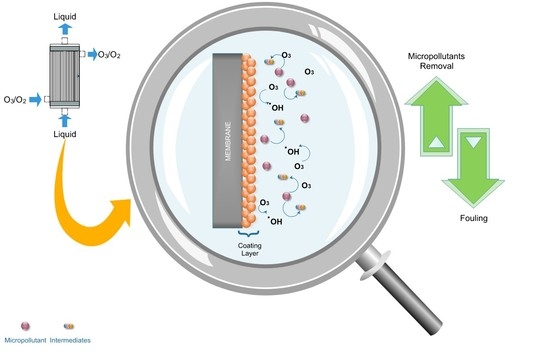
Catalytic Ozonation and Membrane Contactors—A Review Concerning Fouling Occurrence and Pollutant Removal
Abstract
:| Contents | |
| 1. Introduction.................................................................................................................................................................................................................................................... | 3 |
| 2. Membrane Filtration..................................................................................................................................................................................................................................... | 4 |
| 2.1 Membrane Materials............................................................................................................................................................................................................................... | 4 |
| 2.1.1 Organic Membranes.......................................................................................................................................................................................................................... | 5 |
| 2.1.2 Ceramic Membranes......................................................................................................................................................................................................................... | 5 |
| 2.2 Fouling Occurrence................................................................................................................................................................................................................................. | 7 |
| 3. Ozonation....................................................................................................................................................................................................................................................... | 9 |
| 3.1 Membrane Contactors............................................................................................................................................................................................................................ | 10 |
| 3.2 Catalytic Ozonation.................................................................................................................................................................................................................................. | 12 |
| 4. Applications of Hybrid Catalytic Membrane—Ozonation Systems......................................................................................................................................................... | 13 |
| 4.1 Fouling Control......................................................................................................................................................................................................................................... | 14 |
| 4.2 Removal of Micropollutants ................................................................................................................................................................................................................... | 17 |
| 4.2.1 Factors Affecting the Removal of MPs............................................................................................................................................................................................ | 17 |
| 4.2.2 Removal Efficiencies and Mechanistic Aspects.............................................................................................................................................................................. | 19 |
| 4.3 Removal of Bacteria.................................................................................................................................................................................................................................. | 25 |
| 4.4 Special Applications................................................................................................................................................................................................................................. | 25 |
| 4.5 Catalytic Membrane Stability.................................................................................................................................................................................................................. | 26 |
| 5. Conclusions..................................................................................................................................................................................................................................................... | 28 |
| References............................................................................................................................................................................................................................................................ | 30 |
1. Introduction
2. Membrane Filtration
2.1. Membrane Materials
2.1.1. Organic Membranes
2.1.2. Ceramic Membranes
2.2. Fouling Occurrence
3. Ozonation
3.1. Membrane Contactors
- Larger membrane area per unit volume of membrane module
- Higher productivity
- High self-mechanical support
- Good flexibility
- Easy handling from fabrication to operation.
3.2. Catalytic Ozonation
4. Applications of Hybrid Catalytic Membrane—Ozonation Systems
4.1. Fouling Control
4.2. Removal of Micropollutants
4.2.1. Factors Affecting the Removal of MPs
- Coating times
- Pore size
- Preparation method of the metal oxide to be used
- Adsorption capacity
Coating Times
Pore Size
Preparation Method of the Metal Oxide
Adsorption Capacity
4.2.2. Removal Efficiencies and Mechanistic Aspects
4.3. Removal of Bacteria
4.4. Special Applications
4.5. Catalytic Membrane Stability
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Activated Carbon |
| AOPs | Advanced Oxidation Processes |
| BP-3 | Benzophenone-3 |
| BPA | Bisphenol-A |
| CA | Cellulose Acetate |
| CCM | Ceramic Catalytic Membrane |
| CMC | Catalytic Membrane Contactor |
| Cm | Micropollutant concentration |
| CO3 | Ozone concentration |
| COD | Chemical Oxygen Demand |
| COP | Catalytic Ozonation Process |
| DBPs | Disinfection by-products |
| DEET | N,N-Diethyl-meta-toluamide |
| EfOM | Effluent Organic Matter |
| GAC | Granular Activated Carbon |
| GO | Graphene Oxide |
| IEP | Isoelectric Point |
| KHP | Potassium Hydrogen Phthalate |
| MIEX | Magnetic Ion Exchange Resins |
| MF | Microfiltration |
| MPs | Micropollutants |
| MTBE | Methyl Tert-Butyl Ether |
| MWCO | Molecular Weight Cut-Off |
| NF | Nanofiltration |
| NOM | Natural Organic Matter |
| O2•− | Superoxide ions |
| HO• | Hydroxyl radicals |
| PAC | Powder Activated Carbon |
| PAN | Poly-acrylonitrile |
| p-CNB | p-chloronitrobenzene |
| PDMS | Poly-dimethyl-siloxane |
| PEI | Polyether-imide |
| PES | Polyether-sulfone |
| pHpzc | Point of zero charge |
| PI | Polyimide |
| PP | Polypropylene |
| PTFE | Polytetrafluoroethylene |
| PVA | Polyvinyl-alcohol |
| PVDF | Polyvinylidene fluoride |
| RO | Reverse Osmosis |
| ROS | Reactive Oxygen Species |
| SA | Salicylic Acid |
| SAP | Single Adsorption Process |
| SBET | Surface Area |
| SOP | Single Ozonation Process |
| T | Temperature |
| TMP | Trans- Membrane Pressure |
| TOC | Total Organic Carbon |
| UF | Ultrafiltration |
| %wt | Weight percent |
| WWTP | Wastewater Treatment Plant |
References
- The World Bank-IBRD + IDA. Available online: https://www.worldbank.org/en/topic/water-in-agriculture (accessed on 30 September 2020).
