Effect of the Surface Charge on the Adsorption Capacity of Chromium(VI) of Iron Oxide Magnetic Nanoparticles Prepared by Microwave-Assisted Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Analysis
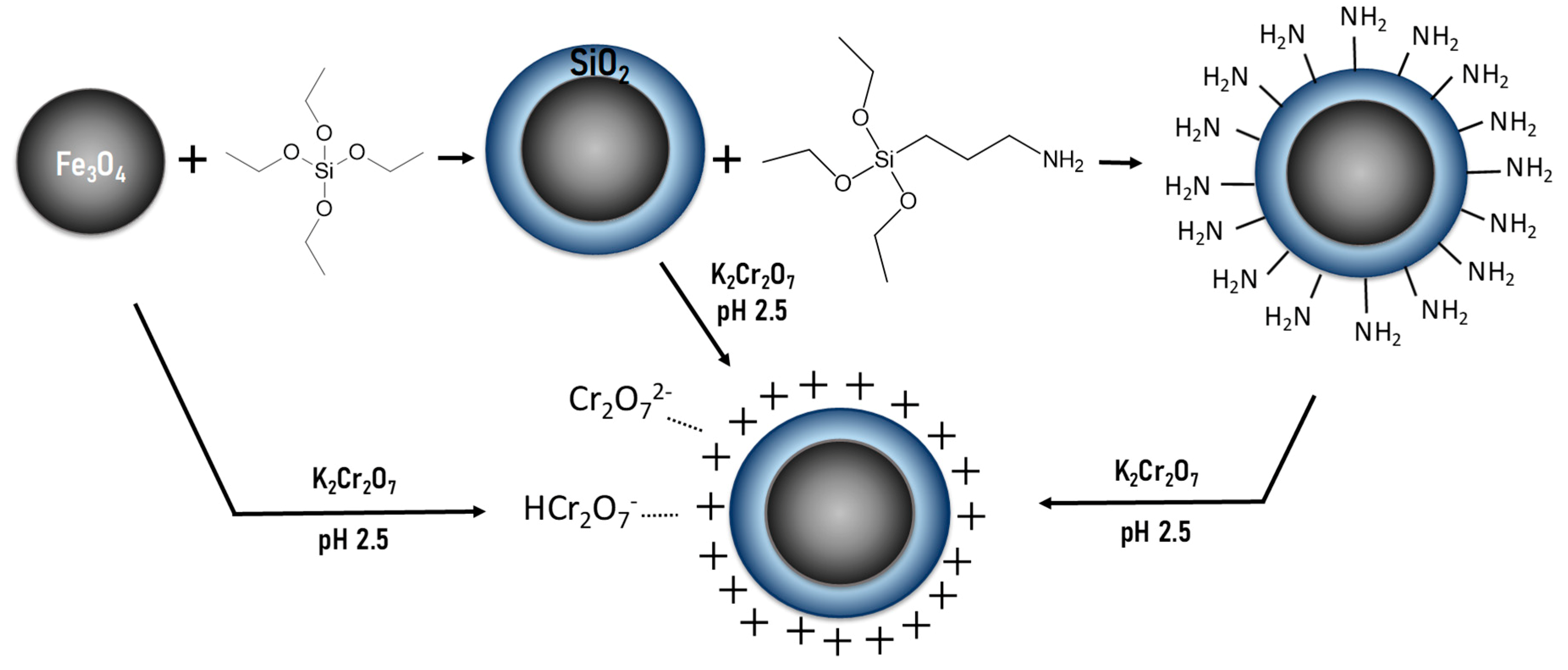
2.2. Magnetic Nanosorbent Preparation
2.3. Characterization
2.4. Kinetic Measurements
2.5. Sorption Experimentation
3. Results and Discussion
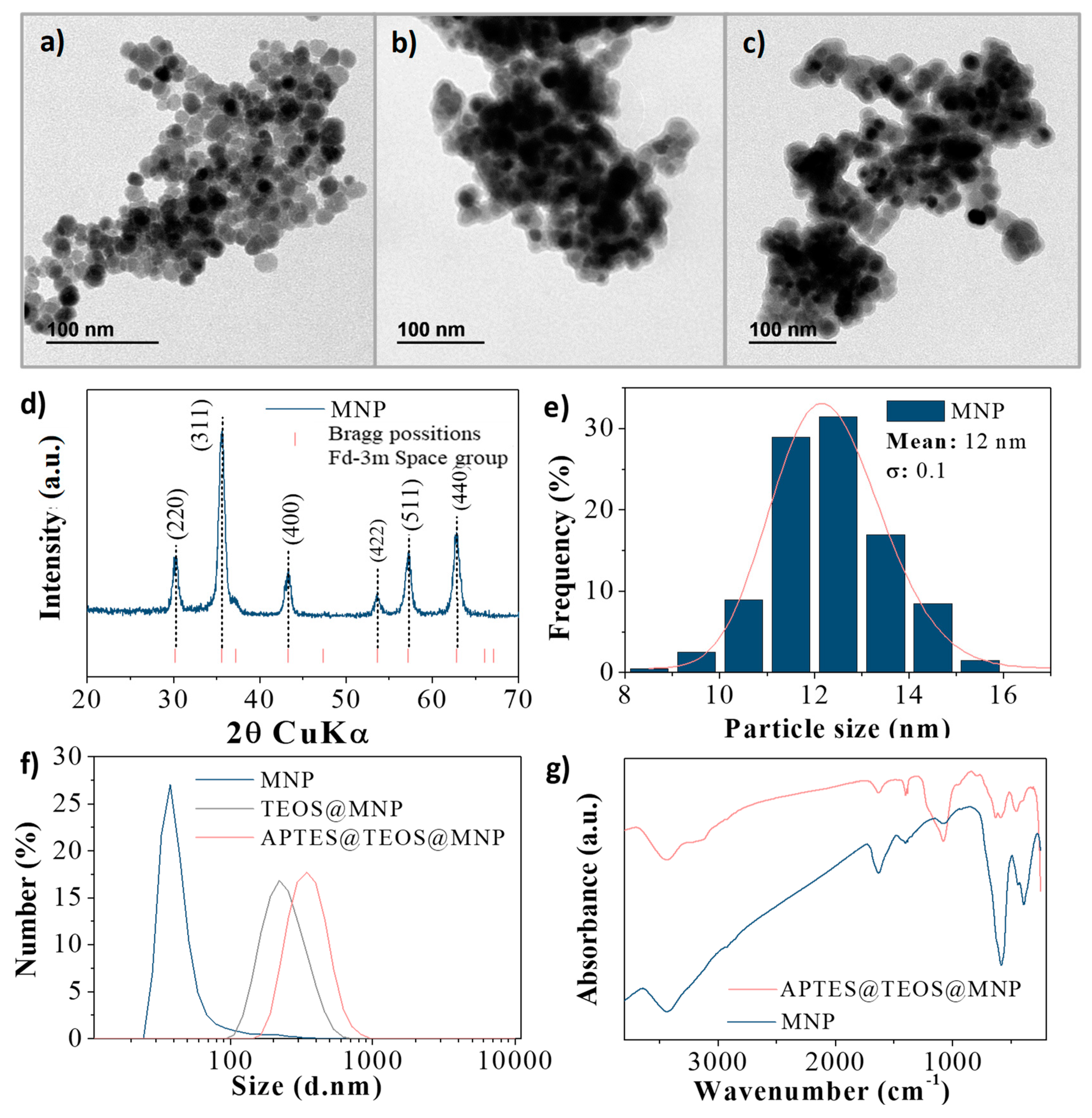
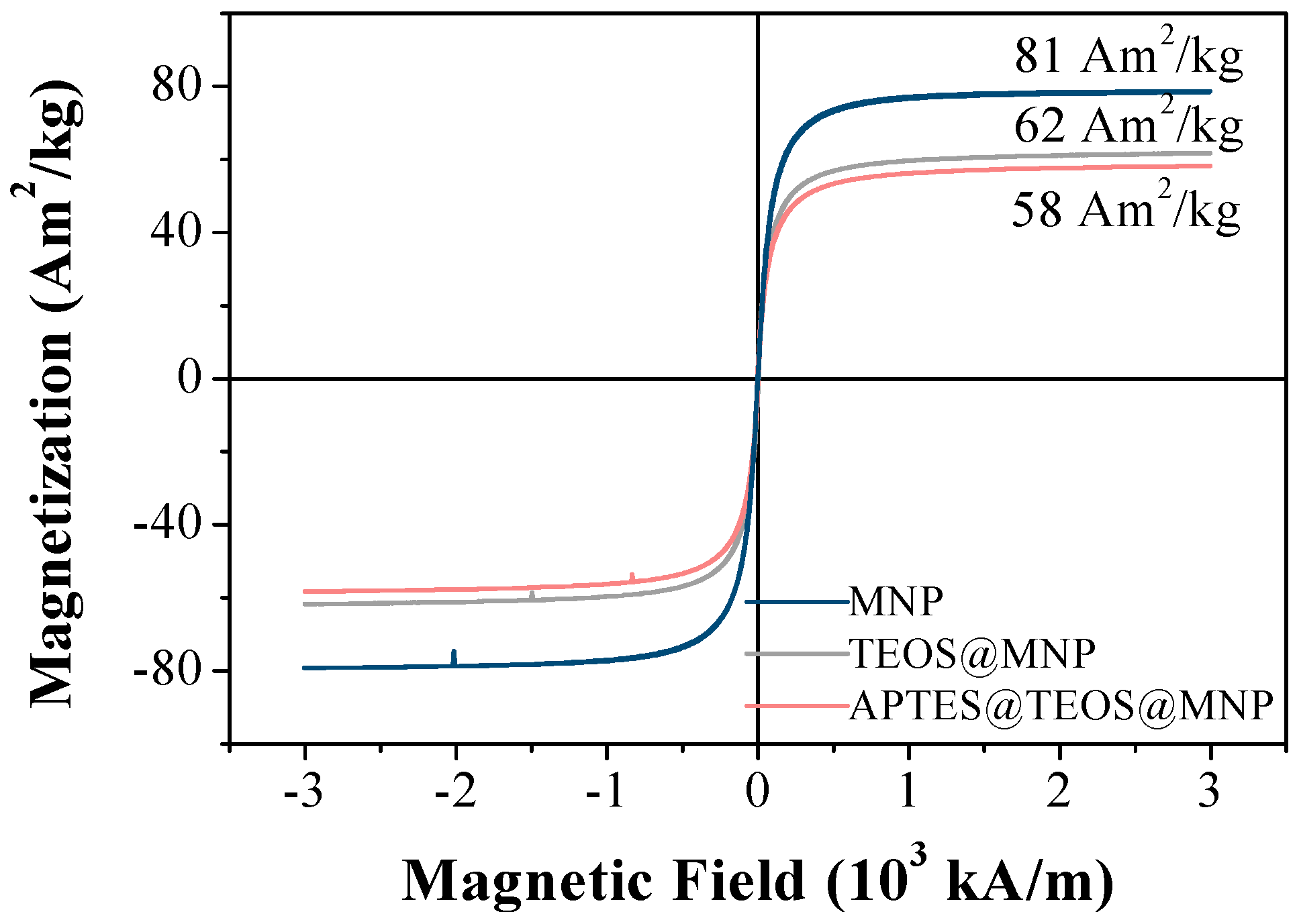
3.1. Characterization
3.2. Adsorptive Measurements
3.2.1. Effect of pH
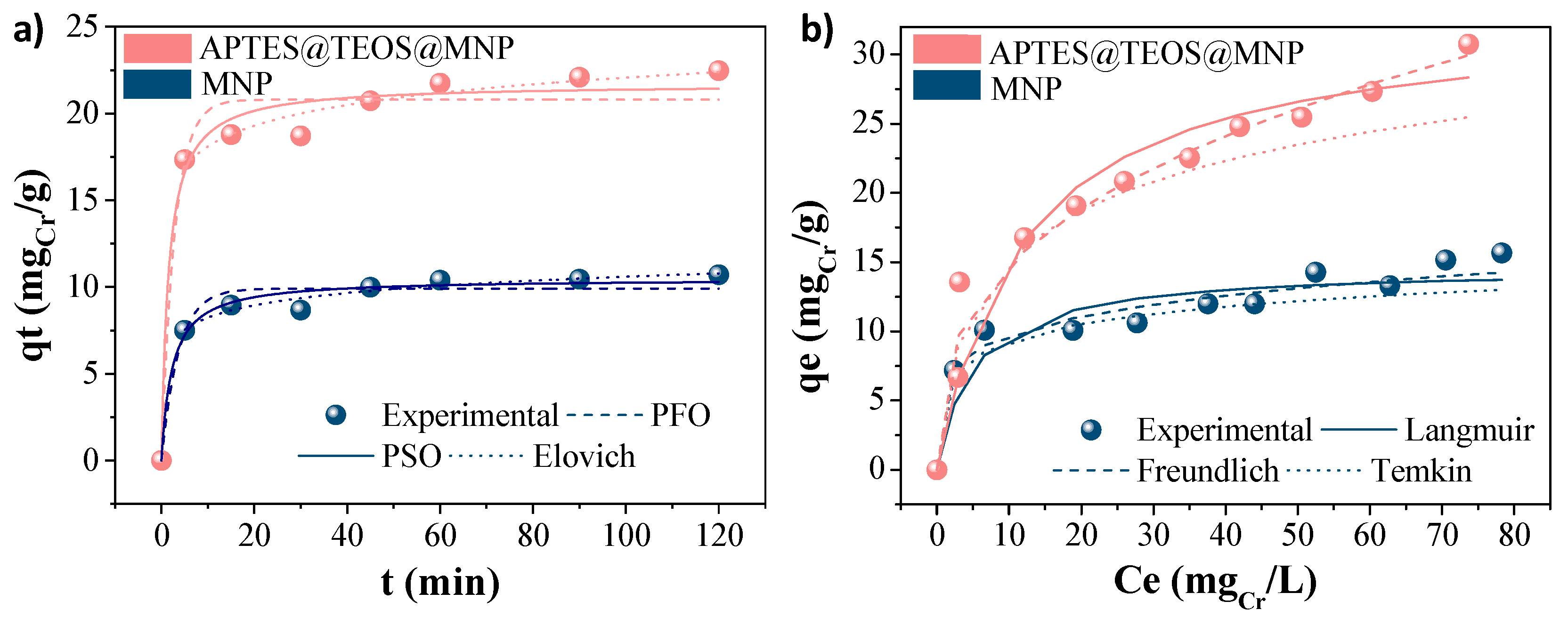
3.2.2. Kinetics and Isotherms Models
3.2.3. Reusability Assays
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gupta, A.B.; Gupta, A.; Pandey, A. Introduction to Water Remediation: Importance and Methods. In Water Remediation; Springer: Singapore, 2018; pp. 3–8. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Almeida, J.C.; Cardoso, C.E.D.; Tavares, D.S.; Freitas, R.; Trindade, T.; Vale, C.; Pereira, E. Chromium removal from contaminated waters using nanomaterials—A review. TrAC Trends Anal. Chem. 2019, 118, 277–291. [Google Scholar] [CrossRef]
- Gheju, M. Progress in Understanding the Mechanism of CrVI Removal in Fe0-Based Filtration Systems. Water 2018, 10, 651. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of Hexavalent Chromium-Contaminated Water and Wastewater: A Review. Water Air Soil Pollut. 2008, 200, 59–77. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Barrera-Diaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223–224, 1–12. [Google Scholar] [CrossRef]
