hautemanière, A.; Hartemann, P. Health effects of disinfection by-products in chlorinated swimming pools. Int. J. Hyg. Environ. Health 2011, 214, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.; MacDonell, M.; Hertzberg, R.C.; Teuschler, L.; Picel, K.; Butler, J.; Chang, Y.-S.; Hartmann, H. An approach for assessing human exposures to chemical mixtures in the environment. Toxicol. Appl. Pharmacol. 2008, 233, 126–136. [Google Scholar] [CrossRef] [PubMed]



| Test Series | Testing Objective | Turb. (NTU) | pH | Temp. (°C) | Test Water Matrix |
|---|---|---|---|---|---|
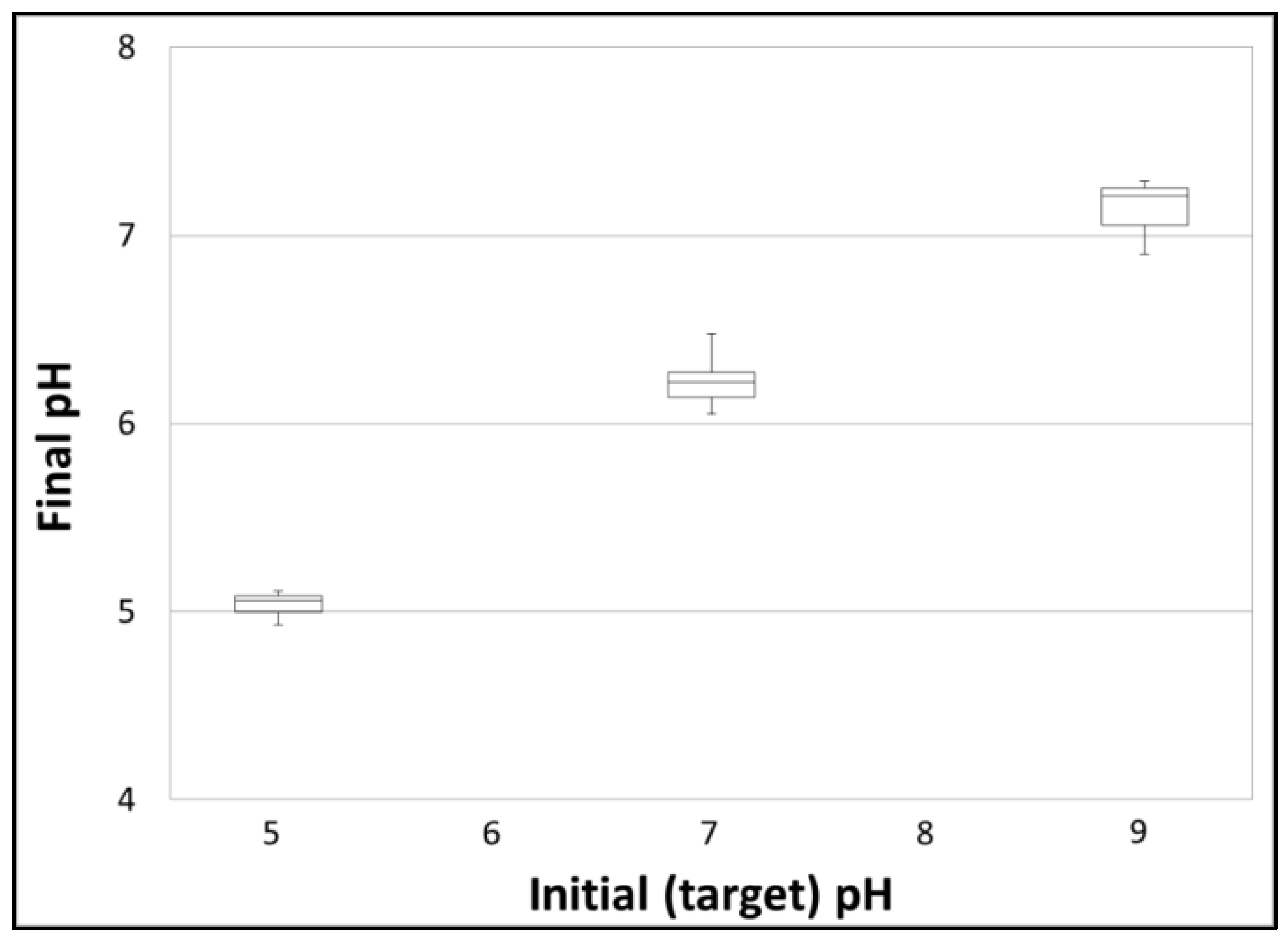
| A | Treatment | 100 | 7.0 | 20 | PSWd—PSW dilution |
| B | Treatment | 100 | 9.0 | 20 | PSWd—PSW dilution |
| C | Treatment | 100 | 5.0 | 20 | PSWd—PSW dilution |
| D | Treatment | 100 | 7.0 | 5 | PSWd—PSW dilution |
| E | Treatment | 800 | 7.0 | 20 | PSWd—PSW dilution |
| F | Treatment/FCR | -- | -- | 20 | SW-Low—St. Lawrence River |
| G | Treatment/FCR | -- | -- | 20 | SW-High1—Chaudière River |
| H | FCR | -- | -- | 20 | DW—Distilled water |
| I | FCR | -- | -- | 20 | DW+HA1—Distilled water + 30 mg/L HA |
| J | DBP | -- | -- | 20 | SW-Low—St. Lawrence River |
| K | DBP | -- | -- | 20 | SW-High2—Marais du Nord |
| L | DBP | -- | -- | 20 | DW+HA2—Distilled water + 11.5 mg/L HA |
| Test | E. coli (MPN/100 mL) | ||
|---|---|---|---|
| Series | Water Matrix | Initial | Final |
| A | PSWd | 3.1 × 105 (1.9 to 5.2) × 105 | 6.6 × 100 (4.5 to 33.1) × 100 |
| B | PSWd | 2.4 × 105 (1.9 to 3.0) × 105 | 2.7 × 100 (<1 to 54.3) × 100 |
| C | PSWd | 1.7 × 105 (1.1 to 5.2) × 105 | <1 × 100 (< 1 to < 1) × 100 |
| D | PSWd | 1.1 × 105 (0.7 to 2.0) × 105 | 1.1 × 100 (< 1 to 4.8) × 100 |
| E | PSWd | 1.5 × 105 (1.1 to 3.2) × 105 | <1 × 100 (<1 to 1.3) × 100 |
| F | SW-Low | 2.6 × 102 (0.9 to 7.9) × 102 | <1 × 100 (< 1 to < 1) × 100 |
| G | SW-High1 | 2.7 × 102 (1.9 to 4.0) × 102 | <1 × 100 (< 1 to 3.9) × 100 |