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marin, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zhang, L.; Lei, J.; Zong, L.; Li, J.; Wu, Z.; Wang, Z. Overview of Emerging Contaminants and Associated Human Health Effects. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Zinicovscaia, I. Conventional Methods of Wastewater Treatment. In Cyanobacteria for Bioremediation of Wastewaters; Zinicovscaia, I., Cepoi, L., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Zhu, Y.; Quan, X.; Chen, F.; Fan, X.; Feng, Y. CeO2-TiO2 Coated Ceramic Membrane with Catalytic Ozonation Capability for Treatment of Tetracycline in Drinking Water. Sci. Adv. Mater. 2012, 4, 1–9. [Google Scholar] [CrossRef]
- Mansas, C.; Mendret, J.; Brosillon, S.; Ayral, A. Coupling Catalytic Ozonation and Membrane Separation: A Review Coupling. Sep. Purif. Technol. 2020, 236, 116221. [Google Scholar] [CrossRef]
- Scaratti, G.; De Noni Júnior, A.; José, H.J.; Moreira, R.D.P.M. 1,4-Dioxane removal from water and membrane fouling elimination using CuO-coated ceramic membrane coupled with ozone. Environ. Sci. Pollut. Res. 2020, 27, 22144–22154. [Google Scholar] [CrossRef]
- Hilal, N.; Wright, C.J. Exploring the current state of play for cost-effective water treatment by membranes. NPJ Clean. Water 2018, 1, 8. [Google Scholar] [CrossRef]
- Ozdemir, S.S.; Buonomenna, G.M.; Drioli, E. Catalytic polymeric membranes: Preparation and application. Appl. Catal. A Gen. 2006, 307, 167–183. [Google Scholar] [CrossRef]
- Zoumpouli, G.A.; Baker, R.; Taylor, C.M.; Chippendale, M.J.; Smithers, C.; Ho, S.S.X.; Mattia, D.; Chew, Y.M.J.; Wenk, J. A Single Tube Contactor for Testing Membrane Ozonation. Water 2018, 10, 1416. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.R.A.D.; Borges, C.P.; Fonseca, F.V.D. Polymeric Materials for Membrane Contactor Devices Applied to Water Treatment by Ozonation. Mater. Res. 2015, 18, 1015–1022. [Google Scholar] [CrossRef] [Green Version]
- Khaisri, S.; de Montigny, D.; Tontiwachwuthikul, P.; Jiraratananon, R. Comparing membrane resistance and absorption performance of three different membranes in a gas absorption membrane contactor. Sep Purif. Technol. 2009, 65, 290–297. [Google Scholar] [CrossRef]
- Wang, X.; Davies, S.H.; Masten, S.J. Energy cost analysis of Mn oxide coated ozone catalytic membrane filtration system. Sep. Purif. Technol. 2017, 186, 182–187. [Google Scholar] [CrossRef]
- Hu, Y.; Milne, N.; Gray, S.; Morris, G.; Jin, W.; Duke, M.; Zhu, B. Combined TiO2 membrane filtration and ozonation for efficient water treatment to enhance the reuse of wastewater. Desalin. Water Treat. 2011, 34, 57–62. [Google Scholar] [CrossRef]
- Hofs, B.; Ogier, J.; Vries, D.; Beerendonk, E.F.; Cornelissen, E.R. Comparison of ceramic and polymeric membrane permeability and fouling using surface water. Sep. Purif. Technol. 2011, 79, 365–374. [Google Scholar] [CrossRef]
- Wang, X.; Anctil, A.; Masten, S.J. Energy Consumption and Environmental Impact Analysis of Ozonation Catalytic Membrane Filtration System for Water Treatment. Environ. Eng. Sci. 2018, 36, 149–157. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, B.; Qi, F. A novel ceramic membrane coated with MnO2–Co3O4 nanoparticles catalytic ozonation for benzophenone-3 degradation in aqueous solution: Fabrication, characterization and performance. Chem. Eng. J. 2016, 287, 381–389. [Google Scholar] [CrossRef]
- Park, H.; Kim, Y.; An, B.; Choi, H. Characterization of natural organic matter treated by iron oxide nanoparticle incorporated ceramic membrane-ozonation process. Water Res. 2012, 46, 5861–5870. [Google Scholar] [CrossRef]
- Stylianou, S.K.; Sklari, S.D.; Zamboulis, D.; Zaspalis, V.T.; Zouboulis, A.I. Development of bubble-less ozonation and membrane filtration process for the treatment of contaminated water. J. Membr. Sci. 2015, 492, 40–47. [Google Scholar] [CrossRef]
- Kukuzaki, M.; Fujimoto, K.; Kai, S.; Ohe, K.; Oshima, T.; Baba, Y. Ozone mass transfer in an ozone–water contacting process with Shirasu porous glass (SPG) membranes—A comparative study of hydrophilic and hydrophobic membranes. Sep. Purif. Technol. 2010, 72, 347–356. [Google Scholar] [CrossRef]
