Impact of Advanced Oxidation Products on Nanofiltration Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Experimental Set Up
2.2.1. UV/H2O2 Oxidation
2.2.2. Membrane Filtration
2.3. Analytical Methods Used
2.3.1. HPLC—High-Performance Liquid Chromatography
2.3.2. SEM Microscopic Analysis
2.3.3. Determination of Contact Angle and Free Surface Energy (FSE)
- Diiodomethane, DIM (γL = 50.80 mJ/m2: γLLW = 50.80 mJ/m2, γL+ = 0 mJ/m2, γL− = 0 mJ/m2)
- Water, W (γL = 72. 80 mJ/m2: γLLW = 21.80 mJ/m2, γL+ = 25.50 mJ/m2, γL− = 25.50 mJ/m2)
- Formamide, F (γL = 58.00 mJ/m2: γLLW = 39.00 mJ/m2, γL+ = 2.28 mJ/m2, γL− = 39.60 mJ/m2)
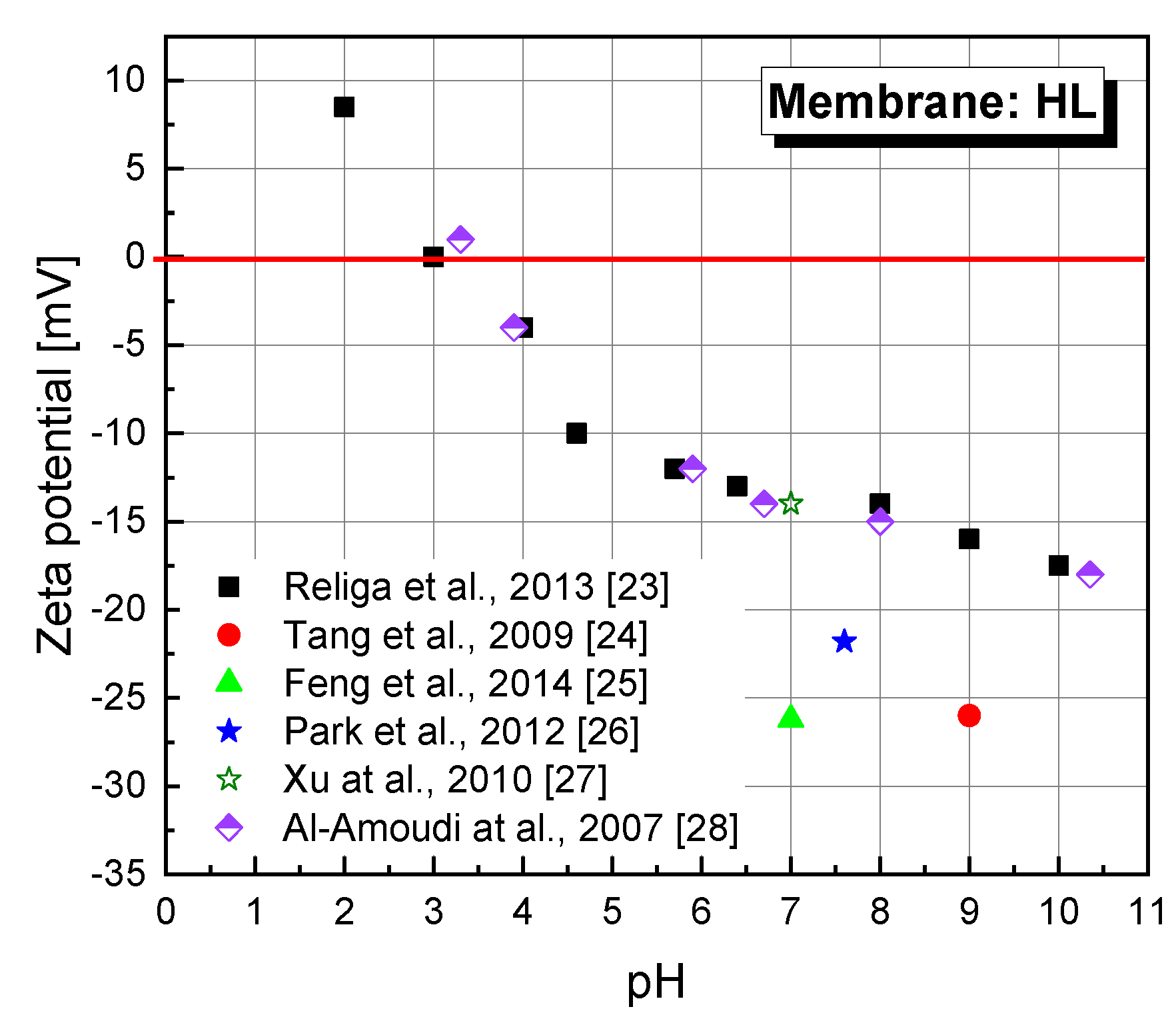
2.3.4. Zeta Potential Measurements
3. Results and Discussion
3.1. UV/H2O2 Oxidation
3.2. Nanofiltration
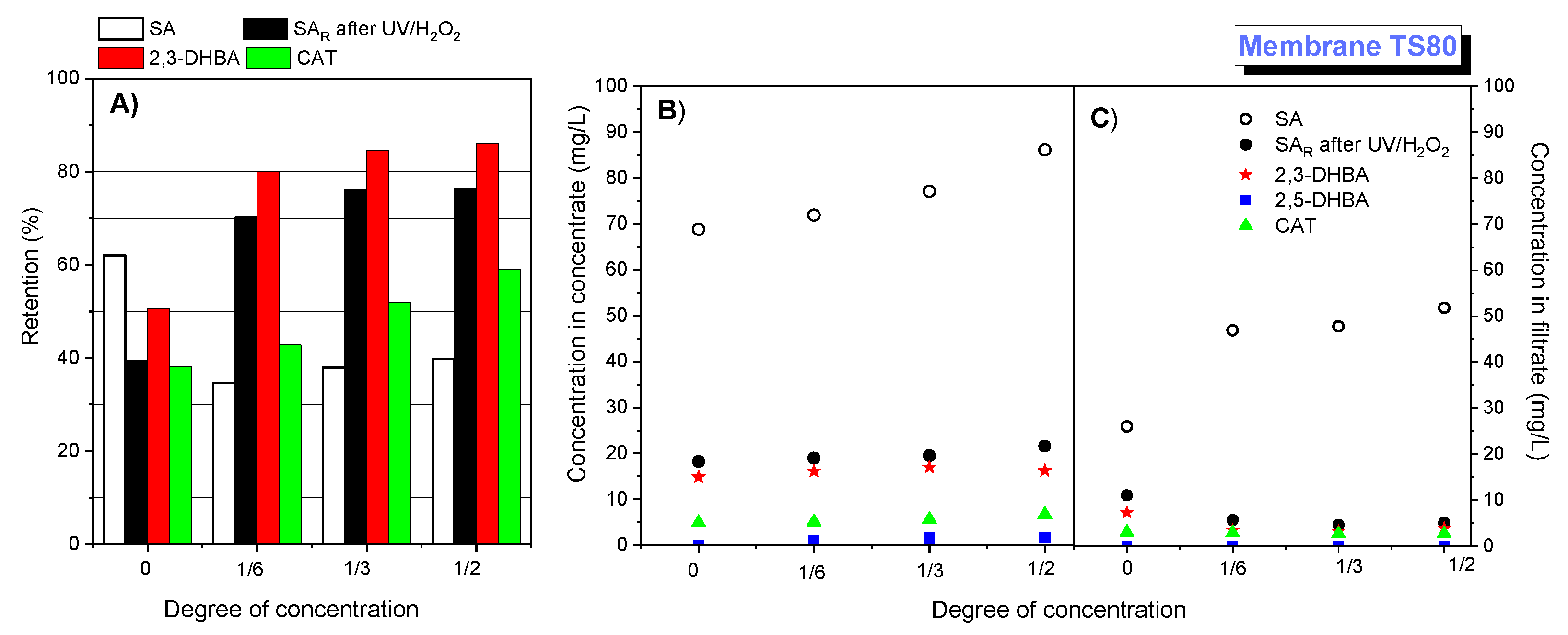
3.3. Retention of Oxidation Products
3.4. The Influence of Oxidation Products on the Retention of Residual Salicylic Acid
3.5. Determination of Contact Angles and Free Surface Energy
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Yang, P. Membrane adsorption of endocrine disrupting compounds and pharmaceutically active compounds. J. Membr. Sci. 2007, 303, 267–277. [Google Scholar] [CrossRef]
- Ternes, T.A.; Meisenheimer, M.; McDowell, M.; Brauch, D.S.F.; Haist-Gudle, B.; Pruess, G.; Wilme, U.; Zulei-Seibert, N. Removal of pharmaceuticals during drinking water treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in US drinking water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Fontela, M.; Galceran, T.M.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2001, 45, 1432–1442. [Google Scholar] [CrossRef]
- Vulliet, E.; Cren-Olivé, C. Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption. Environ. Pollut. 2011, 159, 2929–2934. [Google Scholar] [CrossRef]
- Żyłła, R.; Sójka-Ledakowicz, J.; Michalska, K.; Kos, L.; Ledakowicz, S. Effect of UV/H2O2 oxidation on fouling in textile wastewater nanofiltration. Fibres Text. East. Eur. 2012, 20, 99–104. [Google Scholar]
- Tian, M.; Adams, B.; Wen, J.; Asmussen, R.M.; Chen, A. Photoelectrochemical oxidation of salicylic acid and salicylaldehyde on titanium dioxide nanotube arrays. Electrochim. Acta 2009, 54, 3799–3805. [Google Scholar] [CrossRef]
- Chen, X.M.; Ribeiro da Silva, D.; Martínez-Huitle, C.A. Application of advanced oxidation processes for removing salicylic acid from synthetic wastewater. Chin. Chem. Lett. 2010, 21, 101–104. [Google Scholar] [CrossRef]
