Contribution of Internal Nutrients Loading on the Water Quality of a Reservoir
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Benthic Chamber Experiment
2.3. Model Application
2.4. Statistical Evaluation of Calibration and Verification
3. Results and Discussion
3.1. Nutrient Release Fluxes from the Sediment
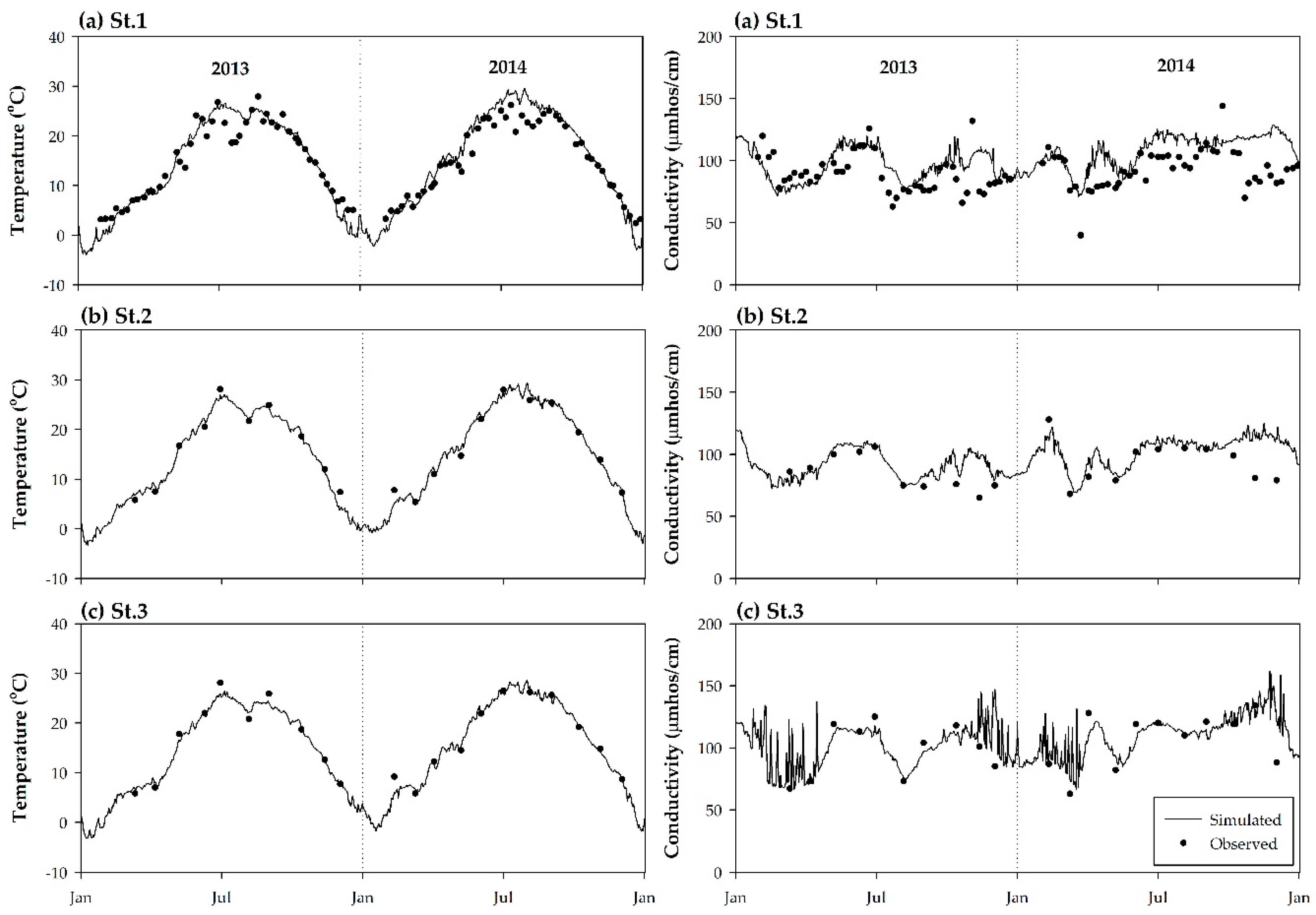
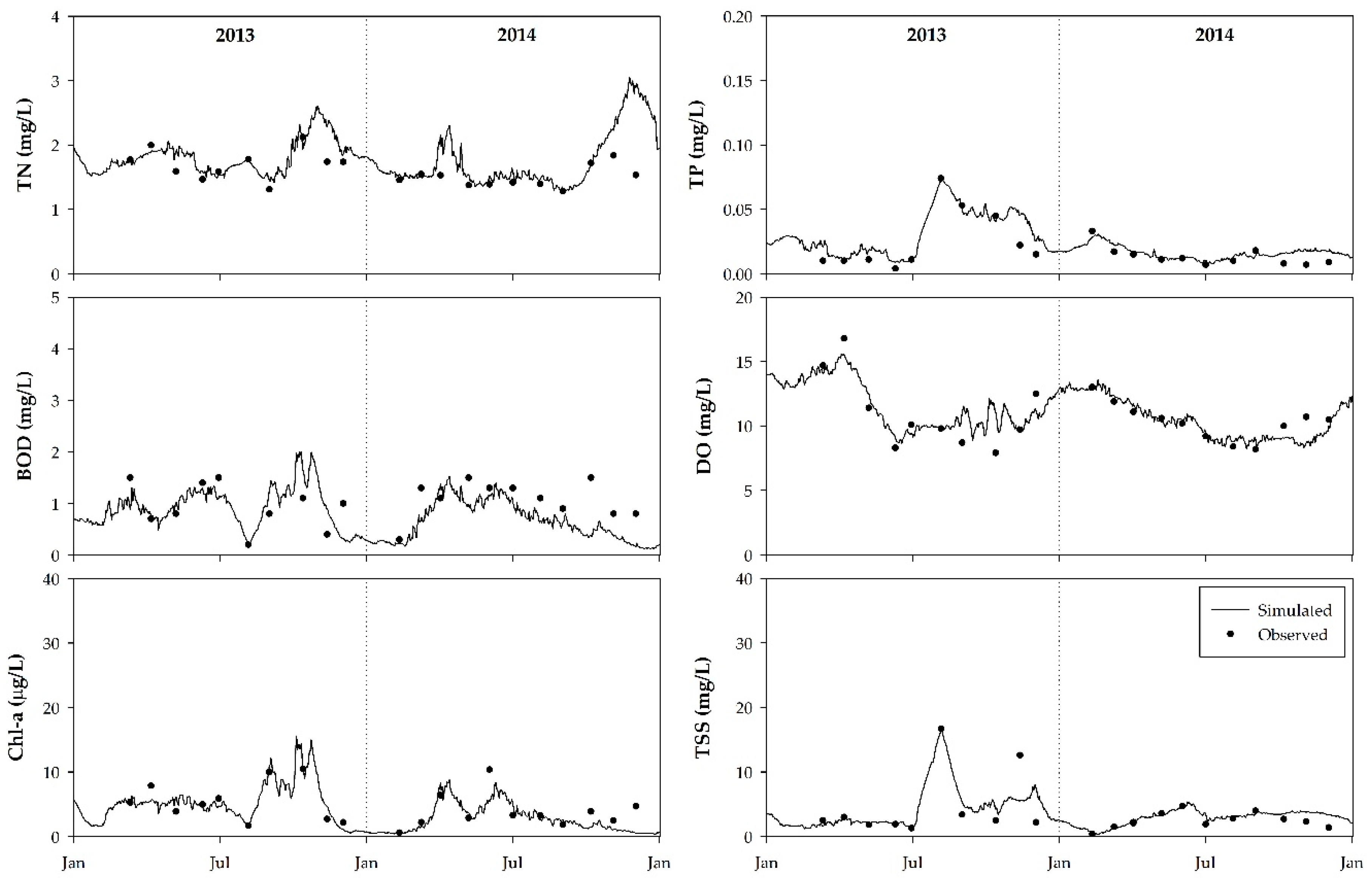
3.2. Model Calibration and Verification
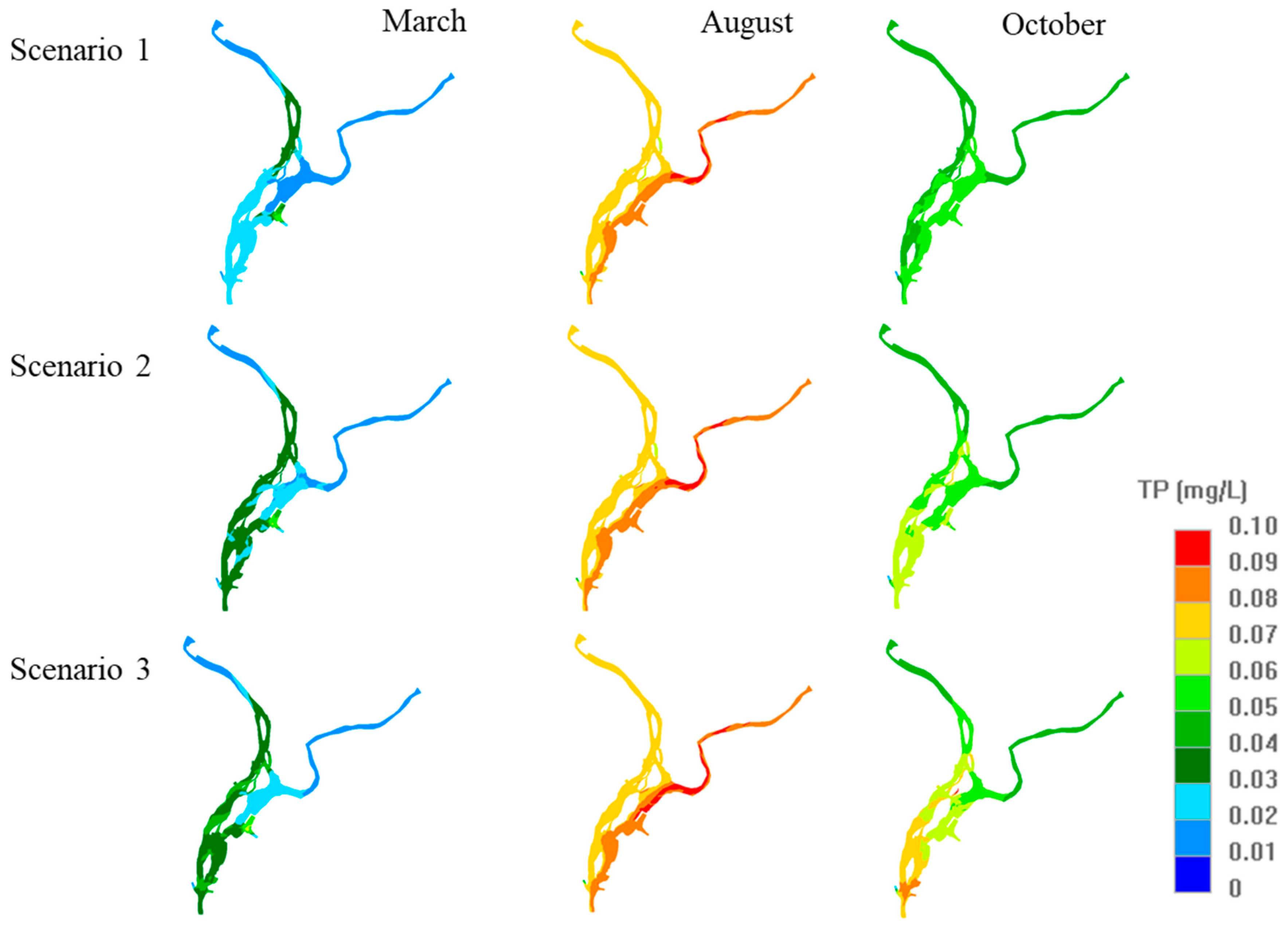
3.3. Assessment of Water Quality Affected by Sediment Flux
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Howarth, R.W.; Marino, R. Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnol. Oceanogr. 2006, 51, 364–376. [Google Scholar] [CrossRef]
- Schindler, D.W. Recent advances in the understanding and management of eutrophication. Limnol. Oceanogr. 2006, 51, 356–363. [Google Scholar] [CrossRef]
- Sinha, E.; Michalak, A.M.; Balaji, V. Eutrophication will increase during the 21st century as a result of precipitation changes. Science 2017, 357, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, M.; Jeppesen, E.; Lauridsen, T.L.; Skov, C.; Van Nes, E.H.; Roijackers, R.; Lammens, E.; Portielje, R. Lake restoration: Successes, failures and long-term effects. J. Appl. Ecol. 2007, 44, 1095–1105. [Google Scholar] [CrossRef]
- Granlund, K.; Räike, A.; Ekholm, A.; Rankinen, K.; Rekolainen, S. Assessment of water protection targets for agricultural nutrient loading in Finland. J. Hydrol. 2005, 304, 251–260. [Google Scholar] [CrossRef]
- Mozeto, A.A.; Silvério, P.F.; Soares, A. Estimates of benthic fluxes of nutrients across the sediment—Water interface (Guarapiranga reservoir, São Paulo, Brazil). Sci. Total Environ. 2001, 266, 135–142. [Google Scholar] [CrossRef]
- Lv, J.; Wu, H.; Chen, M. Effects of nitrogen and phosphorus on phytoplankton composition and biomass in 15 subtropical, urban shallow lakes in Wuhan, China. Limnologica 2011, 41, 48–56. [Google Scholar] [CrossRef]
