Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Chemicals and Standards
2.3. Sample Cleanup and Pre-Concentration
2.4. Instrumental Analysis
3. Results
3.1. Instrumental Analysis Results
3.2. Occurrence of API Cocktails in the Effluent, SPM, Surface Water and River Sediments
4. Discussion
4.1. Cocktails of APIs in the Natural Environment within Low- and Medium-Income Countries
4.2. Risk of APIs in the Environment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vasquez, M.I.; Lambrianides, A.; Schneider, M.; Kümmerer, K.; Fatta-Kassinos, D. Environmental side effects of pharmaceutical cocktails: What we know and what we should know. J. Hazard. Mater. 2014, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks; Springer: Berlin, Germany, 2008; Volume 1. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Tambosi, J.L.; Yamanaka, L.Y.; José, H.J.; De Fátima Peralta Muniz Moreira, R.; Schröder, H.F. Recent research data on the removal of pharmaceuticals from sewage treatment plants (STP). Quim. Nova 2010, 33, 411–420. [Google Scholar] [CrossRef]
- Tran, N.H.; Chen, H.; Reinhard, M.; Mao, F.; Yew-Hoong Gin, K. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016, 104, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the european scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Silva, L.; Laranjeiro, C.; Lino, C.; Pena, A. Selected pharmaceuticals in different aquatic compartments: Part I—Source, fate and occurrence. Molecules 2020, 25, 1026. [Google Scholar] [CrossRef] [Green Version]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef] [Green Version]
- Yakubu, O.H. Pharmaceutical wastewater effluent-source of contaminants of emerging concern: Phytotoxicity of metronidazole to soybean (Glycine Max). Toxics 2017, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef]
- Larsen, T.A.; Maurer, M.; Udert, K.M.; Lienert, J. Nutrient cycles and resource management: Implications for the choice of wastewater treatment technology. Water Sci. Technol. 2007, 56, 229–237. [Google Scholar] [CrossRef]
- KNBS. 2019 Kenya Population and Housing Census: Volume IV—Distibution of Population by Socio-Economic Characteristics; KNBS: Nairobi, Kenya, 2019.
- Kairigo, P.; Ngumba, E.; Sundberg, L.; Gachanja, A.; Tuhkanen, T. Occurrence of antibiotics and risk of antibiotic resistance evolution in selected Kenyan wastewaters, surface waters and sediments. Sci. Total Environ. 2020, 720, 137580. [Google Scholar] [CrossRef] [PubMed]
- Ngumba, E.; Anthony, G.; Tuhkanen, T. Occurrence of selected antibiotics and antiretroviral drugs in Nairobi River Basin, Kenya. Sci. Total Environ. 2016, 539, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Balakrishna, K.; Joshua, D.I.; Kannan, K. Mass loading and removal of pharmaceuticals and personal care products including psychoactives, antihypertensives, and antibiotics in two sewage treatment plants in Southern India. Chemosphere 2017, 167, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngumba, E.; Kosunen, P.; Gachanja, A.; Tuhkanen, T. A multiresidue analytical method for trace level determination of antibiotics and antiretroviral drugs in wastewater and surface water using spe-lc-ms/ms and matrix-matched standards. Anal. Methods 2016, 8, 6720–6729. [Google Scholar] [CrossRef] [Green Version]
- Danner, M.-C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPS), endocrine disrupting contaminants (EDCS), metabolites and illicit drugs in a wwtw and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef]
- Lindberg, R.H.; Wennberg, P.; Johansson, M.I.; Tysklind, M.; Andersson, B.A.V. Screening of human antibiotic substances and determination of weekly mass flows in five sewage treatment plants in Sweden. Environ. Sci. Technol. 2005, 39, 3421–3429. [Google Scholar] [CrossRef]
- Ignatev, A.; Tuhkanen, T. Monitoring WWTP performance using size-exclusion chromatography with simultaneous uv and fluorescence detection to track recalcitrant wastewater fractions. Chemosphere 2019, 214, 587–597. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [Green Version]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO|HIV/AIDS. Available online: https://www.who.int/gho/hiv/en/ (accessed on 22 March 2019).
- World Health Organization. WHO|Antiretroviral Therapy (ART) Coverage among All Age Groups. Available online: https://www.who.int/gho/hiv/epidemic_response/ART/en/ (accessed on 22 March 2019).
- Swanepoel, C.; Bouwman, H.; Pieters, R.; Bezuidenhout, C. Presence, Concentrations and Potential Implications of Hiv-Anti-Retrovirals in Selected Water Resources in South Africa; Water Research Commission: Pretoria, South Africa, 2015.
