Assessment of Key Environmental Factors Influencing the Sedimentation and Aggregation Behavior of Zinc Oxide Nanoparticles in Aquatic Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Stock Solutions
2.2.1. Preparation of ZnO NPs Stock Solution
2.2.2. Preparation of Inorganic Salt and NOM Solutions
2.3. Characterization and Measurement
2.4. Sedimentation Experimental Procedure
2.4.1. Aggregation Kinetics
2.4.2. Influence and Interaction of Multi-Environmental Factors
2.5. Preparation of Environmental Water Samples
2.6. Data Analysis
3. Results and Discussions
3.1. Characterization of ZnO NPs Solution
3.2. Single Factor Effect on Sedimentation
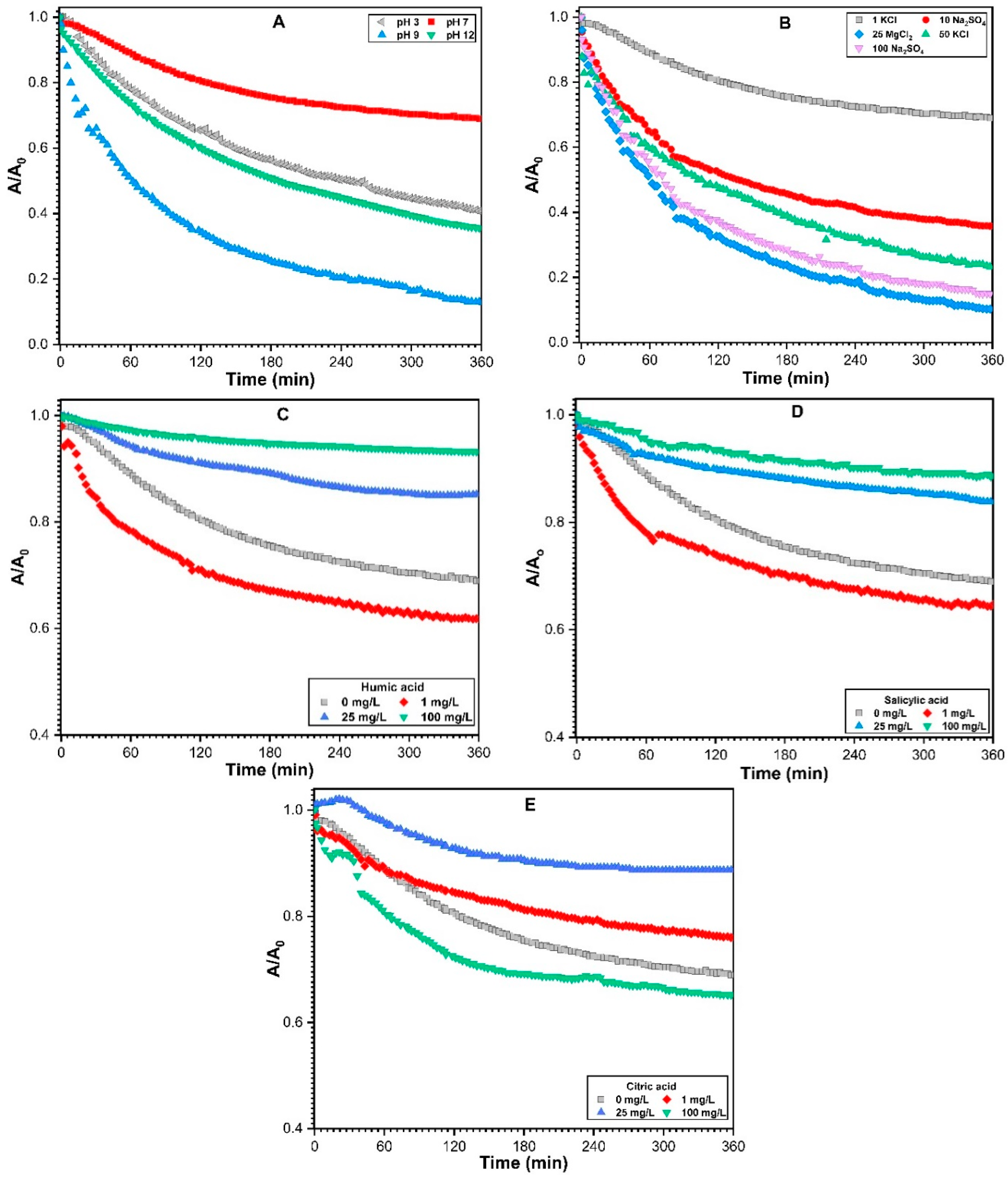
3.2.1. Effect of Solution pH on Sedimentation of ZnO NPs
3.2.2. Effect of Monovalent and Divalent Cations on Sedimentation of ZnO NPs
3.2.3. Impact of NOM on the Sedimentation of ZnO NPs
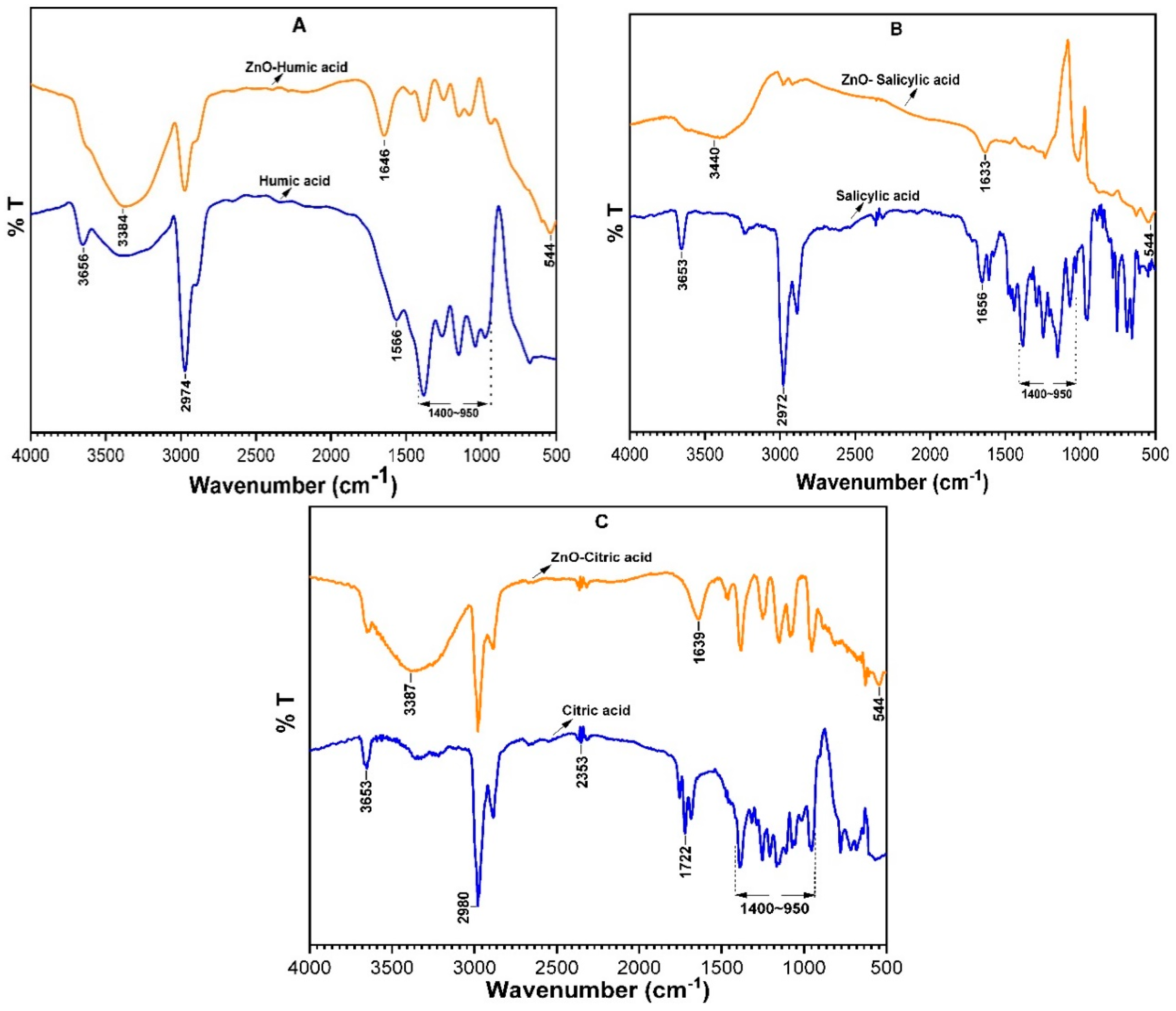
3.2.4. FT-IR Analysis Pristine NOM and ZnO-NOM Complexes
3.2.5. ZnO NPs Aggregation Kinetics Exposed to Various Environmental Factors
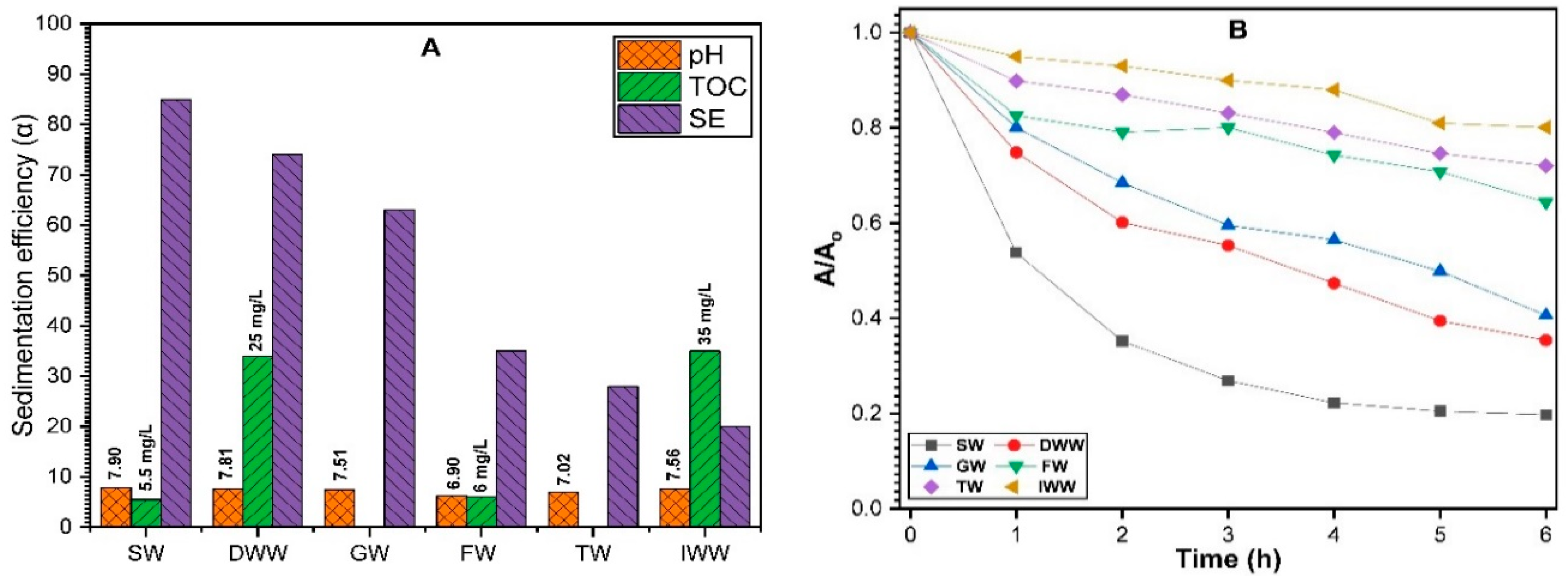
3.2.6. Influence of Multi-Environmental Parameters on ZnO NPs Sedimentation
3.2.7. Sedimentation Behavior of ZnO NPs in Aquatic Environments
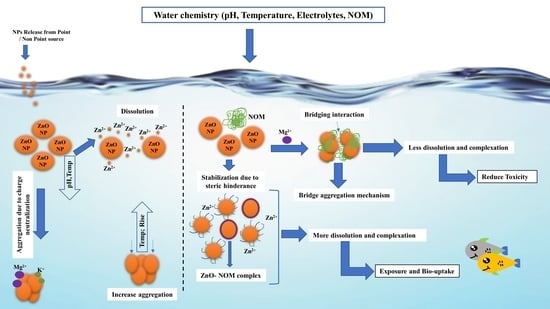
3.3. Environmental Implications
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Handy, R.D.; Von Der Kammer, F.; Lead, J.R.; Hassellöv, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Mädler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the Mechanism of toxicity of Zinc and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. Am. Chem. Soc. Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Root Uptake and Phytotoxicity of ZnO Nanoparticles. Environ. Sci. Technol. 2008, 42, 5580–5585. [Google Scholar] [CrossRef] [PubMed]
- Miao, A.J.; Zhang, X.Y.; Luo, Z.; Chen, C.S.; Chin, W.C.; Santschi, P.H.; Quigg, A. Zinc oxide-engineered nanoparticles: Dissolution and toxicity to marine phytoplankton. Environ. Toxicol. Chem. 2010, 29, 2814–2822. [Google Scholar] [CrossRef] [PubMed]