- Moss, B. Cogs in the endless machine: Lakes, climate change and nutrient cycles: A review. Sci. Total. Environ. 2012, 434, 130–142. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Maeda, M. Effects of pH and oxygen on phosphorus release from agricultural drainage ditch sediment in reclaimed land, Kasaoka Bay, Japan. J. Water Environ. Technol. 2016, 14, 228–235. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Bu, Q.; Jiao, L.; Wu, F. Effects of dissolved oxygen supply level on phosphorus release from lake sediments. Colloid. Surface A 2008, 316, 245–252. [Google Scholar] [CrossRef]
- Wu, J.; Buchak, E.M.; Endinger, J.E.; Kolluru, V.S. Simulation of cooling-water discharge from power plants. J. Environ. Manage. 2001, 61, 77–92. [Google Scholar] [CrossRef]
- Fisher, M.M.; Reddy, K.R.; James, R.T. Long-term changes in the sediment chemistry of a large shallow subtropical lake. Lake Reserv. Manag. 2001, 17, 217–232. [Google Scholar] [CrossRef]
- Fisher, M.M.; Reddy, K.R.; James, R.T. Internal nutrient loads from sediments in a shallow, subtropical lake. Lake Reserv. Manag. 2005, 21, 338–349. [Google Scholar] [CrossRef]
- Khare, Y.P.; Naja, G.M.; Stainback, A.; Martinez, R.; Paudel, R.; Van Lent, T. A Phased Assessment of Restoration Alternatives to Achieve Phosphorus Water Quality Targets for Lake Okeechobee, Florida, USA. Water 2019, 11, 327. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Palmer-Felgate, E.J.; Mortimer, R.J.; Krom, M.D.; Jarvie, H.P.; Williams, R.J.; Spraggs, R.E. Internal loading of phosphorus in a sedimentation pond of a treatment wetland: Effect of a phytoplankton crash. Sci. Total Environ. 2011, 409, 2222–2232. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Asawa, G.L.; Lone, M.A. Experimental study of sediment transport hysteresis. J. Hydraul. Res. 2008, 46, 628–635. [Google Scholar] [CrossRef]