| Test | Turbidity (NTU) | FCR (mg/L) | pH | Temp. (°C) | |||
|---|---|---|---|---|---|---|---|
| Series | Water Matrix | Initial | Final | Final | Initial | Final | Final |
| A | PSWd | 96 ± 2 | 5.1 ± 0.7 | 0.08 ± 0.04 | 7.0 ± 0.1 | 6.2 ± 0.1 | 19.2 ± 2.0 |
| B | PSWd | 97 ± 2 | 7.9 ± 2.8 | 0.08 ± 0.02 | 9.0 ± 0.0 | 7.1 ± 0.2 | 19.2 ± 1.7 |
| C | PSWd | 98 ±7 | 6.4 ± 0.4 | 0.05 ± 0.01 | 5.0 ± 0.0 | 5.0 ± 0.1 | 18.1 ± 0.6 |
| D | PSWd | 101 ± 5 | 5.3 ± 0.9 | 0.05 ± 0.01 | 7.0 ± 0.0 | 6.1 ± 0.1 | 5.6 ± 0.2 |
| E | PSWd | 764 ± 27 | 11 ± 6 | 0.07 ± 0.01 | 7.0 ± 0.1 | 6.3 ± 0.2 | 18.2 ± 0.3 |
| F | SW-Low | 4.8 ± 0.7 | 7.4 ± 4.8 | 0.54 ± 0.52 | 7.1 ± 0.1 | 6.1 ± 0.1 | 18.7 ± 1.6 |
| G | SW-High1 | 3.9 ± 3.2 | 12 ± 5 | 0.03 ± 0.01 | 6.7 ± 0.2 | 6.0 ± 0.2 | 18.8 ± 1.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Légaré-Julien, F.; Lemay, O.; Vallée-Godbout, U.; Bouchard, C.; Dorea, C.C. Laboratory Efficacy and Disinfection by-Product Formation of a Coagulant/Disinfectant Tablet for Point-of-Use Water Treatment. Water 2018, 10, 1567. https://doi.org/10.3390/w10111567
Légaré-Julien F, Lemay O, Vallée-Godbout U, Bouchard C, Dorea CC. Laboratory Efficacy and Disinfection by-Product Formation of a Coagulant/Disinfectant Tablet for Point-of-Use Water Treatment. Water. 2018; 10(11):1567. https://doi.org/10.3390/w10111567
Chicago/Turabian StyleLégaré-Julien, Félix, Olivier Lemay, Ulysse Vallée-Godbout, Christian Bouchard, and Caetano C. Dorea. 2018. "Laboratory Efficacy and Disinfection by-Product Formation of a Coagulant/Disinfectant Tablet for Point-of-Use Water Treatment" Water 10, no. 11: 1567. https://doi.org/10.3390/w10111567
APA StyleLégaré-Julien, F., Lemay, O., Vallée-Godbout, U., Bouchard, C., & Dorea, C. C. (2018). Laboratory Efficacy and Disinfection by-Product Formation of a Coagulant/Disinfectant Tablet for Point-of-Use Water Treatment. Water, 10(11), 1567. https://doi.org/10.3390/w10111567