- Madsen, H.T. Membrane Filtration in Water Treatment-Removal of Micropollutants. In Chemistry of Advanced Environmental Purification Processes of Water; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Huang, H.; Young, T.A.; Jacangelom, J.G. Unified Membrane Fouling Index for Low Pressure Membrane Filtration of Natural Waters: Principles and Methodology. Environ. Sci. Technol. 2008, 42, 714–720. [Google Scholar] [CrossRef]
- Zhu, B.; Hu, Y.; Kennedy, S.; Milne, N.; Morris, G.; Jin, W.; Gray, S.; Duke, M. Dual function filtration and catalytic breakdown of organic pollutants in wastewater using ozonation with titania and alumina membranes. J. Membr. Sci. 2011, 378, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Bokhary, A.; Tikka, A.; Leitch, M.; Liao, B. Membrane Fouling Prevention and Control Strategies in Pulp and Paper Industry Applications: A Review. J. Membr. Sci. Res. 2018, 4, 181–197. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, H.; Qu, F.; Ding, A.; Chang, H.; Liu, B.; Tang, X.; Wu, D.; Li, G. Fabrication of Mn oxide incorporated ceramic membranes for membrane fouling control and enhanced catalytic ozonation of p-chloronitrobenzene. Chem. Eng. J. 2017, 308, 1010–1020. [Google Scholar] [CrossRef]
- Vrijenhoek, E.M.; Hong, S.; Elimelech, M. Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2001, 188, 115–128. [Google Scholar] [CrossRef]
- Huang, H.; Schwab, K.; Jacangelo, J.G. Pretreatment for Low Pressure Membranes in Water Treatment: A Review. Environ. Sci. Technol. 2009, 43, 3011–3019. [Google Scholar] [CrossRef]
- Yu, W.; Brown, M.; Graham, N.J.D. Prevention of PVDF ultrafiltration membrane fouling by coating MnO2 nanoparticles with ozonation. Sci. Rep. 2016, 6, 30144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Liu, J.; Chu, H.; Dong, B. Pretreatment and Membrane Hydrophilic Modification to Reduce Membrane Fouling. Membranes 2013, 3, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Arhin, S.G.; Banadda, N.; Komakech, A.J.; Kabenge, I.; Wanyama, J. Membrane fouling control in low pressure membranes: A review on pretreatment techniques for fouling abatement. Environ. Eng. Res. 2016, 21, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.; Han, Z.; Li, G. Membrane fouling control in ultra filtration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Wolf, P.H.; Siverns, S.; Monti, S. UF membranes for RO desalination pretreatment. Desalination 2005, 182, 293–300. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Li, Z.; Yangali-Quintanilla, V.; Ghaffour, N.; Amy, G.; Leiknes, T.; Vrouwenvelder, J.S. Life cycle cost of a hybrid forward osmosis—Low cost pressure reverse osmosis system for seawater desalination and wastewater recovery. Water Res. 2016, 88, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.; Rosal, R.; Perdigón-Melón, J.A.; Mezcua, M.; Agüera, A.; Hernando, M.D.; Letón, P.; Fernández-Alba, A.R.; García-Calvo, E. Ozone-Based Technologies in Water and Wastewater Treatment. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2008; Volume 5, pp. 127–175. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Wastewater. A Practical Guide to Understanding Ozone and Its Application; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- von Sonntag, C.; von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment. From Basic Principles to Applications; IWA Publishing: London, UK, 2012. [Google Scholar]
- Mundy, B.; Kuhnel, B.; Hunter, G.; Jarnis, R.; Funk, D.; Walker, S.; Burns, N.; Drago, J.; Nezgod, W.; Huang, J.; et al. A Review of Ozone Systems Costs for Municipal Applications. Report by the Municipal Committee—IOA Pan American Group. Ozone Sci. Eng. 2018, 40, 266–274. [Google Scholar] [CrossRef]
- Kaprara, E.; Kostoglou, M.; Koutsiantzi, C.; Psaltou, S.; Zouboulis, A.I.; Mitrakas, M. Enhancement of ozonation efficiency employing dead-end hollow fiber membrane. Environ. Sci. Water Res. Technol. 2020, 6, 2619. [Google Scholar] [CrossRef]
- Berry, M.J.; Taylor, C.M.; King, W.; Chew, Y.M.J.; Wenk, J. Modelling of Ozone Mass-Transfer through Non-Porous Membranes for Water Treatment. Water 2017, 9, 452. [Google Scholar] [CrossRef]
