Distribution Profile of Chemical Elements during the Last 13 Thousand Years from the Sediments of Maloye Yarovoe Lake (Western Siberia, Russia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Studied Area
2.2. Water Sampling and Analysis Methods
2.3. Sediments Sampling and Analysis Methods
2.4. Geochemical Modelling
3. Results
3.1. Lake Water Chemistry
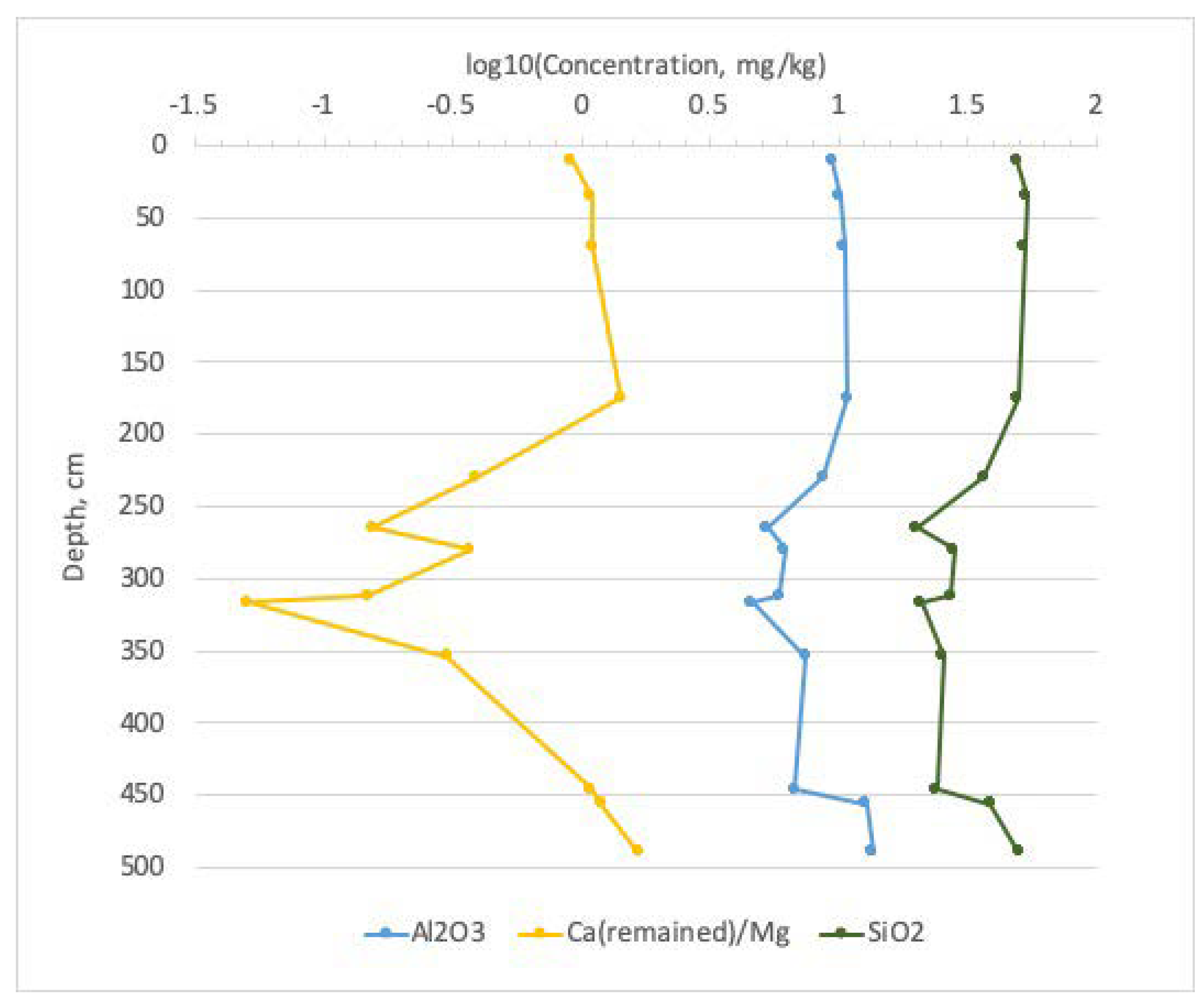
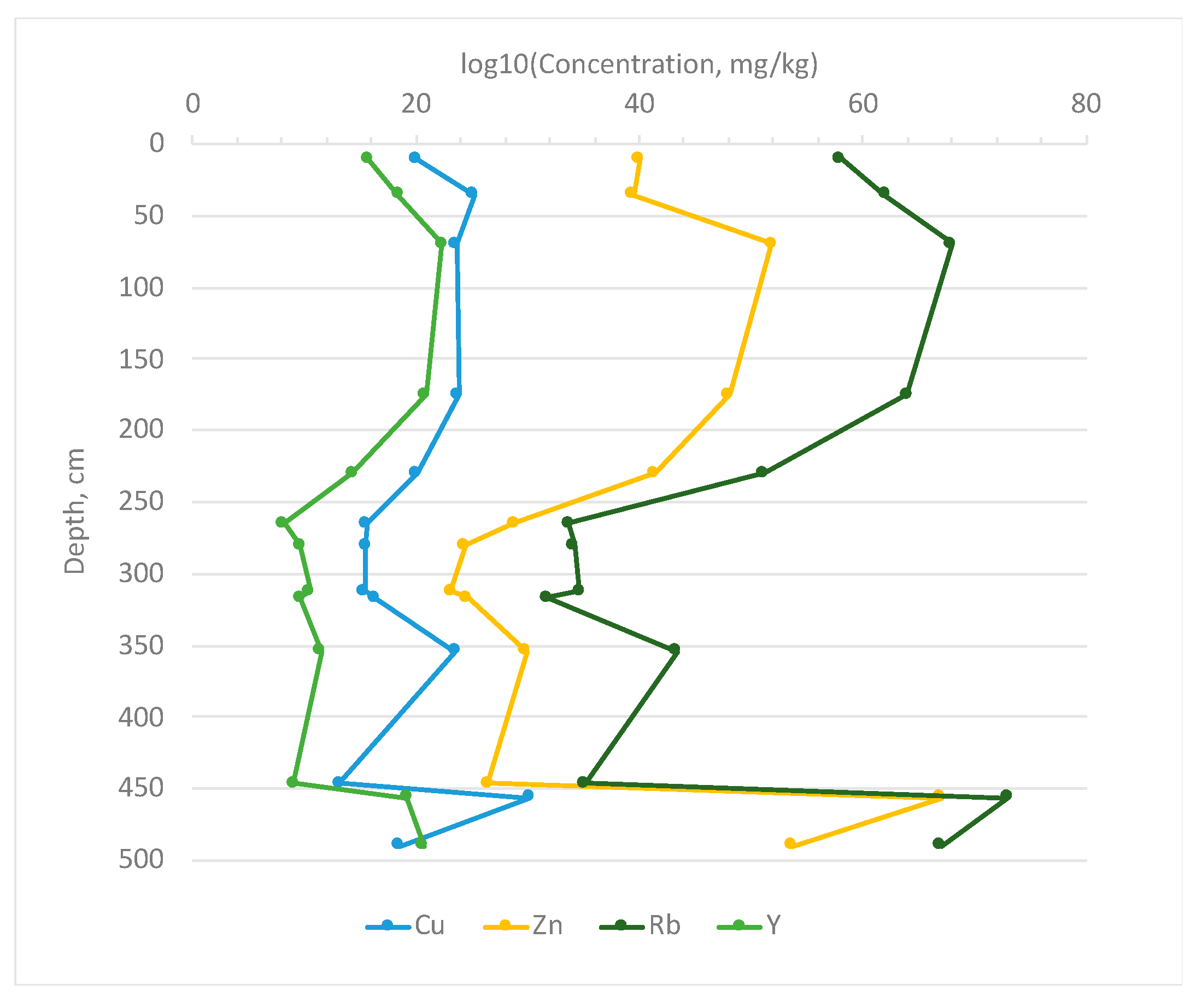
3.2. Bottom Sediments
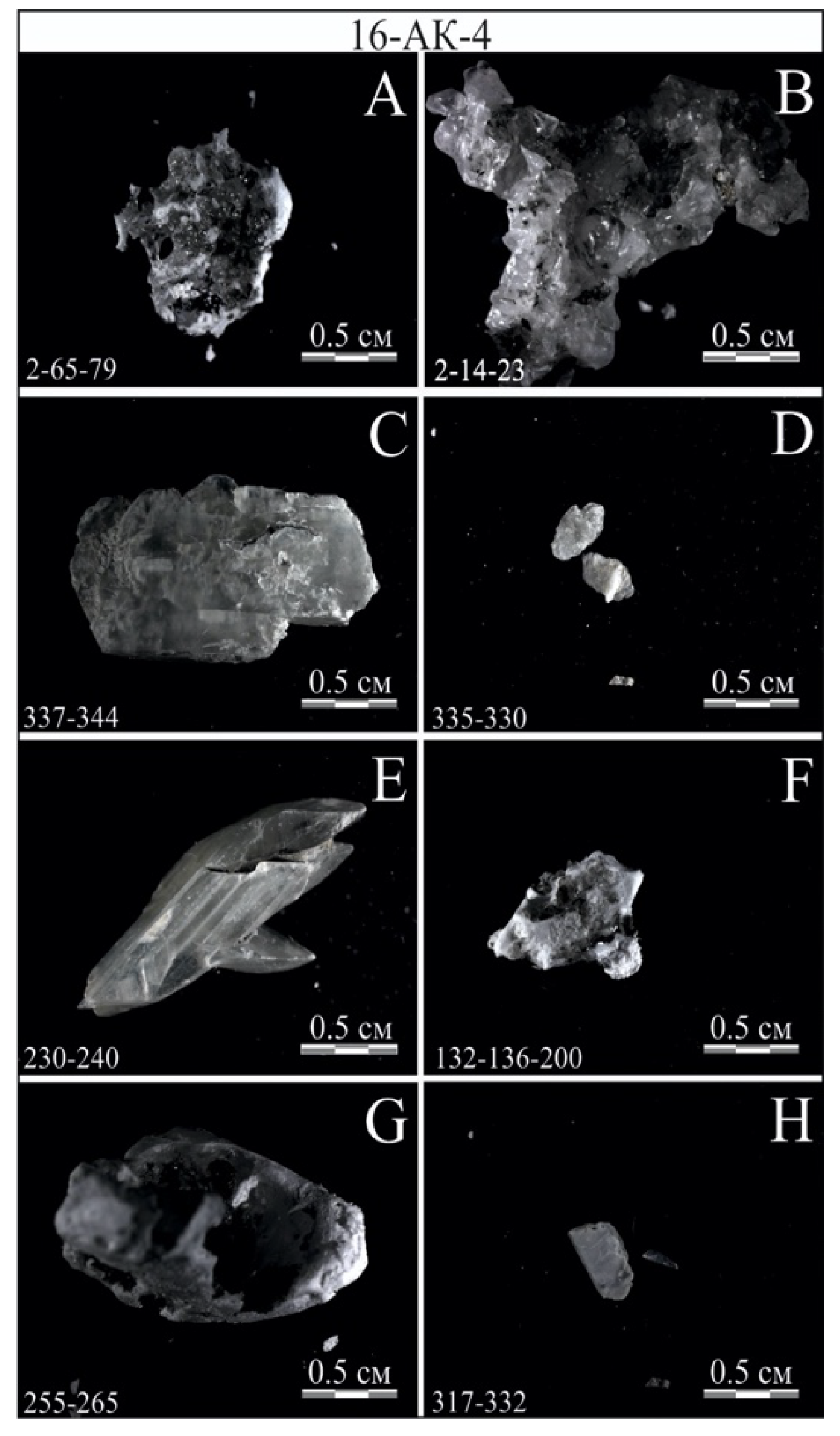
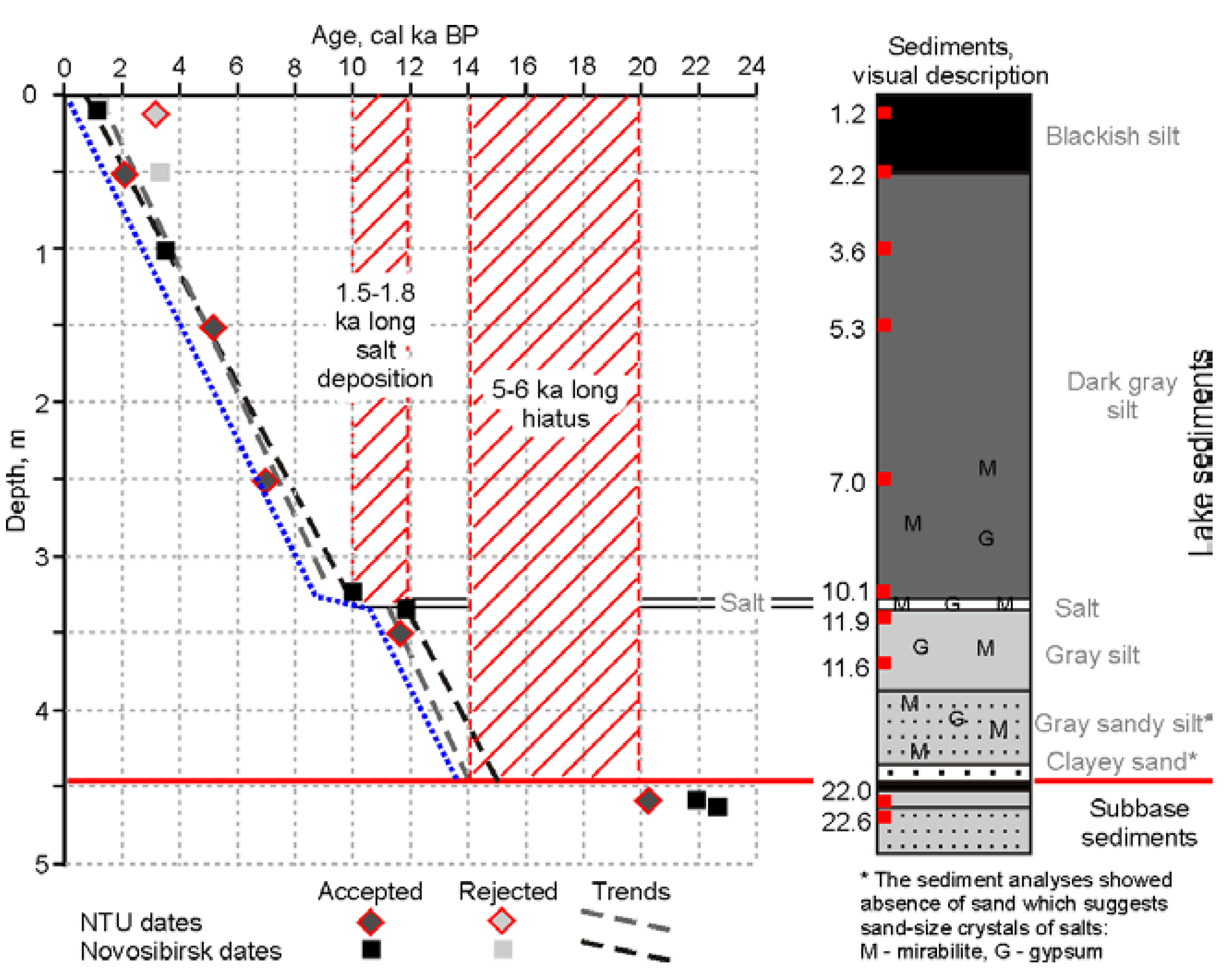
3.2.1. Radiocarbon Dating
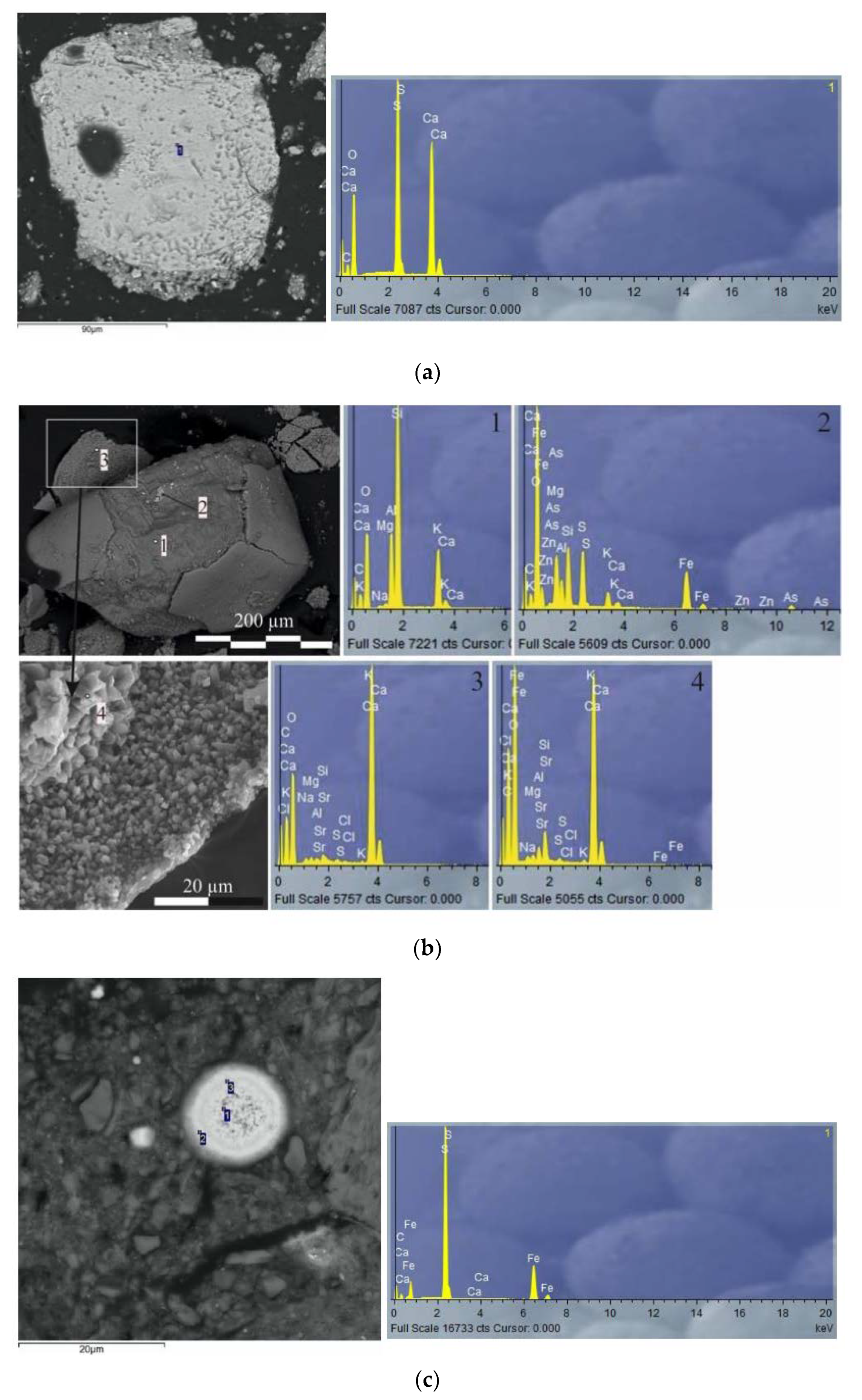
3.2.2. Lithostratigraphy and Mineralogy