- Čelić, M.; Gros, M.; Farré, M.; Barceló, D.; Petrović, M. Pharmaceuticals as chemical markers of wastewater contamination in the vulnerable area of the Ebro Delta (Spain). Sci. Total Environ. 2019, 652, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Mezzelani, M.; Gorbi, S.; Regoli, F. Pharmaceuticals in the aquatic environments: Evidence of emerged threat and future challenges for marine organisms. Mar. Environ. Res. 2018, 140, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.; Tóth, T.; Homolok, R.; Rácz, G.; Takács, E. Hydroxyl radical induced degradation of salicylates in aerated aqueous solution. Radiat. Phys. Chem. 2014, 97, 239–245. [Google Scholar] [CrossRef]
- Karnik, B.S.; Davies, S.H.; Baumann, M.J.; Masten, S.J. Use of salicylic acid as a model compound to integrate hydroxyl radical reaction in an ozonation-membrane filtration hybrid process. Environ. Engng. Sci. 2007, 24, 852–860. [Google Scholar] [CrossRef]
- Punchard, N.; Kelly, F. (Eds.) Free Radicals: A Practical Approach; Oxford University Press: Oxford, UK, 1996; ISBN 0199635609. [Google Scholar]
- Albarran, G.; Schuler, R.H. Concerted effects in the reaction of OH radicals with aromatics: Radiolytic oxidation of salicylic acid. Radiat. Phys. Chem. 2003, 67, 79–285. [Google Scholar] [CrossRef]
- Kim, H.-A.; Choi, J.-H.; Takizawa, S. Comparison of initial filtration resistance by pretreatment processes in the nanofiltration for drinking water treatment. Sep. Purif. Technol. 2007, 56, 354–362. [Google Scholar] [CrossRef]
- Belkacem, M.; Bensadok, K.; Refes, A.; Charvier, P.M.; Nezzal, G. Water produce for pharmaceutical industry: Role of reverse osmosis stage. Desalination 2008, 221, 298–302. [Google Scholar] [CrossRef]
- Al-Rifai, J.H.; Khabbaz, H.; Schäfer, A.I. Removal of pharmaceuticals and endocrine disrupting compounds in a water recycling process using reverse osmosis systems. Sep. Purif. Technol. 2011, 77, 60–67. [Google Scholar] [CrossRef]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Zhang, T.C.; Valero, J.R. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewater. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Van den Berg, G.B.; Smolders, C.A. Diffusional phenomena in membrane separation processes. J. Membr. Sci. 1992, 73, 103–118. [Google Scholar] [CrossRef]
- Chang, S.; Waite, T.D.; Schäfer, A.I.; Fane, A.G. Adsorption of endocrine-active compound estrone on microfiltration hollow fiber membranes. Environ. Sci. Technol. 2003, 37, 3158–3163. [Google Scholar] [CrossRef] [PubMed]