- Corral-Bobadilla, M.; González-Marcos, A.; Vergara-González, E.; Alba-Elías, F. Bioremediation of Waste Water to Remove Heavy Metals Using the Spent Mushroom Substrate of Agaricus bisporus. Water 2019, 11, 454. [Google Scholar] [CrossRef]
- Kumari, M.; Pittman, C.U., Jr.; Mohan, D. Heavy metals [chromium (VI) and lead (II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres. J. Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Dolgormaa, A.; Lv, C.-J.; Li, Y.; Yang, J.; Yang, J.-X.; Chen, P.; Wang, H.-P.; Huang, J. Adsorption of Cu(II) and Zn(II) Ions from Aqueous Solution by Gel/PVA-Modified Super-Paramagnetic Iron Oxide Nanoparticles. Molecules 2018, 23, 2982. [Google Scholar] [CrossRef] [PubMed]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2016, 2, 43–70. [Google Scholar] [CrossRef]
- Martínez, L.J.; Muñoz-Bonilla, A.; Mazario, E.; Recio, F.J.; Palomares, F.J.; Herrasti, P. Adsorption of chromium(VI) onto electrochemically obtained magnetite nanoparticles. Int. J. Environ. Sci. Technol. 2015, 12, 4017–4024. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Sui, H.; He, L. One-Step Fabrication of Dual Responsive Lignin Coated Fe3O4 Nanoparticles for Efficient Removal of Cationic and Anionic Dyes. Nanomaterials 2018, 8, 162. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Ortega, D.; Southern, P.; Pankhurst, Q.A.; Thanh, N.T. High performance multi-core iron oxide nanoparticles for magnetic hyperthermia: Microwave synthesis, and the role of core-to-core interactions. Nanoscale 2015, 7, 1768–1775. [Google Scholar] [CrossRef]
- Lastovina, T.A.; Budnyk, A.P.; Kubrin, S.P.; Soldatov, A.V. Microwave-assisted synthesis of ultra-small iron oxide nanoparticles for biomedicine. Mendeleev Commun. 2018, 28, 167–169. [Google Scholar] [CrossRef]