- Wood, T.P.; Duvenage, C.S.J.; Rohwer, E. The occurrence of anti-retroviral compounds used for HIV treatment in south african surface water. Environ. Pollut. 2015, 199, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ncube, S.; Madikizela, L.M.; Chimuka, L.; Nindi, M.M. Environmental fate and ecotoxicological effects of antiretrovirals: A current global status and future perspectives. Water Res. 2018, 145, 231–247. [Google Scholar] [CrossRef] [PubMed]
- K’oreje, K.O.; Vergeynst, L.; Ombaka, D.; De Wispelaere, P.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu City, Kenya. Chemosphere 2016, 149, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kandie, F.J.; Krauss, M.; Beckers, L.M.; Massei, R.; Fillinger, U.; Becker, J.; Liess, M.; Torto, B.; Brack, W. Occurrence and risk assessment of organic micropollutants in freshwater systems within the Lake Victoria South Basin, Kenya. Sci. Total Environ. 2020, 714, 136748. [Google Scholar] [CrossRef] [PubMed]
- Segura, P.A.; Takada, H.; Correa, J.A.; El Saadi, K.; Koike, T.; Onwona-Agyeman, S.; Ofosu-Anim, J.; Sabi, E.B.; Wasonga, O.V.; Mghalu, J.M.; et al. Global occurrence of anti-infectives in contaminated surface waters: Impact of income inequality between countries. Environ. Int. 2015, 80, 89–97. [Google Scholar] [CrossRef]
- Bagnis, S.; Boxall, A.; Gachanja, A.; Fitzsimons, M.; Murigi, M.; Snape, J.; Tappin, A.; Wilkinson, J.; Comber, S. Characterization of the Nairobi River catchment impact zone and occurrence of pharmaceuticals: Implications for an impact zone inclusive environmental risk assessment. Sci. Total Environ. 2020, 703. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elwafa Abdallah, M.; Nguyen, K.H.; Ebele, A.J.; Atia, N.N.; Ali, H.R.H.; Harrad, S. A single run, rapid polarity switching method for determination of 30 pharmaceuticals and personal care products in waste water using Q-Exactive Orbitrap high resolution accurate mass spectrometry. J. Chromatogr. A 2019, 1588, 68–76. [Google Scholar] [CrossRef]
- Oluwatosin, O.; Adekunle, B.; Obih, U.; Arne, H. Quantification of pharmaceutical residues in wastewater impacted surface waters and sewage sludge from Lagos, Nigeria. J. Environ. Chem. Ecotoxicol. 2016, 8, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Analysis, Occurrence and removal of pharmaceuticals in african water resources: A current status. J. Environ. Manag. 2020, 253, 109741. [Google Scholar] [CrossRef]
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Antiviral drugs in aquatic environment and wastewater treatment plants: A review on occurrence, fate, removal and ecotoxicity. Sci. Total Environ. 2020, 134322. [Google Scholar] [CrossRef] [PubMed]