- Religa, P.; Kowalik-Klimczak, A.; Gierycz, P. Study on the behavior of nanofiltration membranes using for chromium(III) recovery from salt mixture solution. Desalination 2013, 315, 115–123. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes II. Membrane physiochemical properties and their dependence on polyamide and coating layers. Desalination 2009, 242, 168–182. [Google Scholar] [CrossRef]
- Feng, G.; Chu, H.; Dong, B. Fouling effects of algogenic organic matters during nanofiltration of naproxen. Desalination 2014, 350, 69–78. [Google Scholar] [CrossRef]
- Park, S.K.; Choi, J.H.; Hu, J.Y. Assessing bacterial growth potential in a model distribution system receiving nanofiltration membrane treated water. Desalination 2012, 296, 7–15. [Google Scholar] [CrossRef]
- Xu, B.; Li, D.-P.; Li, W.; Xie, S.J.; Lin, Y.-L.; Hu, C.-Y.; Zhang, C.-Y.; Gao, N.-Y. Measurements of dissolved organic nitrogen (DON) in water samples with nanofiltration pretreatment. Water Res. 2010, 44, 5376–5384. [Google Scholar] [CrossRef]
- Al-Amoudi, A.; Williams, P.; Mandele, S.; Lovitt, R.W. Cleaning results of new and fouled nanofiltration membrane characterized by zeta potential and permeability. Sep. Purif. Technol. 2007, 54, 234–240. [Google Scholar] [CrossRef]
- Park, N.; Kwon, B.; Kim, I.S.; Cho, J. Biofouling potential of various NF membranes with respect to bacteria and their soluble microbial products (SMP): Characterizations, flux decline, and transport parameters. J. Membr. Sci. 2005, 258, 43–54. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Acero, J.L.; Benitez, F.J.; Teva, F.; Leal, A.I. Retention of emerging micropollutants from UP water and a municipal secondary effluent by ultrafiltration and nanofiltration. Chem. Eng. J. 2010, 163, 264–272. [Google Scholar] [CrossRef]
- Al-Amoudi, A.; Williams, P.; Al-Hobaib, A.S.; Lovitt, R.W. Cleaning results of new and fouled nanofiltration membrane characterized by contact angle, updated DSPM, flux and salts rejection. Appl. Surf. Sci. 2008, 254, 3983–3992. [Google Scholar] [CrossRef]
- Oh, B.S.; Jang, H.Y.; Hwang, T.M.; Kang, J.-W. Role of ozone for reducing fouling due to pharmaceuticals in MF (microfiltration) process. J. Membr. Sci. 2007, 289, 178–186. [Google Scholar] [CrossRef]
- Abdelmelek, S.B.; Greaves, J.; Ishida, K.P.; Cooper, W.J.; Song, W. Removal of pharmaceutical and personal care products from reverse osmosis retentate using advanced oxidation processes. Environ. Sci. Technol. 2011, 45, 3665–3671. [Google Scholar] [CrossRef] [PubMed]