- Dhas, S.P.; Shiny, P.J.; Khan, S.; Mukherjee, A.; Chandrasekaran, N. Toxic behavior of silver and zinc oxide nanoparticles on environmental microorganisms. J. Basic Microbiol. 2014, 54, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, Y.; Chen, C.S.; Spurgin, J.; Schwehr, K.A.; Quigg, A.; Chin, W.C.; Santschi, P.H. Aggregation, dissolution, and stability of quantum dots in marine environments: Importance of extracellular polymeric substances. Environ. Sci. Technol. 2012, 46, 8764–8772. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Bligh, M.W.; Waite, T.D. Effects of aggregate structure on the dissolution kinetics of citrate-stabilized silver nanoparticles. Environ. Sci. Technol. 2013, 47, 9148–9156. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to escherichia Coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.W.; Adeleye, A.; Ji, Z.; Keller, A.A. Stability, metal leaching, photoactivity and toxicity in freshwater systems of commercial single wall carbon nanotubes. Water Res. 2013, 47, 4074–4085. [Google Scholar] [CrossRef] [PubMed]
- Quik, J.T.K.; Velzeboer, I.; Wouterse, M.; Koelmans, A.A.; van de Meent, D. Heteroaggregation and sedimentation rates for nanomaterials in natural waters. Water Res. 2014, 48, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Degen, A.; Kosec, M. Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. J. Eur. Ceram. Soc. 2000, 20, 667–673. [Google Scholar] [CrossRef]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, andcation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir 2012, 28, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Odzak, N.; Kistler, D.; Behra, R.; Sigg, L. Dissolution of metal and metal oxide nanoparticles in aqueous media. Environ. Pollut. 2014, 191, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Schaumann, G.E. Interactions of dissolved organic matter with natural and engineered inorganic colloids: A review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef] [PubMed]