- Sabri, N.A.; Schmitt, H.; Van der Zaan, B.; Gerritsen, H.W.; Zuidema, T.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in the Netherlands. J. Environ. Chem. Eng. 2018, 102245. [Google Scholar] [CrossRef]
- Vilchèze, C.; Jacobs, W.R. The combination of sulfamethoxazole, trimethoprim, and isoniazid or rifampin is bactericidal and prevents the emergence of drug resistance in mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 5142–5148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 1057–1098. [Google Scholar] [CrossRef] [Green Version]
- CDC. Antibiotic Resistance Threats in the United States; Center for Disease Control and Prevention: Atlanta, GA, USA, 2019. [CrossRef] [Green Version]
- WHO. Global Tuberculosis Report 2017; WHO: Geneva, Switzerland, 2017. [Google Scholar]
| Sample | pH | Temp (°C) | EC (dS/m) | TDS (ppm) | TSS (mg L−1) |
|---|---|---|---|---|---|
| Effluent | 7.88 | 30.2 | 5610 | 3.73 | 72.8 |
| Surface water | 6.36 | 27.6 | 1140 | 2.86 | 66.4 |
| Target Compound | RT a | Precursor Ion [M + H]+ (m/z) (CV) b | Quantifier Ion (m/z) (CE) c | Qualifier Ion (CE) |
|---|---|---|---|---|
| 3TC | 1.5 | 229.9 (17) | 112.0 (18) | 95.0 (29) |
| ZDV | 2.3 | 268.2 (16) | 127.0 (17) | 110.1 (25) |
| NVP | 4.1 | 267.2 (40) | 226.2 (29) | 198 (29) |
| CIP | 2.2 | 332.1 (34) | 288.0 (19) | 314.1 (19) |
| TMP | 2.2 | 291.1 (34) | 123.0 (19) | 230.0 (19) |
| NOR | 2.1 | 320.3 (30) | 276.0 (18) | 302.0 (25) |
| SMX | 5.1 | 254.0 (28) | 156.0 (18) | 108.0 (17) |
| API | ILIS | r2 | % Recovery (RSD) | DF (%) | LOQ ng L−1 |
|---|---|---|---|---|---|
| NOR | (2H8)-CIP | 0.996 | 92.6 (3.2) | 100 | 12 |
| TMP | (2H9)-TMP | 0.999 | 111.3 (4.1) | 100 | 9 |
| CIP | (2H8)-CIP | 0.993 | 84.3 (8.3) | 100 | 10 |
| SMX | (2H4)-SMX | 0.997 | 101 (7.2) | 100 | 17 |
| 3TC | (13C2H2 15N2)-3TC | 0.993 | 98.8 (3.7) | 100 | 15 |
| ZDV | (13C2H3)-ZDV | 0.988 | 98.7 (19.4) | 100 | 53 |
| NVP | (2H4)-NVP | 0.989 | 87.7 (9.3) | 100 | 19 |
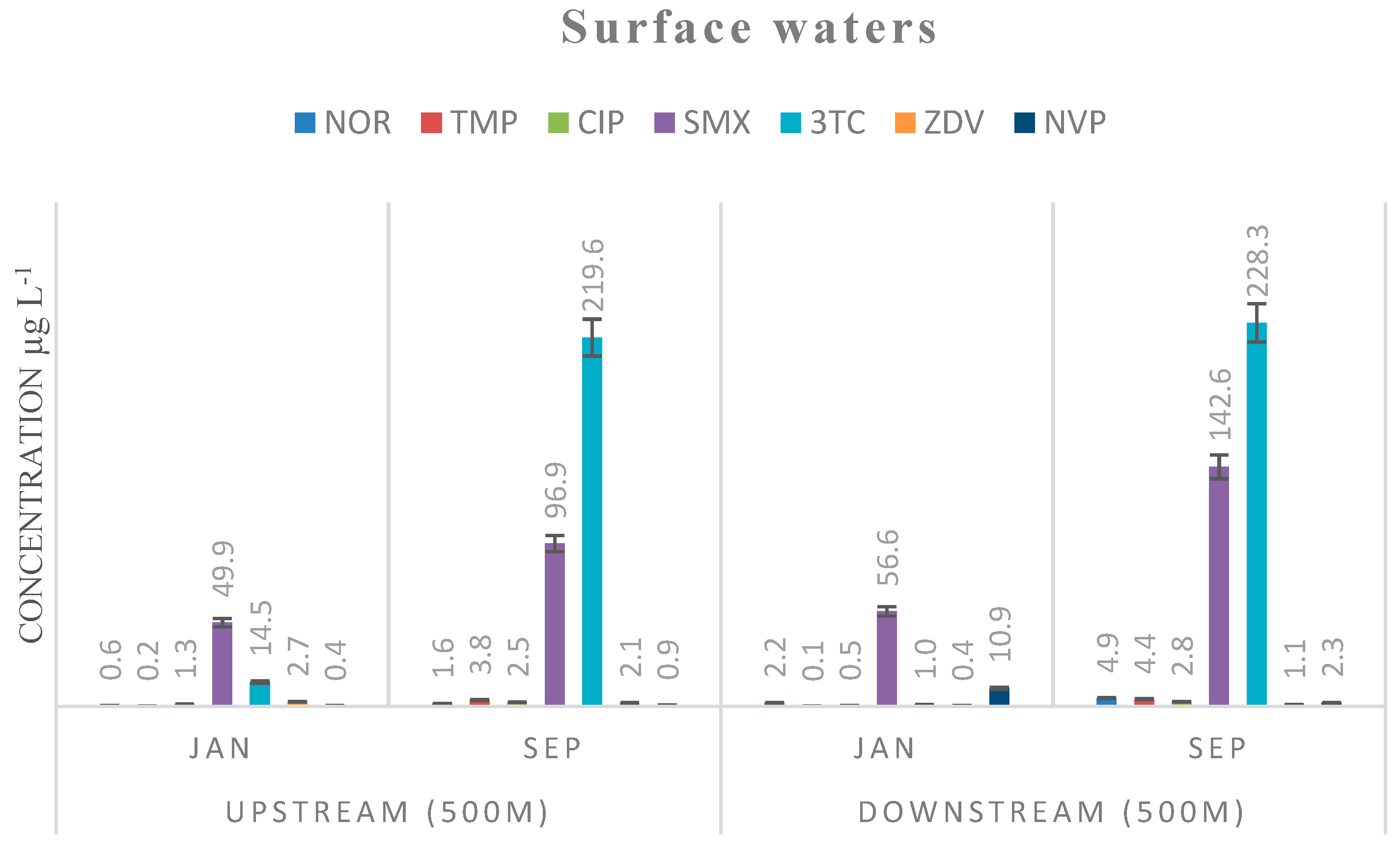
| Compound | Effluent Aqueous Phase | Effluent SPM Phase | Water µg L−1 | Sediments µg kg−1 | PNEC [15] | ||
|---|---|---|---|---|---|---|---|
| µg L−1 | µg kg−1 | Upstream 500 M | Downstream 500 M | Upstream 500 M | Downstream 500 M | µg L−1 | |
| NOR | 4.2 (0.8) | 82,267 (559) | 1.6 (0.4) | 4.9 (1.2) | 776 (22) | 248 (35) | 0.5 |
| TMP | 15.8 (1.1) | 3080 (845) | 3.8 (1.2) | 4.4 (1.5) | 11 (3.2) | 90 (19) | 0.5 |
| CIP | 5.3 (0.6) | 5017 (344) | 2.5 (0.9) | 2.8 (1.1) | 4125 (236) | 1275 (67) | 0.064 |