- Brollo, M.E.F.; Veintemillas-Verdaguer, S.; Salvan, C.M.; Morales, M.P. Key Parameters on the Microwave Assisted Synthesis of Magnetic Nanoparticles for MRI Contrast Agents. Contrast Media Mol. Imaging 2017, 2017, 8902424. [Google Scholar] [CrossRef]
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louër, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Cryst. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Li, H.; Hou, R.; Chen, Y.; Chen, H. Removal of Hexavalent Chromium from Aqueous Solutions Using Sulfonated Peat. Water 2019, 11, 1980. [Google Scholar] [CrossRef]
- Deng, R.-J.; Jin, C.-S.; Ren, B.-Z.; Hou, B.-L.; Hursthouse, A. The Potential for the Treatment of Antimony-Containing Wastewater by Iron-Based Adsorbents. Water 2017, 9, 794. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Silva-Gordillo, M.d.M.; Muñoz, G.A.; Arboleda-Faini, X.; Almeida Streitwieser, D. Comparison of the adsorption capacity of organic compounds present in produced water with commercially obtained walnut shell and residual biomass. J. Environ. Chem. Eng. 2017, 5, 4041–4050. [Google Scholar] [CrossRef]
- Arriortua, O.K.; Insausti, M.; Lezama, L.; Gil de Muro, I.; Garaio, E.; de la Fuente, J.M.; Fratila, R.M.; Morales, M.P.; Costa, R.; Eceiza, M.; et al. RGD-Functionalized Fe3O4 nanoparticles for magnetic hyperthermia. Colloids Surf. B Biointerfaces 2018, 165, 315–324. [Google Scholar] [CrossRef]
- Iyengar, S.J.; Joy, M.; Ghosh, C.K.; Dey, S.; Kotnala, R.K.; Ghosh, S. Magnetic, X-ray and Mössbauer studies on magnetite/maghemite core–shell nanostructures fabricated through an aqueous route. RSC Adv. 2014, 4, 64919–64929. [Google Scholar] [CrossRef]