- Majedi, S.M.; Kelly, B.C.; Lee, H.K. Combined effects of water temperature and chemistry on the environmental fate and behavior of nanosized zinc oxide. Sci. Total Environ. 2014, 496, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, K.; De Schamphelaere, K.A.C.; Van Der Meeren, P.; Smagghe, G.; Janssen, C.R. Aggregation and ecotoxicity of CeO2 nanoparticles in synthetic and natural waters with variable pH, organic matter concentration and ionic strength. Environ. Pollut. 2011, 159, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Romanello, M.B.; Fidalgo de Cortalezzi, M.M. An experimental study on the aggregation of TiO2 nanoparticles under environmentally relevant conditions. Water Res. 2013, 47, 3887–3898. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Shen, C.; Zheng, S.; Yang, W.; Hu, H.; Liu, J.; Shi, J. Transformation of CuO Nanoparticles in the Aquatic Environment: Influence of pH, Electrolytes and Natural Organic Matter. Nanomaterials 2017, 7, 326. [Google Scholar] [CrossRef] [PubMed]
- Guzman, K.A.D.; Finnegan, M.P.; Banfield, J.F. Influence of surface potential on aggregation and transport of Titania nanoparticles. Environ. Sci. Technol. 2006, 40, 7688–7693. [Google Scholar] [CrossRef] [PubMed]
- Domingos, R.F.; Tufenkji, N.; Wilkinson, K.J. Aggregation of Titanium Dioxide Nanoparticles: Role of a Fulvic Acid Aggregation of Titanium Dioxide Nanoparticles: Role of a Fulvic Acid. Environ. Sci. Technol. 2009, 43, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Gondikas, A.P.; Morris, A.; Reinsch, B.C.; Marinakos, S.M.; Lowry, G.V.; Hsu-Kim, H. Cysteine-induced modifications of zero-valent silver nanomaterials: Implications for particle surface chemistry, aggregation, dissolution, and silver speciation. Environ. Sci. Technol. 2012, 46, 7037–7045. [Google Scholar] [CrossRef] [PubMed]
- Phadke, M.S. Uauty Engineering Using Robust Design; Prentice Hall: Upper Saddle River, NJ, USA, 1989; ISBN 0137451679. [Google Scholar]
- Raj, C.B.C.; Quen, H. Advanced oxidation processes for wastewater treatment: Optimization of UV/H2O2 process through a statistical technique. Chem. Eng. Sci. 2005, 60, 5305–5311. [Google Scholar] [CrossRef]