- Stylianou, S.K.; Szymanska, K.; Katsoyiannis, I.A.; Zouboulis, A.I. Novel Water Treatment Processes Based on Hybrid Membrane-Ozonation Systems: A Novel Ceramic Membrane Contactor for Bubbleless Ozonation of Emerging Micropollutants. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Janknecht, P.; Picard, C.; Larbot, A.; Wilderer, P.A. Membrane Ozonation in Wastewater Treatment. Acta Hydrochim. Hydrobiol. 2004, 32, 33–39. [Google Scholar] [CrossRef]
- Li, K.; Xu, L.; Zhang, Y.; Cao, A.; Wang, Y.; Huang, H.; Wang, J. A novel electro-catalytic membrane contactor for improving the efficiency of ozone on wastewater treatment. Appl. Catal. B Environ. 2019, 249, 316–321. [Google Scholar] [CrossRef]
- Miachon, S.; Perez, V.; Crehan, G.; Torp, E.; Raeder, H.; Bredesen, R.; Dalmon, J.-A. Comparison of a contactor catalytic membrane reactor with a conventional reactor: Example of wet air oxidation. Catal. Today 2003, 82, 75–81. [Google Scholar] [CrossRef]
- Bazhenov, S.D.; Bildyukevich, A.V.; Volkov, A.V. Gas-Liquid Hollow Fiber Membrane Contactors for Different Applications. Fibers 2018, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Plumlee, M.H.; Stanford, B.D.; Debroux, J.F.; Hopkins, D.C.; Snyder, S.A. Costs of Advanced Treatment in Water Reclamation. Ozone Sci. Eng. 2014, 36, 485–495. [Google Scholar] [CrossRef]
- Mosadegh-Sedghi, S.; Rodrigue, D.; Brisson, J.; Iliuta, M.C. Wetting phenomenon in membrane contactors—Causes and prevention. J. Membr. Sci. 2014, 452, 332–353. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Chang, J.; Shen, J.; Kang, J.; Chen, Q. Fabrication of a low-cost cementitious catalytic membrane for p-chloronitrobenzene degradation using a hybrid ozonation-membrane filtration system. Chem. Eng. J. 2015, 262, 904–912. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Wang, F.; Xiong, X.; Tian, K.; Sun, Y. Application of Heterogeneous Catalytic Ozonation for Refractory Organics in Wastewater. Catalysts 2019, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Vittenet, J.; Wael, A.; Julie, M.; Pic, J.; Debellefontaine, H.; Lesage, N.; Faucher, K.; Manero, M.; Thibault-Starzyk, F.; Leclerc, H.; et al. Catalytic ozonation with γ-Al2O3 to enhance the degradation of refractory organics in water. Appl. Catal. A Gen. 2015, 504, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Shokri, A.; Mahanpoor, K. Degradation of ortho-toluidine from aqueous solution by the TiO2/O3 process. Int. J. Ind. Chem. 2017, 8, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Yan, X.; Xu, H.; Li, D.; Sun, L.; Cao, G.; Xia, D. Enhanced ozonation degradation of atrazine in the presence of nano-ZnO: Performance, kinetics and effects. J. Environ. Sci. 2017, 61, 3–13. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Tang, L.; Lu, P.; Sun, F.; Li, L. Catalytic ozonation of p-chlorobenzoic acid by activated carbon and nickel supported activated carbon prepared from petroleum coke. J. Hazard. Mater. 2009, 163, 115–120. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, M.; Ben, Y.; Shen, J.; Qi, F.; Chen, Z. Efficiency and mechanism of atenolol decomposition in Co-FeOOH catalytic ozonation. J. Hazard. Mater. 2019, 365, 146–154. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Catalytic ozonation for the removal of organic contaminants in water on ZSM-5 zeolites. Appl. Catal. B Environ. 2014, 154, 110–122. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Waheed, S.; Joya, K.S. Catalytic ozonation of paracetamol on zeolite A: Non-radical mechanism. Catal. Commun. 2018, 112, 15–20. [Google Scholar] [CrossRef]
- Khataee, A.; Rad, T.S.; Fathinia, M. The role of clinoptilolitenanosheets in catalytic ozonation process: Insights into the degradation mechanism, kinetics and the toxicity. J. Taiwan Inst. Chem. Eng. 2017, 77, 205–215. [Google Scholar] [CrossRef]
- Ahn, Y.; Oh, H.; Yoon, Y.; Park, W.K.; Yang, W.S.; Kang, J.W. Effect of graphene oxidation degree on the catalytic activity of graphene for ozone catalysis. J. Environ. Chem. Eng. 2017, 5, 3882–3894. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Kermani, M.; Kakavandi, B.; Farzadkia, M.; Esrafili, A.; Jokandan, S.F.; Shahsavani, A. Catalytic ozonation of high concentrations of catechol over TiO2@Fe3O4 magnetic core-shell nanocatalyst: Optimization, toxicity and degradation pathway studies. J. Clean. Prod. 2018, 192, 597–607. [Google Scholar] [CrossRef]