- Medina, R.P.; Nadres, E.T.; Ballesteros, F.C.; Rodrigues, D.F. Incorporation of graphene oxide into a chitosan–poly (acrylic acid) porous polymer nanocomposite for enhanced lead adsorption. Environ. Sci. Nano 2016, 3, 638–646. [Google Scholar] [CrossRef]
- Sodipo, B.K.; Aziz, A.A. A sonochemical approach to the direct surface functionalization of superparamagnetic iron oxide nanoparticles with (3-aminopropyl) triethoxysilane. Beilstein J. Nanotechnol. 2014, 5, 1472–1476. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.B.; Fang, Z.Q.; Tsang, P.K.E.; Cheng, W.; Yan, X.M.; Zheng, L.C. Selective adsorption of Cr(VI) from aqueous solution by EDA-Fe3O4 nanoparticles prepared from steel pickling waste liquor. Appl. Surf. Sci. 2014, 314, 655–662. [Google Scholar] [CrossRef]
- Srivastava, V.; Sharma, Y.C. Synthesis and Characterization of Fe3O4@n-SiO2 Nanoparticles from an Agrowaste Material and Its Application for the Removal of Cr(VI) from Aqueous Solutions. Water Air Soil Pollut. 2013, 225, 1776. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, D.; Fan, M.; Yang, D.; Zhu, R.; Ge, F.; Zhu, J.; He, H. Removal of hexavalent chromium [Cr(VI)] from aqueous solutions by the diatomite-supported/unsupported magnetite nanoparticles. J. Hazard. Mater. 2010, 173, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Obike, A.; Igwe, J.; Emeruwa, C.; Uwakwe, K.; Aghalibe, C. Diffusion-Chemisorption and Pseudo-Second Order Kinetic Models for Heavy Metal Removal from Aqueous Solutions Using Modified and Unmodified Oil Palm Fruit Fibre. Chem. Sci. Int. J. 2018, 23, 1–13. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Shen, H.Y.; Pan, S.D.; Hu, M.Q. Synthesis, characterization and properties of ethylenediamine-functionalized Fe3O4 magnetic polymers for removal of Cr(VI) in wastewater. J. Hazard. Mater. 