- Lan, W.G.; Wongt, M.K.; Chen, N.; Sin, Y.M. Orthogonal array design as a chemometric method for the optimization of analytical procedures. Part 5. Three-level design and its application in microwave dissolution of biological samples. Analyst 1995, 120, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wang, H.; Keller, A.A.; Wang, T.; Li, F. The effect of humic acid on the aggregation of titanium dioxide nanoparticles under different pH and ionic strengths. Sci. Total Environ. 2014, 487, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.R. Introduction to Chemical Kinetics; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 047002593X. [Google Scholar]
- Mudunkotuwa, I.A.; Rupasinghe, T.; Wu, C.-M.; Grassian, V.H. Dissolution of ZnO Nanoparticles at Circumneutral pH: A Study of Size Effects in the Presence and Absence of Citric Acid. Langmuir 2012, 28, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.D.; Son, J.I.; Kim, H.H.; Yun, H.S.; Cho, N.H. Variation of the nanostructural feature of nc-SiC:H thin films with post-deposition thermal annealing. Thin Solid Films 2014, 571, 238–244. [Google Scholar] [CrossRef]
- Peng, Y.H.; Tsai, Y.C.; Hsiung, C.E.; Lin, Y.H.; Shih, Y.H. Influence of water chemistry on the environmental behaviors of commercial ZnO nanoparticles in various water and wastewater samples. J. Hazard. Mater. 2017, 322, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, G.; Borg, J.; Raynal, P.I.; Djouadi, Z.; D’Hendecourt, L.; Flynn, G.J.; Deboffle, D. FTIR and Raman analyses of the Tagish Lake meteorite: Relationship with the aliphatic hydrocarbons observed in the Diffuse Interstellar Medium. Astron. Astrophys. 2004, 416, 983–990. [Google Scholar] [CrossRef]
- Kumar, H.; Rani, R. Structural and Optical Characterization of ZnO Nanoparticles Synthesized by Microemulsion Route. Int. Lett. Chem. Phys. Astron. 2013, 19, 26–36. [Google Scholar] [CrossRef]