- Montreuil, A.-L.; Levoy, F.; Bretel, P.; Anthony, E.J. Morphological diversity and complex sediment recirculation on the ebb delta of a macrotidal inlet (Normandy, France): A multiple LiDAR dataset approach. Geomorphology 2014, 2019, 114–125. [Google Scholar] [CrossRef]
- Waters, K.A.; Curran, J.C. Linking bed morphology changes of two sediment mixtures to sediment transport predictions in unsteady flows. Water Resour. Res. 2015, 51, 2724–2741. [Google Scholar] [CrossRef]
- Burger, D.F.; Hamilton, D.P.; Pilditch, C.A. Modelling the relative importance of internal and external nutrient loads on water column nutrient concentrations and phytoplankton biomass in a shallow polymictic lake. Ecol. Model. 2008, 211, 411–423. [Google Scholar] [CrossRef]
- Qin, B.; Hu, W.; Gao, G.; Luo, L.; Zhang, J. Dynamics of sediment resuspension and the conceptual schema of nutrient release in the large shallow Lake Taihu, China. Chin. Sci. Bull. 2004, 49, 54–64. [Google Scholar] [CrossRef]
- Reddy, K.R.; Fisher, M.M.; Ivanoff, D. Resuspension and diffusive flux of nitrogen and phosphorus in a hypereutrophic lake. J. Environ. Qual. 1996, 25, 363–371. [Google Scholar] [CrossRef]
- Schadlow, S.G.; Hamilton, D.P. Effect of major flow diversion on sediment nutrient release in a stratified reservoir. Mar. Freshwater Res. 1995, 46, 189–195. [Google Scholar] [CrossRef]
- Hu, W.F.; Lo, W.; Chua, H.; Sin, S.N.; Yu, P.H.F. Nutrient release and sediment oxygen demand in a eutrophic land-locked embayment in Hong Kong. Environ. Int. 2001, 26, 369–375. [Google Scholar] [CrossRef]
- Rozan, T.F.; Taillefert, M.; Trouwborst, R.E.; Glazer, B.T.; Ma, S.; Herszage, J.; Valdes, L.M.; Price, K.S.; Luther, G.W., III. Iron-sulfur-phosphrous cycling in the sediments of a shallow coastal bay: Implications for sediment nutrient release and benthic macroalgal blooms. Limnol. Oceanogr. 2002, 47, 1346–1354. [Google Scholar] [CrossRef]