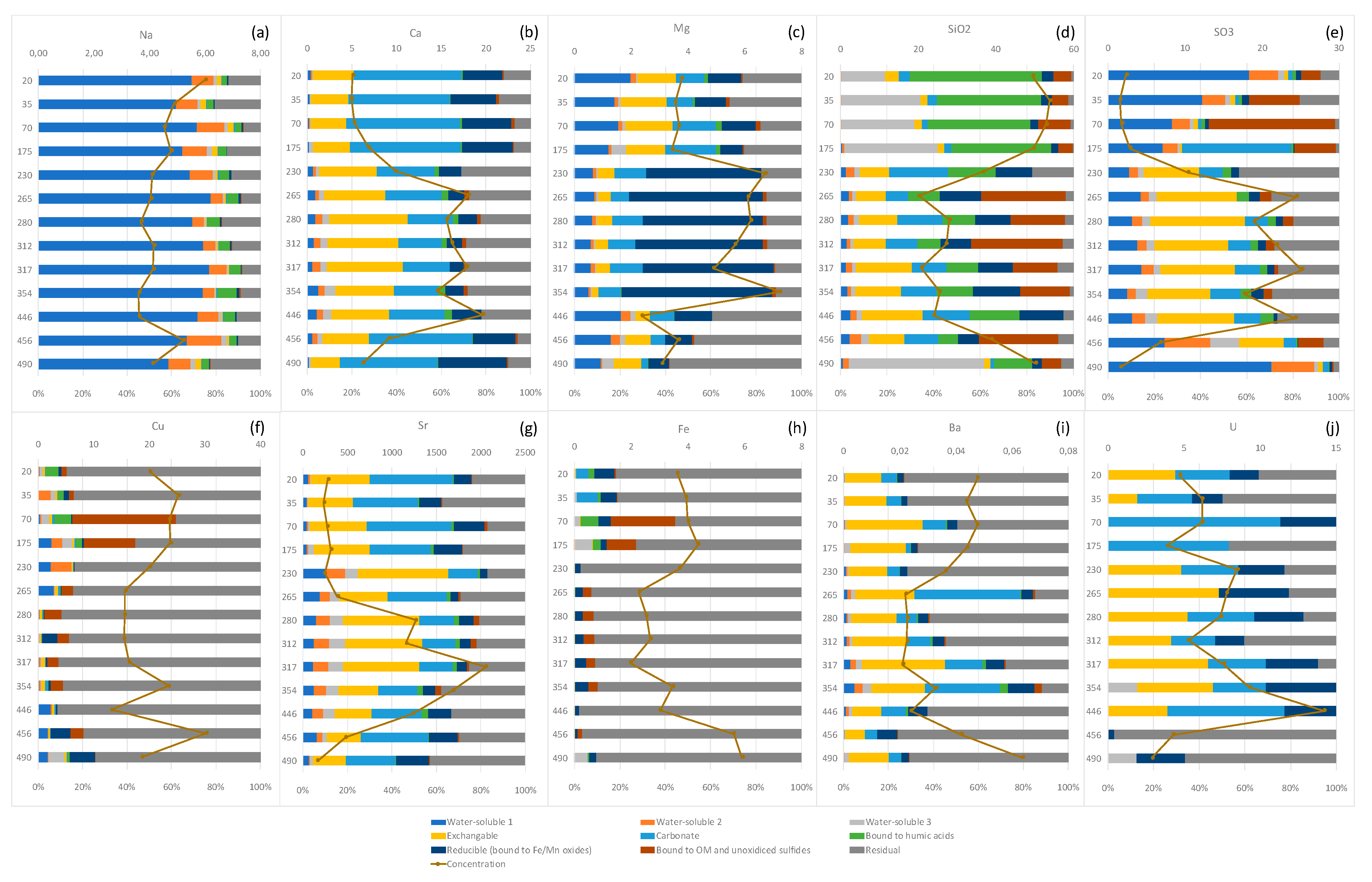
3.2.3. Sequential Extractions
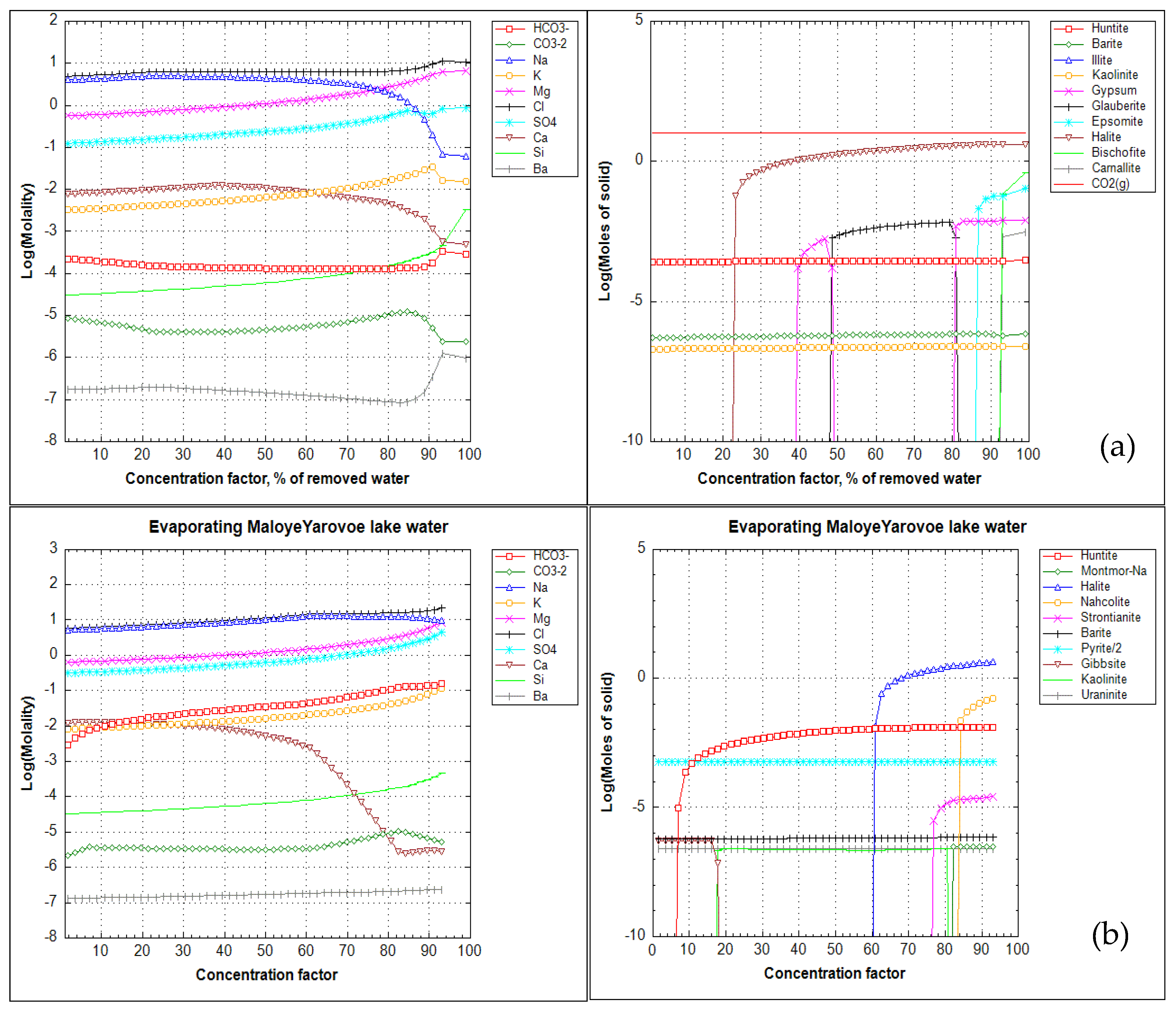
3.3. Geochemical Modelling of Evaporite Sedimentation
3.3.1. Oxic Conditions of Lake-Water
3.3.2. Reducing Conditions of Lake-Water
4. Discussion
4.1. Factors Controlling the Chemical Evolution of Brines
4.1.1. Water-Rock Interaction and Evaporation
4.1.2. Biogeochemical Processes
4.1.3. Stability of Chemical State
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Donchyts, G.; Baart, F.; Winsemius, H.; Gorelick, N.; Kwadijk, J.; Van De Giesen, N. Earth’s surface water change over the past 30 years. Nat. Clim Chang. 2016, 6, 810–813. [Google Scholar] [CrossRef]