- Bian, S.W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.S.; Peng, Y.H.; Shiung, C.E.; Shih, Y.H. The effect of cations on the aggregation of commercial ZnO nanoparticle suspension. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Zhaoxia, J.I. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Crittenden, J. Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res. 2009, 43, 4249–4257. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mashayekhi, H.; Bhowmik, P.; Xing, B. Colloidal Stability of Al2O3 Nanoparticles as Affected by Coating of Structurally Different Humic Acids. Langmuir 2010, 26, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Erhayem, M.; Sohn, M. Stability studies for titanium dioxide nanoparticles upon adsorption of Suwannee River humic and fulvic acids and natural organic matter. Sci. Total Environ. 2014, 468–469, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Mudunkotuwa, I.A.; Grassian, V.H. Citric Acid Adsorption on TiO2 Nanoparticles in Aqueous Suspensions at Acidic and Circumneutral pH: Surface Coverage, Surface Speciation, and Its Impact on Nanoparticle–Nanoparticle Interactions. J. Am. Chem. Soc. 2010, 132, 14986–14994. [Google Scholar] [CrossRef] [PubMed]
- Giasuddin, A.B.M.; Kanel, S.R.; Choi, H. Adsorption of humic acid onto nanoscale zerovalent iron and its effect on arsenic removal. Environ. Sci. Technol. 2007, 41, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, N.; Skopelitis, Y.; Psaroudaki, M.; Konstantinidou, V.; Chatzilazarou, A.; Tegou, E. Applications of Fourier transform-infrared spectroscopy to edible oils. Anal. Chim. Acta 2006, 573–574, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Alberts, J.J.; Filip, Z. Metal binding in estuarine humic and fulvic acids: Ftir analysis of humic acid-metal complexes. Environ. Technol. (UK) 1998, 19, 923–931. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Franck, R.; Cabuil, V.; Massart, R. Surfacted ferrofluids: Interactions at the surfactant-magnetic iron oxide interface. Leuk. Res. 1987, 5, 126–127. [Google Scholar]
- Vermeer, A.W.P.; van Riemsdijk, W.H.; Koopal, L.K. Adsorption of Humic Acid to Mineral Particles. 1. Specific and Electrostatic Interactions. Langmuir 1998, 14, 2810–2819. [Google Scholar] [CrossRef]