2010, 182, 295–302. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.; Chen, G. Performance and mechanism of chromate (VI) adsorption by δ-FeOOH-coated maghemite (γ-Fe2O3) nanoparticles. Sep. Purif. Technol. 2007, 58, 76–82. [Google Scholar] [CrossRef]
- Burks, T.; Avila, M.; Akhtar, F.; Gothelid, M.; Lansaker, P.C.; Toprak, M.S.; Muhammed, M.; Uheida, A. Studies on the adsorption of chromium(VI) onto 3-Mercaptopropionic acid coated superparamagnetic iron oxide nanoparticles. J. Colloid Interface Sci. 2014, 425, 36–43. [Google Scholar] [CrossRef]


| Isotherm | Linearized Equation | Plot |
|---|---|---|
| Langmuir | Ce/qe = 1/b0qm + Ce/qm | Ce/qe vs. Ce |
| Freundlich | lnqe = lnKf + (lnCe)/n | lnqe vs. lnCe |
| Temkin | qe = (RT/bT)lnKT + (RT/bT)lnCe | qt vs. lnCe |
| Langmuir | Freundlich | Temkin | ||||||
| Constant | MNP | APTES | Constant | MNP | APTES | Constant | MNP | APTES |
| b0 (L/mg) | 0.19 | 0.08 | Kf (mg/g)/(L/g)1/n | 6.32 | 6.55 | bt (J/mol) | 1259.37 | 440.05 |
| qm (mg/g) | 15.00 | 35.00 | 1/n | 0.19 | 0.35 | kt (L/g) | 17.38 | 1.90 |
| R2 | 0.98 | 0.97 | R2 | 0.88 | 0.84 | R2 | 0.85 | 0.93 |
| Pseudo-First Order | Pseudo-Second Order | Elovich | ||||||
| Constant | MNP | APTES | Constant | MNP | APTES | Constant | MNP | APTES |
| qe (mg/g) | 9.9 | 20.8 | qe (mg/g) | 10.5 | 21.7 | β (mg/g) | 0.98 | 0.58 |
| k1 | 0.27 | 0.35 | k2 | 0.04 | 0.03 | α (mg/g.min) | 325 | 6445 |
| R2 | 0.96 | 0.95 | R2 | 0.98 | 0.98 | R2 | 0.89 | 0.88 |
| Adsorbent | Surface Charge | pH (-) | qm (mg/g) | Reference |
|---|---|---|---|---|
| TEOS@MNP | Slightly positive | 2.5 | 0 | This work |