- Real, F.J.; Benitez, F.J.; Acero, J.L.; Roldan, G. Combined chemical oxidation and membrane filtration techniques applied to the removal of some selected pharmaceuticals from water systems. J. Environ. Sci. Healthpart A 2012, 42, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Benner, J.; Salhi, E.; Ternes, T.; von Gunten, U. Ozonation of reverse osmosis concentrate: Kinetics and efficiency of beta blockers oxidation. Water Res. 2008, 42, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Justo, A.; Gonzáles, O.; Aceña, J.; Pérez, S.; Barceló, D.; Sans, C.; Esplugas, S. Pharmaceuticals and organic pollution mitigation in reclamation osmosis brines by UV/H2O2 and ozone. J. Hazard. Mater. 2013, 263, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Miralles-Cuevas, S.; Oller, I.; Pérez, J.A.; Malato, S. Removal of pharmaceuticals from MWTP effluent by nanofiltration and solar photo-Fenton using two different iron complexes at neutral pH. Water Res. 2016, 64, 23–31. [Google Scholar] [CrossRef]
- Oh, B.S.; Oh, S.; Kim, S.-J.; Choi, Y.; Hwang, T.-M. Optimization of wastewater reclamation and reuse system using membrane filtration and oxidation processes: Removal of pharmaceuticals. Desalin. Water Treat. 2016, 57, 10146–10151. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Mandale, S.J.; Williams, P.M.; Lovitt, R.W. Nanofiltration of treated digested agricultural wastewater for recovery of carboxylic acids. J. Clean. Prod. 2016, 112, 4749–4761. [Google Scholar] [CrossRef]
- Acero, J.L.; Benitez, F.J.; Real, F.J.; Teva, F. Micropollutants removal from retentates generated in ultrafiltration and nanofiltration treatments of municipal secondary effluents by means of coagulation, oxidation, and adsorption processes. Chem. Eng. J. 2016, 289, 48–58. [Google Scholar] [CrossRef]
- Religa, P.; Kowalik, A.; Gierycz, P. Effect of membrane properties on chromium(III) recirculation from concentrate salt mixture solution by nanofiltration. Desalination 2011, 274, 164–170. [Google Scholar] [CrossRef]
- Gautam, A.K.; Menkhaus, T.J. Performance evaluation and fouling analysis for reverse osmosis and nanofiltration membranes during processing of lignocellulosic biomass hydrolysate. J. Membr. Sci. 2014, 451, 252–265. [Google Scholar] [CrossRef]
- Petersen, R.J. Composite reverse-osmosis and nanofiltration membranes. J. Membr. Sci. 1993, 83, 81–150. [Google Scholar] [CrossRef]