- Huangfu, X.; Jiang, J.; Ma, J.; Liu, Y.; Yang, J. Aggregation kinetics of manganese dioxide colloids in aqueous solution: Influence of humic substances and biomacromolecules. Environ. Sci. Technol. 2013, 47, 10285–10292. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H. The derjaguin–landau–verwey–overbeek (dlvo) theory of colloid stability. In Electrical Phenomena at Interfaces and Biointerfaces Electrical Phenomena at Interfaces and Biointerfaces: Fundamentals and Applications in Nano-, Bio-, and Environmental Sciences, 1; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 27. [Google Scholar]



| Experiment Type | Solution Chemistry |
|---|---|
| A (pH) | 3.0 to 12.0 |
| B (Electrolyte type and concentration) | KCl (0.01 to 100 mM) Na2SO4 (0.01 to 100 mM) MgCl2 (0.01 to 25 mM) |
| C (NOM type and concentration) | Humic acid (0.5 to 100 mg/L) Salicylic acid (0.5 to 100 mg/L) Citric acid (0.5 to 100 mg/L) |
| Factor | pH | Electrolyte Conc; (mM) | NOM Conc: (mg/L) | Electrolyte Type | NOM Type | Temperature (°C) |
|---|---|---|---|---|---|---|
| Level 1 | 7.0 | 25 | 5 | KCl | Humic acid | 15 |
| Level 2 | 8.0 | 50 | 10 | Na2SO4 | Salicylic acid | 25 |
| Level 3 | 9.5 | 100 | 25 | MgCl2 | Citric acid | 35 |
| Trial No | A | B | C | D | E | F | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Elec. Conc; (mM) | (A × B)1 | (A × B)2 | NOM Conc; (mg/L) | # | # | (B × C)1 | Electrolyte Type | NOM Type | (B × C)2 | Temperature (°C) | # | SE% | |
| 1 | 7.0 | 25 | 10 | KCl | Humic acid | 15 | 22.12 | |||||||
| 2 | 7.0 | 25 | 25 | Na2SO4 | Salicylic acid | 25 | 20.15 | |||||||
| 3 | 7.0 | 25 | 50 | MgCl2 | citric acid | 35 | 47.06 | |||||||
| 4 | 7.0 | 50 | 10 | Na2SO4 | Salicylic acid | 35 | 30.4 | |||||||
| 5 | 7.0 | 50 | 25 | MgCl2 | citric acid | 15 | 55.56 | |||||||
| 6 | 7.0 | 50 | 50 | KCl | Humic acid | 25 | 6.56 | |||||||
| 7 | 7.0 | 100 | 10 | MgCl2 | citric acid | 25 | 75.02 | |||||||
| 8 | 7.0 | 100 | 25 | KCl | Humic acid | 35 | 36.8 | |||||||
| 9 | 7.0 | 100 | 50 | Na2SO4 | Salicylic acid | 15 | 29.78 | |||||||