- DadbanShahamat, Y.; Sadeghi, M.; Shahryari, A.; Okhovat, N.; Bahrami, A.F.; Baneshi, M.M. Heterogeneous catalytic ozonation of 2,4-dinitrophenol in aqueous solution by magnetic carbonaceous nanocomposite: Catalytic activity and mechanism. Desalin. Water Treat. 2015, 57, 20447–20456. [Google Scholar] [CrossRef]
- Farzadkia, M.; DadbanShahamat, Y.; Nasseri, S.; Mahvi, A.H.; Gholami, M.; Shahryari, A. Catalytic Ozonation of Phenolic Wastewater: Identification and Toxicity of Intermediates. J. Eng. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, P.; Li, J.; Hou, D.; Wang, J.; Liu, H. A hybrid process combining homogeneous catalytic ozonation and membrane distillation for wastewater treatment. Chemosphere 2016, 160, 134–140. [Google Scholar] [CrossRef]
- Gu, L.; Tang, X.; Sun, Y.; Kou, H. Bioavailability of dissolved organic matter in biogas slurry enhanced by catalytic ozonation combined with membrane separation. Ecotoxicol. Environ. Saf. 2020, 196, 110547. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Kong, L.; Tu, Y.; Lu, J.; Xiong, Y. Efficient degradation of nitrobenzene by an integrated heterogeneous catalytic ozonation and membrane separation system with active MgO (111) catalyst. Desalin. Water Treat. 2015, 56, 2168–2180. [Google Scholar] [CrossRef]
- Corneal, L.M.; Baumann, M.J.; Masten, S.J.; Davies, S.H.R.; Tarabara, V.V.; Byun, S. Mn oxide coated catalytic membranes for hybrid ozonation-membrane filtration: Membrane microstructural characterization. J. Membr. Sci. 2011, 369, 182–187. [Google Scholar] [CrossRef]
- Karnik, B.S.; Davies, S.H.; Baumann, M.J.; Masten, S.J. Fabrication of Catalytic Membranes for the Treatment of Drinking Water Using Combined Ozonation and Ultrafiltration. Environ. Sci. Technol. 2005, 39, 7656–7661. [Google Scholar] [CrossRef] [PubMed]
- Karnik, B.S.; Davies, S.H.; Baumann, M.J.; Masten, S.J. Removal of Escherichia coli after Treatment Using Ozonation-Ultrafiltration with Iron Oxide-Coated Membranes. Ozone-Sci. Eng. 2007, 29, 75–84. [Google Scholar] [CrossRef]
- Davies, S.H.; Baumann, M.J.; Byun, S.; Corneal, L.M.; Tarabara, V.V.; Masten, S.J. Fabrication of catalytic ceramic membranes for water filtration. Water Sci. Technol. Water Supply. 2010, 10, 81–86. [Google Scholar] [CrossRef]
- Lee, W.J.; Bao, Y.; Hu, X.; Lim, T. Hybrid catalytic ozonation-membrane filtration process with CeOx and MnOx impregnated catalytic ceramic membranes for micropollutants degradation. Chem. Eng. J. 2019, 378, 121670. [Google Scholar] [CrossRef]
- Guo, Y.; Song, Z.; Xu, B.; Li, Y.; Qi, F.; Croue, J. A novel catalytic ceramic membrane fabricated with CuMn2O4 particles for emerging UV absorbers degradation from aqueous and membrane fouling elimination. J. Hazard Mater. 2018, 344, 1229–1239. [Google Scholar] [CrossRef]
- Wells, G.F.; Bottero, J.; Barron, A.R.; Wiesner, M.R. Ceramic membranes derived from ferroxane nanoparticles: A new route for the fabrication of iron oxide ultrafiltration membranes. J. Membr. Sci. 2003, 227, 207–217. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Zhang, X. Study on Catalytic Ozone Oxidation with Nano-TiO2 Modified Membrane for Treatment of Municipal Wastewater. Biomim. Biomater. Tissue Eng. 2013, 18, 113. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, C.; Yang, C.; Zhang, T. Degradation of Nitrobenzene by Nano-TiO2 /PVDF Membrane Catalytic Ozonation. In Advances in Intelligent and Soft Computing, Proceedings of the 2011 International Conference on Informatics, Cybernetics and Computer Engineering (ICCE2011), Melbourne, Australia, 19–20 November 2011; Jiang, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 112. [Google Scholar] [CrossRef]
- Feng, B.X.; Jiang, L. Design and Creation of Superwetting/Antiwetting Surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Agbaba, J.; Jazic, J.M.; Tubić, A.; Watson, M.; Maletić, S.; Isakovski, M.K.; Dalmacija, B. Oxidation of natural organic matter with processes involving O3, H2O2 and UV light: Formation of oxidation and disinfection by-products. RSC Adv. 2016, 6, 86212–86219. [Google Scholar] [CrossRef]