| Fe3O4@n–SiO2 NPs | Slightly positive | 2 | 3.8 | [33] |
| Fe3O4 (PAA-coated and amino-functionalized) | Negative | 3 | 11.2 | [36] |
| Diatomite-supported magnetite NPs | Positive | 2 | 11.4 | [34] |
| Iron oxide magnetic nanoparticles (MNPs) | Positive | 2.5 | 15 | This work |
| Maghemite nanoparticles | Positive | 2.5 | 17 | [37] |
| APTES@TEOS@MNP | Positive | 2.5 | 35 | This work |
| (3-MPA)-functionalized iron oxide NPs | Positive | 1 | 45 | [38] |
| Chitosan-coated Fe3O4 nanocomposites | Positive | 2 | 81.5 | [32] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo-Cordova, A.; Morales, M.d.P.; Mazarío, E. Effect of the Surface Charge on the Adsorption Capacity of Chromium(VI) of Iron Oxide Magnetic Nanoparticles Prepared by Microwave-Assisted Synthesis. Water 2019, 11, 2372. https://doi.org/10.3390/w11112372
Gallo-Cordova A, Morales MdP, Mazarío E. Effect of the Surface Charge on the Adsorption Capacity of Chromium(VI) of Iron Oxide Magnetic Nanoparticles Prepared by Microwave-Assisted Synthesis. Water. 2019; 11(11):2372. https://doi.org/10.3390/w11112372
Chicago/Turabian StyleGallo-Cordova, Alvaro, María del Puerto Morales, and Eva Mazarío. 2019. "Effect of the Surface Charge on the Adsorption Capacity of Chromium(VI) of Iron Oxide Magnetic Nanoparticles Prepared by Microwave-Assisted Synthesis" Water 11, no. 11: 2372. https://doi.org/10.3390/w11112372
APA StyleGallo-Cordova, A., Morales, M. d. P., & Mazarío, E. (2019). Effect of the Surface Charge on the Adsorption Capacity of Chromium(VI) of Iron Oxide Magnetic Nanoparticles Prepared by Microwave-Assisted Synthesis. Water, 11(11), 2372. https://doi.org/10.3390/w11112372