- Song, K.; Adams, C.J.; Burgin, A.J. Relative importance of external and internal phosphorus loadings on affecting lake water quality in agricultural landscapes. Ecol. Eng. 2017, 108, 482–488. [Google Scholar] [CrossRef]
- He, Y.; Men, B.; Yang, X.; Li, Y.; Xu, H.; Wang, D. Investigation of heavy metals release from sediment with bioturbation/bioirrigation. Chemosphere 2017, 184, 235–243. [Google Scholar] [CrossRef]
- Parsons, C.T.; Rezanezhad, F.; O’Connell, D.W.; Cappellen, P.V. Sediment phosphorus speciation and mobility under dynamic redox conditions. Biogeosciences 2017, 14, 3585–3602. [Google Scholar] [CrossRef]
- Petersen, W.; Willer, E.; Willamowski, C. Remobilization of trace elements from polluted anoxic sediments after resuspension in oxic water. Water Air Soil Pollut. 1997, 99, 515–522. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Sim, Y.-B.; Choi, B.-G.; Kim, K.; Park, C.; Soe, W.; Park, M.-H.; Lee, S.-W.; Shin, J.-K. Rainfall and hydrological comparative analysis of water quality variability in Euiam reservoir, the North-Han River, Korea. Korean J. Limnol. 2017, 50, 29–45. [Google Scholar] [CrossRef]
- Jung, S.; Shin, M.; Kim, J.; Eum, J.; Lee, Y.; Lee, J.; Choi, Y.; You, K.; Owen, J.; Kim, B. The effects of Asian summer monsoons on algal blooms in reservoirs. Inland Waters 2016, 6, 406–413. [Google Scholar] [CrossRef]
- Choi, J.K.; Min, J.-H.; Kim, D.-W. Three-dimensional algal dynamics modeling study in lake Euiam Based on limited monitoring data. J. Korean Soc. Water Environ. 2015, 31, 181–195. [Google Scholar] [CrossRef]
- Lee, G.; Lee, H.W.; Lee, Y.S.; Choi, J.H.; Yang, J.E.; Lim, K.J.; Kim, J. The effect of reduced flow on downstream water systems due to the kumgangsan dam under dry conditions. Water 2019, 11, 739. [Google Scholar] [CrossRef]