- Chen, S.; Yu, J.; Wang, H.; Yu, H.; Quan, X. A pilot-scale coupling catalytic ozonation—Membrane filtration system for recirculating aquaculture wastewater treatment. Desalination 2015, 363, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Ganiyu, S.O.; Hullebusch, E.D.V.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Verliefde, A.R.; Heijman, S.G.; Cornelissen, E.R.; Amy, G.; Van der Bruggen, B.; van Dijk, J.C. Influence of electrostatic interactions on the rejection with NF and assessment of the removal efficiency during NF/GAC treatment of pharmaceutically active compounds in surface water. Water Res. 2007, 41, 3227–3240. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Xu, B.; Chen, Z.; Zhang, L.; Zhang, P.; Sun, D. Mechanism investigation of catalyzed ozonation of 2-methylisoborneol in drinking water over aluminum (hydroxyl) oxides: Role of surface hydroxyl group. Chem. Eng. J. 2010, 165, 490–499. [Google Scholar] [CrossRef]
- Valdés, H.; Tardón, R.F.; Zaror, C.A. Role of surface hydroxyl groups of acid-treated natural zeolite on the heterogeneous catalytic ozonation of methylene blue contaminated waters. Chem. Eng. J. 2012, 211, 388–395. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Ma, J.; Tian, H.; Qiang, Z. Surface hydroxyl groups of synthetic α-FeOOH in promoting HO• generation from aqueous ozone: Property and activity relationship. Appl. Catal. B Environ. 2008, 82, 131–137. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, J. Catalytic ozonation of trace nitrobenzene in water with synthetic goethite. J. Mol. Catal. A Chem. 2008, 279, 82–89. [Google Scholar] [CrossRef]
- Heng, S.; Lun, K.; Julbe, A.; Ayral, A.; Schrotter, J. Preparation of composite zeolite membrane separator/contactor for ozone water treatment. Microporous Mesoporous Mater. 2008, 115, 137–146. [Google Scholar] [CrossRef]
- Gao, G.; Kang, J.; Shen, J.; Chen, Z.; Chu, W. Heterogeneous Catalytic Ozonation of Sulfamethoxazole in Aqueous Solution over Composite Iron—Manganese Silicate Oxide. Ozone Sci. Eng. 2017, 39, 24–32. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, X.; Ren, H.; Huang, Z. Preparation of MgO nanocrystals and catalytic mechanism on phenol ozonation. RSC Adv. 2017, 7, 43464–43473. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Shen, J.; Chen, Z.; Liu, Y. Pumice-catalyzed ozonation degradation of p-chloronitrobenzene in aqueous solution. Appl. Catal. B Environ. 2012, 117, 414–419. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.; Ma, J.; Liu, H. Enhancement mechanism of heterogeneous catalytic ozonation by cordierite-supported copper for the degradation of nitrobenzene in aqueous solution. Environ. Sci. Technol. 2009, 43, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Karnik, B.S.; Davies, S.H.; Baumann, M.J.; Masten, S.J. Use of Salicylic Acid as a Model Compound to Investigate Filtration Hybrid Process. Environ. Eng. Sci. 2007, 24, 852–860. [Google Scholar] [CrossRef]
- Li, Y.; Yeung, K.L. Polymeric catalytic membrane for ozone treatment of DEET in water. Catal. Today 2019, 331, 53–59. [Google Scholar] [CrossRef]
- Nalepa, T.; Stankiewicz, R.; Ammono, S.A.; Witkiewicz, Z. Rate of dibutylsulfide decomposition by ozonation and the O3/H2O2 advanced oxidation process. J. Hazard. Mater. 2009, 164, 1364–1371. [Google Scholar] [CrossRef]
- Chan, W.K.; Jouët, J.; Heng, S.; Lun, K.; Schrotter, J. Membrane contactor/separator for an advanced ozone membrane reactor for treatment of recalcitrant organic pollutants in water. J. Solid State Chem. 2012, 189, 96–100. [Google Scholar] [CrossRef]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human Health Risk Assessment for Aluminium, Aluminium Oxide, and Aluminium Hydroxide. J. Toxicol. Environ. Health Part. B 2011, 10, 1–269. [Google Scholar] [CrossRef]
- Pines, D.S.; Reckhow, D.A. Effect of dissolved cobalt(II) on the ozonation of oxalic acid. Environ. Sci. Technol. 2002, 36, 4046–4051. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating First Addendum, 4th ed + 1st Add. World Health Organization, License: CC BY-NC-SA 3.0 IGO. Available online: apps.who.int/iris/handle/10665/254637 (accessed on 20 August 2020).