- Verliefde, A.R.D.; Cornelissen, E.R.; Heijman, S.G.J.; Petrinic, I.; Luxbacher, T.; Amy, G.I.; Van der Bruggen, B.; van Dijk, J.C. Influence of membrane fouling by (pretreated) surface water on rejection of pharmaceutically active compounds (PhACs) by nanofiltration membranes. J. Membr. Sci. 2009, 330, 90–103. [Google Scholar] [CrossRef]
- Oss van, C.J.; Good, R.J.; Chaudhury, M.K. The role of van der Waals forces and hydrogen bonds in “hydrophobic interactions” between biopolymers and low energy surfaces. J. Colloid Interface Sci. 1986, 111, 378–390. [Google Scholar] [CrossRef]
- Bangham, D.H.; Razouk, R.I. Adsorption and the wettability of solid surfaces. Trans. Faraday Soc. 1937, 33, 1459–1463. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Lee, Y.; Neumann, A.W. Evaluation of the Lifshitz-van Der Waals/Acid-Base Approach to determine interfacial tensions of liquid—liquid systems. Langmuir 1998, 14, 2548–2553. [Google Scholar] [CrossRef]






| Compound | CAS | Molar Concentration (mol/L) | Mass Concentration (mg/L) | Molar Mass of Compound (g/mol) |
|---|---|---|---|---|
| SA | 69-72-7 | 5 × 10−4 | 69.06 | 138.12 |
| 2,3-DHBA | 303-38-8 | 3.24 × 10−4 | 50.0 | 154.12 |
| CAT | 120-80-9 | 3.24 × 10−4 | 35.72 | 110.1 |
| Characteristics | Membrane Type | |
|---|---|---|
| HL | TS80 | |
| Use | Water softening, Purification of industrial waters, wastewater decolorization | Water softening, Wastewater treatment, food industry, other industrial processes |
| Polymer | Thin film (composite: Piperazine-based polyamide, microporous polysulfone as a carrier layer) [23,25,40,41] | Polyamide |
| pH range | 3–9 | 2–11 |
| MWCO * (Da) | 150–300 | ~150 |
| Retention | 98.0% (MgSO4) | 99.0% (MgSO4)/80–90% (NaCl) |
| Typical flow rate L/(m2·h MPa) | 96 | 45 |
| Pressure range | 4 MPa [42] | |
| Temperature range | Max. 50 °C | Max. 45 °C |
| Surface | Smooth, roughness (RMS **) 10 nm [24,43] | RMS 89 nm [1] |
| Manufacturer | GE Osmonics | TriSepTM |
| Compound | SA | 2,3-DHBA | 2,5-DHBA | Catechol |
|---|---|---|---|---|
| Concentration (mg/L) | 17.5 | 14.8 | trace amounts | 5.1 |
| Molar concentration (mol/L) | 1.27 × 10−4 | 0.96 × 10−4 | -- | 0.46 × 10−4 |
| Degree of conversion (%) | 75 | 19.2 | -- | 9.2 |
| Concentration ratio | Concentrate | Filtrate | ||
|---|---|---|---|---|
| pH | Conductivity, μS/cm | pH | Conductivity, μS/cm | |
| Salicylic acid | ||||