- Lim, B.; Ki, B.; Choi, J.H. Evaluation of nutrient release from sediments of Artificial Lake. J. Environ. Eng. 2011, 137, 347–354. [Google Scholar] [CrossRef]
- Edinger, J.E.; Buchak, E.M. Numerical hydrodynamics of estuaries. In Estuarine and Wetland Processes with Emphasis on Modeling; Hamilton, P., Macdonald, K.B., Eds.; Plenum Press: New York, NY, USA, 1980; pp. 115–146. [Google Scholar]
- Edinger, J.E.; Buchak, E.M. Numerical waterbody dynamics and small computers. Proceeding of the ASCE 1985 Hydraulic Division Specialty Conference on Hydraulics and Hydrology in the Small Computer Age, American Society of Civil Engineers, Lake Buena Vista, FL, USA, 12–17 August 1985; pp. 705–710. [Google Scholar]
- Edinger, J.E.; Buchak, E.M.; Kolluru, V.S. Modeling flushing and mixing in a deep estuary. Water Air Soil Poll. 1998, 102, 345–353. [Google Scholar] [CrossRef]
- Camp, J.S.; LeBoeuf, E.J.; Abkowitz, M.D. Application of an enhanced spill management information system to inland waterways. J. Hazard. Mater. 2010, 175, 583–592. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, S.S. A hydrodynamic modeling study to estimate the flushing rate in a large coastal embayment. J. Environ. Manag. 2013, 115, 278–286. [Google Scholar] [CrossRef]
- Na, E.H.; Park, S.S. A hydrodynamic modeling study to determine the optimum water intake location in Lake Paldang, Korea. J. Am. Water Resour. As. 2005, 41, 1315–1332. [Google Scholar] [CrossRef]
- Na, E.H.; Park, S.S. A hydrodynamic and water quality modeling study of spatial and temporal patterns of phytoplankton growth in a stratified lake with buoyant incoming flow. Ecol. Model. 2006, 199, 298–314. [Google Scholar] [CrossRef]
- Edinger, J.E. Waterbody hydrodynamic and water quality modeling. In An Introductory Workbook on Three Dimensional Water Body Hydrodynamics and Water Quality Modeling Including Modeling Software; ASCE Press: Reston, VA, USA, 2001. [Google Scholar]