- USEPA. 2018 Ed. of the Drinking Water Standards and Health Advisories; EPA 822-F-18–001; Office of Water. U.S. Environmental Protection Agency: Washington, DC, USA, 2018.
- BIS. Indian Standard for Drinking Water: Specification (Second Revision); Bureau of Indian Standards: New Delhi, India, 2012; IS: 10500: 2012.











| Organic Membranes | Ceramic Membranes |
|---|---|
| Lower cost | Good selectivity |
| Flexibility | Robustness |
| Scalability | High productivity |
| Elasticity | Chemical, mechanical, and thermal stability |
| Resistance to fatigue | Controlled pore size |
| Pre-Treatment Process | Foulants | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Coagulation | Viruses, humic/fluvic acids, proteins, polysaccharides with acid groups, colloids | Flux improvement | Requires proper dose | [25,28,29,30,31] |
| Reversible fouling reduction | May exacerbate fouling | |||
| Decrease colloids and NOM concentration | Produce solid wastes | |||
| Low cost | Ineffective in mitigating the fouling by hydrophilic neutral organics | |||
| Easy operation | No removal of small molecules | |||
| Adsorption | Humic/fluvic acids, small organic acids, some DBPs, pesticides, and other synthetic organic compounds | Flux improvement | Possible exacerbation of membrane fouling | [25,28] |
| Small molecules removal | Difficult in removing PAC powders from treatment facilities | |||
| Cost competitive method | ||||
| Pre-oxidation | Viruses and organic contaminants | Flux improvement | Formation of by-products, such asDBPs (chlorination), bromated (ozonation), precipitates (permanganate) | [25,28,30,31] |
| Decrease of organic pollutants concentration | May damage membranes incompatible with oxidants | |||
| Suppress microbial growth | May be ineffective in suppressing the growth of some microbiota resistant to oxidation | |||
| Pre-filtration | Particulate and colloidal organic/inorganic substances, microbiota | Flux improvement | Performance of pre-filters may deteriorate and be difficult to recover | [25,28] |
| Can remove colloidal matter and suspended solids | May require pre-treatment (e.g., coagulation or pre-oxidation) to enhance the efficacy |
| Advantages | Disadvantages |
|---|---|
| Higher mass transfer | Mass-transfer resistance from the membrane itself |
| Operating flexibility | Wetting of membrane pores |
| Scale-up simplicity | Sensitive to impurities |
| Absence of dispersion between the fluid phases | Membrane fouling |
| Compact structure | Life duration of a membrane (quicker replacement) |
| Operation with low pressure drops | Only mild operating conditions can be applied (polymeric) membranes) |
| Higher interfacial area | |
| Easy recycling of effluent mixture | |
| Increase conversion in equilibrium limited reactions | |
| Overall higher efficiency |
| MP | Catalytic Membrane | Membrane Characteristics | Conditions | Efficiency | Reference |
|---|---|---|---|---|---|
| Tetracycline | CeO2-TiO2/α-Al2O3 | Thickness = 1.5 μm Particle size = 13 nm SBET = 71.3 m2/g Pore size = 9.0 nm MWCO = 80 kDa | Cm = 5 mg/L CO3 = 2.5 mg/L TMP = 2 bar | SAP < 10% (600 min) COP > 80% (200 min) | [6] |
| 1,4-dioxane | CuO/α-Al2O3-ZrO2 | One channel L = 25 cm D = 1 cm Pore diameter = 50 nm A = 47.12 cm2 MWCO = 10 kDa Cover density = 0.90 mg/cm2 | Cm = 200 mg/L CO3 = 60 mg/L pH = 4–5.5 T = 25 ± 1 °C TMP = 0.25 bar | SAP = ng (1) COP ≈ 65% (350 min) | [8] |