| SMX | 956.4 (9.4) | 23,448 (1959) | 96.9 (4.6) | 142.6 (8.3) | 542 (13) | 896 (25) | 16 |
| 3TC | 847.1 (25.3) | 69,681 (5824) | 219.6 (16.9) | 228.3 (11) | 491 (18.2) | 107 (12) | n.a |
| ZDV | 1.4 (1) | 3336 (119) | 2.1 (1.3) | 1.1 (0.9) | 510 (40) | 118 (18) | n.a |
| NVP | 9.5 (2.2) | 3214 (146) | 0.9 (0.4) | 2.3 (1) | 95 (14) | 101 (11) | n.a |
| Category | Compound | Sample | Concentration Range µg L−1 | Country | Ref. |
|---|---|---|---|---|---|
| Antibiotics | Sulfamethoxazole | surface waters | <LOQ to 9.64 | Ghana | [31] |
| surface waters | <LOQ to 49.56 | Kenya | |||
| surface waters | 0.511 to 53.83 | Mozambique | |||
| surface waters | 0.0033 to 10.57 | South Africa | |||
| surface waters | 11.25 | Kenya | [32] | ||
| effluent/surface water | <MQL to 0.019 | Egypt | [33] | ||
| surface water | <0.01 to 1.5 | Nigeria | [34] | ||
| Trimethoprim | surface waters | 0.014 to 1.37 | Ghana | [31] | |
| surface waters | <LOQ to 11.38 | Kenya | |||
| surface waters | 0.31 to 6.22 | Mozambique | |||
| surface waters | 0.004 to 5.88 | South Africa | |||
| surface water | 3.35 | Kenya | [32] | ||
| surface water | <0.01 to 0.4 | Nigeria | [34] | ||
| effluent/surface water | 0.21 to 1.06 | Egypt | [33] | ||
| Ciprofloxacin | surface water | 0.51 to 14.33 | South Africa, Ghana, Kenya | [17] | |
| ARVDs | Zidovudine | effluent/surface water | n.d. to 5.3 | South Africa | [35] |
| effluent | 12.1 to 20.13 | Kenya | [36] | ||
| Nevirapine | effluent/surface water | <LOQ to 0.28 | South Africa | [35] | |
| effluent | 0.0053 to 3.3 | Kenya | [36] | ||
| Lamivudine | effluent/surface water | 0.13 to 20.93 | South Africa | [35] | |
| effluent | 0.0325 to 60.68 | Kenya | [36] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kairigo, P.; Ngumba, E.; Sundberg, L.-R.; Gachanja, A.; Tuhkanen, T. Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies. Water 2020, 12, 1376. https://doi.org/10.3390/w12051376
Kairigo P, Ngumba E, Sundberg L-R, Gachanja A, Tuhkanen T. Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies. Water. 2020; 12(5):1376. https://doi.org/10.3390/w12051376
Chicago/Turabian StyleKairigo, Pius, Elijah Ngumba, Lotta-Riina Sundberg, Anthony Gachanja, and Tuula Tuhkanen. 2020. "Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies" Water 12, no. 5: 1376. https://doi.org/10.3390/w12051376
APA StyleKairigo, P., Ngumba, E., Sundberg, L.-R., Gachanja, A., & Tuhkanen, T. (2020). Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies. Water, 12(5), 1376. https://doi.org/10.3390/w12051376