- Ki, B.-M.; Huh, I.A.; Choi, J.H.; Cho, K.S. Relationship of nutrient dynamics and bacterial community structure at the water-sediment interface using a benthic chamber experiment. J. Environ. Sci. Health A 2018, 53, 482–491. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, X.; Yao, Y.; Li, L.; Wu, F. Effects of biological activity, light, temperature and oxygen on phosphorus release processes at the sediment and water interface of Taihu Lake, China. Water Res. 2008, 42, 2251–2259. [Google Scholar] [CrossRef]
- Youn, S.T.; Koh, Y.K.; Ryu, S.O. Distribution characteristics of surface sediments and metal elements in Hampyong Bay, the southwestern coast of Korea. J. Environ. Sci. Int. 1999, 8, 677–684. [Google Scholar]
- Tina, M.-W.; Twilley, R.R. Theory and operation of continuous flow systems for the study of benthic-pelagic coupling. Mar. Ecol. Prog. Ser. 1996, 140, 257–269. [Google Scholar]
- Shim, J.H.; Kang, Y.C.; Choi, J.W. Chemical fluxes at the sediment-water interface below marine fish cages on the coastal waters off Tong-young, south coast of Korea. Sea 1997, 2, 151–159. [Google Scholar]
- Jeong, H.-Y.; Jo, K.-J. SOD and inorganic nutrient fluxes from sediment in the downstream of the Nakdong River. Korean J. Limnol. 2003, 36, 322–335. [Google Scholar]
- Kim, D.-H.; Park, C.-K. Estimation of nutrients released from sediments of Deukryang Bay. J. Korean Environ. Sci. Soc. 1998, 7, 425–431. [Google Scholar]
- McKee, T.B.; Doesken, N.J.; Kleist, J. The relationship of drought frequency and duration to time scale. In Proceedings of the Eighth Conference on Applied Climatology; American Meteorological Society: Boston, MA, USA, 1993; pp. 179–184. [Google Scholar]
- Ambrose, R.B.; Wool, T.A.; Matrin, J.L. The Water Quality Analysis Simulation Program, WASP5. Part A: Model Documentation, Version 5.10.; U.S. Environmental Protection Agency: Athens, GA, USA, 1993.