| Nitrobenzene | Ni foam/PVDF | Flat sheet Thickness = 210 μm Porosity = 76% Pore size = 0.2 μm | Cm = 30 mg/L CO3 = 50 mg/L pH = 7 current density = 1 mA/cm2 | Electrolysis = 23% COP = 55% Electrolysis-COP = 85% (120 min) | [43] |
| p-CNB | Cement (MF) | Pore size = 0.3–8.2 μm 19.97%wt Si 7.57%wt Ca | Cm = 15 mg/L CO3 = 2.5 mg/L pH = 6.5 HRT = 10 min TMP = 60 Kpa | SAP ≈ 0 SOP ≈ 28% COP = 90% | [48] |
| BPA | Mn2O3/Al2O3 | SBET = 35.4 m2/g 4.2 mg/g Mn | Cm = 3 mg/L CO3 = 4 mg/L HRT = 13.7 min Reaction time = 60 min | SAP = 55% (1 h) SOP = 84% COP ≈ 98% | [70] |
| BTZ | SAP < 5% SOP = 57% COP ≈ 55% | ||||
| CA | SAP < 5% SOP = 49% COP ≈ 55% | ||||
| BTA | CeO2/Al2O3 | SBET = 47.4 m2/g 19.8 mg/g Ce Rq = 105.4 nm | SAP < 5% SOP = 84% COP ≈ 80% | ||
| BTZ | SAP < 5% SOP = 57% COP ≈ 57% | ||||
| CA | SAP < 5% SOP = 49% COP ≈ 40% | ||||
| BP-3 | CuMn2O4/ZrO2/α-Al2O3 | Rq = 187 ± 17 nm Thickness = 125–145 μm | Cm = 2 mg/L CO3 = 1 mg/L pH = 7 ± 0.25 | SAP ≈ 8% SOP = 47.4% COP = 76.6% | [71] |
| Nitrobenzene | Nano-TiO2/PVDF | Membrane area = 12 × 15 cm2 | Cm = 48.08 μg/L CO3 = 2.5 mg/L pH = 8 T = 25 °C TMP = 5 × 104 Pa | SAP = 18.4% SOP = 25.7% COP = 59.5% (20 min) | [74] |
| Salicylic acid | Fe2O3/CéRAM | D = 10 mm L = 25 cm A = 41.2 cm2 MWCO = 5 kDa 40 layers of Fe2O3 | Cm = 65 μΜ CO3 = 2.5 mg/L pH = 8 T = 20 °C TMP = 5 × 104 Pa | SOP = 40% (240 min) COP > 95% (240 min) | [89] |
| DEET | PAC/PVDF | SBET = 760 m2/g Pore diameter = 2.4 nm Pore volume = 0.46 cm3/g Thickness = 20 μm (of the coating) | Cm = 200 mg/L CO3/O2 = 120 mg/L | SAP = 83% (10 min) SOP = 45% COP = 39% | [90] |
| Fh (2)-AC/PVDF | SBET = 730 m2/g Pore diameter = 2.7 nm Pore volume = 0.49 cm3/g Thickness = 20 μm (of the coating) | SAP = 80% (10 min) SOP = 45% COP = 60% | |||
| KHP | Alumina/ZSM-5 | Pore diameter = 6.40 nm Pore volume = 0.63 cm3/g SBET = 395 m2/g IEP = 8.2 (of the coating) Surface area(contactor) = 48 m2 | Cm = 100 mg/L CO3 = 100 mg/L pH = 7 T = 40 °C | COP = 100% High adsorption capacity Better TOC removal | [92] |
| Hydrotalcite/ZSM-5 | Pore diameter = 6.23 nm Pore volume = 0.07 cm3/g SBET = 44 m2/g IEP = 11.7 (of the coating) Surface area (contactor) = 90 m2 | COP = 100% High adsorption capacity |
| Heavy Metal | Limit (μg/L) | ||
|---|---|---|---|
| WHO, 2017 | USEPA, 2015 | BIS, 2012 | |
| Iron (Fe) | NGV | 300 | 300 |
| Manganese (Mn) | NGV | 50 | 100 |
| Zinc (Zn) | NGV | 7400 | 5000 |
| Copper (Cu) | 2000 | 1300 | 50 |
| Nickel (Ni) | 70 | 610 | 20 |
| Aluminum (Al) | NGV * | 200 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psaltou, S.; Zouboulis, A. Catalytic Ozonation and Membrane Contactors—A Review Concerning Fouling Occurrence and Pollutant Removal. Water 2020, 12, 2964. https://doi.org/10.3390/w12112964
Psaltou S, Zouboulis A. Catalytic Ozonation and Membrane Contactors—A Review Concerning Fouling Occurrence and Pollutant Removal. Water. 2020; 12(11):2964. https://doi.org/10.3390/w12112964
Chicago/Turabian StylePsaltou, Savvina, and Anastasios Zouboulis. 2020. "Catalytic Ozonation and Membrane Contactors—A Review Concerning Fouling Occurrence and Pollutant Removal" Water 12, no. 11: 2964. https://doi.org/10.3390/w12112964
APA StylePsaltou, S., & Zouboulis, A. (2020). Catalytic Ozonation and Membrane Contactors—A Review Concerning Fouling Occurrence and Pollutant Removal. Water, 12(11), 2964. https://doi.org/10.3390/w12112964