| 10 | 8.0 | 25 | 10 | Na2SO4 | citric acid | 25 | 28.94 | |||||||
| 11 | 8.0 | 25 | 25 | MgCl2 | Humic acid | 35 | 57.78 | |||||||
| 12 | 8.0 | 25 | 50 | KCl | Salicylic acid | 15 | 7.78 | |||||||
| 13 | 8.0 | 50 | 10 | MgCl2 | Humic acid | 15 | 65.56 | |||||||
| 14 | 8.0 | 50 | 25 | KCl | Salicylic acid | 25 | 26.67 | |||||||
| 15 | 8.0 | 50 | 50 | Na2SO4 | citric acid | 35 | 16.50 | |||||||
| 16 | 8.0 | 100 | 10 | KCl | Salicylic acid | 35 | 49.89 | |||||||
| 17 | 8.0 | 100 | 25 | Na2SO4 | citric acid | 15 | 41.40 | |||||||
| 18 | 8.0 | 100 | 50 | MgCl2 | Humic acid | 25 | 53.22 | |||||||
| 19 | 9.5 | 25 | 10 | MgCl2 | Salicylic acid | 35 | 47.41 | |||||||
| 20 | 9.5 | 25 | 25 | KCl | citric acid | 15 | 9.25 | |||||||
| 21 | 9.5 | 25 | 50 | Na2SO4 | Humic acid | 25 | 4.35 | |||||||
| 22 | 9.5 | 50 | 10 | KCl | citric acid | 25 | 39.12 | |||||||
| 23 | 9.5 | 50 | 25 | Na2SO4 | Humic acid | 35 | 28.6 | |||||||
| 24 | 9.5 | 50 | 50 | MgCl2 | Salicylic acid | 15 | 43.44 | |||||||
| 25 | 9.5 | 100 | 10 | Na2SO4 | Humic acid | 15 | 63.13 | |||||||
| 26 | 9.5 | 100 | 25 | MgCl2 | Salicylic acid | 25 | 78.0 | |||||||
| 27 | 9.5 | 100 | 50 | KCl | citric acid | 35 | 52.46 | |||||||
| L1 | 35.90 | 27.21 | 43.49 | 38.28 | 46.70 | 35.69 | 39.30 | 35.64 | 27.90 | 37.58 | 38.26 | 36.60 | 39.20 | |
| L2 | 38.59 | 34.63 | 33.12 | 42.30 | 39.31 | 39.50 | 37.65 | 39.72 | 29.28 | 37.05 | 38.05 | 38.01 | 36.27 | |
| L3 | 40.61 | 53.30 | 38.60 | 34.60 | 29.05 | 39.85 | 38.20 | 38.25 | 58.10 | 40.60 | 38.90 | 40.80 | 37.69 | |
| R | 4.71 | 26.09 | 10.37 | 7.7 | 17.65 | 4.16 | 1.65 | 4.08 | 30.20 | 3.55 | 0.85 | 4.20 | 2.93 |
| Environmental Parameters | k (h−1) | R2 | Environmental Parameters | k (h−1) | R2 | ||
|---|---|---|---|---|---|---|---|
| pH | 3 | 0.1507 | 0.9975 | NOM type | Conc. (mg/L) | ||
| 7 | 0.0620 | 0.9986 | Humic Acid | 1 | 0.0808 | 0.9865 | |
| 9 | 0.3473 | 0.9876 | 25 | 0.0267 | 0.9275 | ||
| 12 | 0.1738 | 0.9981 | 100 | 0.0118 | 0.8509 | ||
| Electrolyte Conc. (mM) | Salicylic Acid | 1 | 0.0722 | 0.9781 | |||