- Bowie, G.L.; Mills, W.B.; Porcella, D.B.; Campbell, C.L.; Pagenkopt, J.R.; Rupp, G.L.; Johnson, K.M.; Chan, P.W.H.; Gherini, S.A. Rates, Constants and Kinetics Formulation in Surface Water Quality Modeling, 2nd ed.; U.S. Environmental Protection Agency: Athens, GA, USA, 1985; p. 475.
- Chapra, S.C. Surface Water-Quality Modeling; McGraw-Hill: New York, NY, USA, 1997; p. 844. [Google Scholar]
- Thomann, R.V.; Mueller, J.A. Principle of Surface Water Quality Modeling and Control; Harper Collins Publishers: New York, NY, USA, 1987; p. 644. [Google Scholar]
- WDOE (State of Washington Department of Environment). Comprehensive Circulation and Water Quality Study at Budd Inlet, Southern Puget Sound Water Quality Assessment Study; URS Corporation for the Washington State Department of Ecology: Washington, DC, USA, 1986; p. 275.
- Vollenweider, R.A.; Kerekes, J.J. Eutrophication of Waters: Monitoring, Assessment and Control; Organization for Economic Cooperation and Development: Paris, France, 1982; p. 156. [Google Scholar]
- Cralson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361. [Google Scholar] [CrossRef]







| Description | Symbol | Unit | Range | Value |
|---|---|---|---|---|
| Phytoplankton | ||||
| Maximum growth rate | K1c | day−1 | 0.01–4.0 | 2.5 |
| Death rate | K1D | day−1 | 0.015–0.2 | 0.015 |
| Endogenous respiration at 20 °C | K1R | day−1 | 0.05–0.20 | 0.1 |
| Half-saturation constant for nitrogen | KmN | mgN m−3 | 10–20 | 13 |
| Half-saturation constant for phosphorus | KmP | mgP m−3 | 1–2 | 1 |
| Settling velocity of phytoplankton | Vs4 | m day−1 | 0.05–0.5 | 0.05 |
| Nitrogen and phosphorus | ||||
| Nitrification rate | K12 | day−1 | 0.09–0.13 | 0.11 |
| Denitrification rate at 20 °C | K2D | day−1 | 0.09 | 0.09 |
| Organic nitrogen mineralization rate | K71 | day−1 | 0.01–0.15 | 0.15 |
| Dissolved organic phosphorus mineralization rate at 20 °C | K83 | day−1 | 0.1–0.3 | 0.3 |
| Settling velocity of particulate organic nitrogen | Vs7 | m day−1 | 0.05–0.5 | 0.18 |
| Settling velocity of particulate organic phosphorus | Vs8 | m day−1 | 0.05–0.5 | 0.05 |
| CBODand DO | ||||
| Deoxygenation rate at 20 °C | Kd | day−1 | 0.02–0.20 | 0.05 |
| Settling velocity of particulate CBOD | Vs5 | m day−1 | 0.05–0.5 | 0.05 |
| Parameter | Temperature | Conductivity | BOD | DO | |||||||||