- Erler, A.R.; Frey, S.K.; Khader, O.; d’Orgeville, M.; Park, Y.-J.; Hwang, H.-T.; Lapen, D.R.; Peltier, W.R.; Sudicky, E.A. Simulating Climate Change Impacts on Surface Water Resources Within a Lake-Affected Region Using Regional Climate Projections. Water Resour. Res. 2018, 55. [Google Scholar] [CrossRef]
- Maberly, S.C.; O’Donnell, R.A.; Woolway, R.I.; Cutler, M.E.; Gong, M.; Jones, I.D.; Merchant, C.J.; Miller, C.A.; Politi, E.; Marian Scott, E. Global lake thermal regions shift under climate change. Nat. Commun. 2020, 11, 1232. [Google Scholar] [CrossRef]
- Jones, B.F.; Naftz, D.L.; Spencer, R.J.; Oviatt, C.G. Geochemical evolution of Great Salt Lake, Utah, USA. Aquat. Geochem. 2009, 15, 95–121. [Google Scholar] [CrossRef]
- Tweed, S.; Grace, M.; Leblanc, M.; Cartwright, I.; Smithyman, D. The individual response of saline lakes to a severe drought. Sci. Total Environ. 2011, 409, 3919–3933. [Google Scholar] [CrossRef] [PubMed]
- Wahed, A.M.S.M.; Mohamed, E.A.; El-Sayed, M.I.; M’nif, A.; Sillanpää, M. Geochemical modeling of evaporation process in Lake Qarun, Egypt. J. Afr. Earth Sci. 2014, 97, 322–330. [Google Scholar] [CrossRef]