| KCl | 1 | 0.0620 | 0.9987 | 25 | 0.0295 | 0.9322 | |
| 50 | 0.2431 | 0.9879 | 100 | 0.0202 | 0.7876 | ||
| Na2SO4 | 10 | 0.1714 | 0.9932 | Citric Acid | 1 | 0.0457 | 0.9237 |
| 100 | 0.3172 | 0.9943 | 25 | 0.0199 | 0.8401 | ||
| MgCl2 | 25 | 0.3786 | 0.9914 | 100 | 0.0713 | 0.8048 | |
| Variance Parameters | Degree of Freedom | Sum of Squares (SS) | Mean Squared (MS) | F Ratio | % Contribution |
|---|---|---|---|---|---|
| pH (A) | 2 | 100.18 | 50.09 | 0.80 a | 0.90% |
| Electrolyte conc. (B) | 2 | 3248.55 | 1624.28 | 25.95 b | 29.08% |
| NOM conc. (C) | 2 | 1442.27 | 721.13 | 11.52 b | 12.91% |
| Electrolyte Type (D) | 2 | 5253.95 | 2626.97 | 41.98 c | 47.03% |
| NOM Type (E) | 2 | 65.59 | 32.80 | 0.52 a | 0.59% |
| Temperature (F) | 2 | 77.25 | 38.63 | 1.85 a | 0.69% |
| (A × B) | 4 | 753.9 | 188.47 | 3.01* | 6.75% |
| (B × C) | 4 | 104.27 | 26.06 | 0.41* | 0.93% |
| Error | 6 | 125.14 | 62.57 | 1.12% | |
| Total | 26 | 11171.1 | 5329.285 | 100.00% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.; Inam, M.A.; Zam, S.Z.; Park, D.R.; Yeom, I.T. Assessment of Key Environmental Factors Influencing the Sedimentation and Aggregation Behavior of Zinc Oxide Nanoparticles in Aquatic Environment. Water 2018, 10, 660. https://doi.org/10.3390/w10050660
Khan R, Inam MA, Zam SZ, Park DR, Yeom IT. Assessment of Key Environmental Factors Influencing the Sedimentation and Aggregation Behavior of Zinc Oxide Nanoparticles in Aquatic Environment. Water. 2018; 10(5):660. https://doi.org/10.3390/w10050660
Chicago/Turabian StyleKhan, Rizwan, Muhammad Ali Inam, Saba Zam Zam, Du Ri Park, and Ick Tae Yeom. 2018. "Assessment of Key Environmental Factors Influencing the Sedimentation and Aggregation Behavior of Zinc Oxide Nanoparticles in Aquatic Environment" Water 10, no. 5: 660. https://doi.org/10.3390/w10050660
APA StyleKhan, R., Inam, M. A., Zam, S. Z., Park, D. R., & Yeom, I. T. (2018). Assessment of Key Environmental Factors Influencing the Sedimentation and Aggregation Behavior of Zinc Oxide Nanoparticles in Aquatic Environment. Water, 10(5), 660. https://doi.org/10.3390/w10050660