| 0 | 3.52 | 153 | 3.83 | 77 |
| 1/6 | 3.54 | 158 | 3.56 | 118 |
| 1/3 | 3.51 | 163 | 3.53 | 139 |
| 1/2 | 3.52 | 165 | 3.54 | 140 |
| Salicylic acid + 2,3-DHBA | ||||
| 0 | 3.43 | 206 | 3.60 | 142 |
| 1/6 | 3.43 | 231 | 3.46 | 208 |
| 1/3 | 3.43 | 248 | 3.46 | 220 |
| 1/2 | 3.43 | 251 | 3.46 | 221 |
| Salicylic acid + Catechol | ||||
| 0 | 3.67 | 150 | 4.04 | 69 |
| 1/6 | 3.70 | 161 | 3.78 | 138 |
| 1/3 | 3.69 | 168 | 3.76 | 141 |
| 1/2 | 3.69 | 177 | 3.76 | 151 |
| No. | Sample | Contact Angle Θ, deg. | Free Surface Energy FSE, mJ/m2 | ||||
|---|---|---|---|---|---|---|---|
| ΘW | ΘF | ΘDIM | γLW | γAB | γ | ||
| 1 | HL membrane before filtration–pure | 59.0 | 51.6 | 34.8 | 42.1 | 1.2 | 43.3 |
| 2 | HL membrane after salicylic acid filtration | 75.9 | 71.3 | 47.6 | 35.6 | 6.9 | 42.5 |
| 3 | HL membrane after filtration of oxidized salicylic acid | 48.6 | 51.1 | 45.1 | 37.0 | 0.0 | 37.0 |
| 4 | HL membrane after filtration of salicylic acid and 2,3-DHBA mixture | 66.4 | 55.1 | 33.8 | 42.6 | 1.6 | 44.2 |
| 5 | HL membrane after filtration of salicylic acid and catechol mixture | 55.1 | 49.7 | 36.5 | 41.3 | 0.3 | 41.6 |
| 6 | HL membrane after filtration of water and H2O2 (0.324 mL/L) | 59.6 | 44.5 | 34.8 | 42.1 | 4.1 | 46.3 |
| No. | Sample | Contact Angle Θ,deg. | Free Surface Energy FSE, mJ/m2 | ||||
|---|---|---|---|---|---|---|---|
| ΘW | ΘF | ΘDIM | γLW | γAB | γ | ||
| 1 | Pure TS80 membrane | 53.4 | 52.5 | 32.9 | 43.5 | 7.5 | 48.3 |
| 2. | TS80 membrane after salicylic acid filtration | 59.7 | 48.1 | 30.0 | 44.2 | 0.3 | 44.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żyłła, R.; Milala, R.; Kamińska, I.; Kudzin, M.; Gmurek, M.; Ledakowicz, S. Impact of Advanced Oxidation Products on Nanofiltration Efficiency. Water 2019, 11, 541. https://doi.org/10.3390/w11030541
Żyłła R, Milala R, Kamińska I, Kudzin M, Gmurek M, Ledakowicz S. Impact of Advanced Oxidation Products on Nanofiltration Efficiency. Water. 2019; 11(3):541. https://doi.org/10.3390/w11030541
Chicago/Turabian StyleŻyłła, Renata, Rafał Milala, Irena Kamińska, Marcin Kudzin, Marta Gmurek, and Stanisław Ledakowicz. 2019. "Impact of Advanced Oxidation Products on Nanofiltration Efficiency" Water 11, no. 3: 541. https://doi.org/10.3390/w11030541
APA StyleŻyłła, R., Milala, R., Kamińska, I., Kudzin, M., Gmurek, M., & Ledakowicz, S. (2019). Impact of Advanced Oxidation Products on Nanofiltration Efficiency. Water, 11(3), 541. https://doi.org/10.3390/w11030541