- Boros, E.; Horváth, Z.; Wolfram, G.; Vörös, L. Salinity and ionic composition of the shallow astatic soda pans in the Carpathian Basin. Ann. De Limnol. Int. J. Limnol. 2014, 50, 59–69. [Google Scholar] [CrossRef]
- Deocampo, D.; Jones, B. Geochemistry of Saline Lakes. In Treatise on Geochemistry. Volume 7: Surface and Groundwater, Weathering, and Soils, 2nd ed.; Drever, J.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 7.13. [Google Scholar] [CrossRef]
- Pitzer, K.S. Activity Coefficients in Electrolyte Solutions, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Lassin, A.; André, L.; Lach, A. Considerations about the Building of a Thermodynamic Database for the Chemical Description of Highly Saline Systems. Procedia Earth Planet. Sci. 2017, 17, 304–307. [Google Scholar] [CrossRef]
- Wollast, R.; Chou, C. The carbon cycle at the ocean margin in the northern Gulf of Biscay. Deep Sea Res. 2001, 48, 3265–3293. [Google Scholar] [CrossRef][Green Version]
- Berg, G.M.; Balode, M.; Purina, I.; Bekere, S.; Bechemin, C.; Maestrini, S.Y. Plankton community composition in relation to availability and uptake of oxidized and reduced nitrogen. Aquat. Microb. Ecol. 2003, 30, 263–274. [Google Scholar] [CrossRef]
- Middelburg, J.J. Reviews and syntheses: To the bottom of carbon processing at the seafloor. Biogeosciences 2018, 15, 413–427. [Google Scholar] [CrossRef]