| RMSE (℃) | MAPE (%) | NSE | RMSE (μs cm−1) | MAPE (%) | NSE | RMSE (mg L−1) | MAPE (%) | NSE | RMSE (mg L−1) | MAPE (%) | NSE | ||
| 2013 | St.1 | 0.37 | 20.0 | 0.88 | 2.35 | 14.9 | 0.18 | 0.15 | 71.2 | 0.03 | 0.22 | 11.9 | 0.42 |
| St.2 | 0.44 | 10.3 | 0.96 | 4.41 | 9.5 | 0.31 | 0.14 | 42.2 | 0.24 | 0.46 | 11.8 | 0.72 | |
| St.3 | 0.37 | 6.5 | 0.98 | 7.21 | 16.9 | 0.67 | 0.21 | 54.8 | 0.63 | 0.50 | 10.3 | 0.45 | |
| 2014 | St.1 | 0.39 | 21.4 | 0.87 | 3.19 | 21.5 | 0.06 | 0.18 | 60.8 | −1.15 | 0.29 | 13.0 | 0.15 |
| St.2 | 0.37 | 7.5 | 0.98 | 5.64 | 15.3 | 0.27 | 0.16 | 40.4 | 0.08 | 0.24 | 5.9 | 0.66 | |
| St.3 | 0.36 | 6.4 | 0.97 | 4.60 | 11.1 | 0.57 | 0.27 | 42.8 | 0.21 | 0.31 | 7.3 | 0.57 | |
| Parameter | TN | TP | Chl-a | TSS | |||||||||
| RMSE (mg L−1) | MAPE (%) | NSE | RMSE (mg L−1) | MAPE (%) | NSE | RMSE (μg L−1) | MAPE (%) | NSE | RMSE (mg L−1) | MAPE (%) | NSE | ||
| 2013 | St.1 | 0.05 | 13.6 | 0.13 | 0.003 | 88.5 | 0.15 | 0.88 | 51.7 | 0.06 | 0.61 | 11.9 | 0.36 |
| St.2 | 0.07 | 9.6 | 0.03 | 0.003 | 51.3 | 0.82 | 0.42 | 24.8 | 0.79 | 0.93 | 54.2 | 0.66 | |
| St.3 | 0.08 | 8.9 | 0.51 | 0.005 | 65.6 | 0.13 | 1.93 | 37.5 | 0.44 | 0.46 | 21.5 | 0.37 | |
| 2014 | St.1 | 0.05 | 16.2 | 0.20 | 0.002 | 45.6 | 0.05 | 0.62 | 59.2 | −1.77 | 0.19 | 42.1 | −0.02 |
| St.2 | 0.14 | 18.3 | 0.10 | 0.002 | 48.3 | 0.39 | 0.69 | 40.6 | 0.18 | 0.28 | 41.6 | 0.39 | |
| St.3 | 0.19 | 15.3 | 0.32 | 0.002 | 38.8 | 0.38 | 1.32 | 44.6 | 0.15 | 0.24 | 72.3 | 0.77 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.W.; Lee, Y.S.; Kim, J.; Lim, K.J.; Choi, J.H. Contribution of Internal Nutrients Loading on the Water Quality of a Reservoir. Water 2019, 11, 1409. https://doi.org/10.3390/w11071409
Lee HW, Lee YS, Kim J, Lim KJ, Choi JH. Contribution of Internal Nutrients Loading on the Water Quality of a Reservoir. Water. 2019; 11(7):1409. https://doi.org/10.3390/w11071409
Chicago/Turabian StyleLee, Hye Won, Yong Seok Lee, Jonggun Kim, Kyoung Jae Lim, and Jung Hyun Choi. 2019. "Contribution of Internal Nutrients Loading on the Water Quality of a Reservoir" Water 11, no. 7: 1409. https://doi.org/10.3390/w11071409
APA StyleLee, H. W., Lee, Y. S., Kim, J., Lim, K. J., & Choi, J. H. (2019). Contribution of Internal Nutrients Loading on the Water Quality of a Reservoir. Water, 11(7), 1409. https://doi.org/10.3390/w11071409