- Mianping, Z. Classification of Saline Lakes and Types of Mineral Deposit. In An Introduction to Saline Lakes on the Qinghai—Tibet Plateau; Monographiae Biologicae; Springer: Dordrecht, The Netherlands, 1997; Volume 76. [Google Scholar]
- Berner, R.A. Principles of Chemical Sedimentology; McGraw-Hill: New York, NY, USA, 1971; 240p. [Google Scholar]
- Jorgensen, B.B. Processes at the sediment-water interface. In The Major Biochemical Cycles and Their Interactions; Bolin, B., Cook, R.B., Eds.; John Wiley: New York, NY, USA, 1983; pp. 477–515. [Google Scholar]
- Santschi, P.H.; Höhener, P.P.; Benoit, G.; Buchholtz-ten Brink, M. Chemical Processes at the Sediment-Water Interface. Mar. Chem. 1990, 30, 269–315. [Google Scholar] [CrossRef]
- Canfield, D.; Raiswell, R.; Bottrell, S.H. The reactivity of sedimentary iron minerals toward sulfide. Am. J. Sci. 1992, 292, 659–683. [Google Scholar] [CrossRef]
- Chilingar, G.V.; Larsen, G. Diagenesis in Sediments and Sedimentary Rocks; Elsevier: Amsterdam, The Netherlands, 1983; Volume 2. [Google Scholar]
- Davydova, N.; Subetto, D.; Belkina, N.; Simola, H.; Kukkonen, M. Paleolimnology and sediments of Lake Ladoga: Monitoring programmer proposal. Environ. Monit. Lake Ladoga. Joensuu 2000, 1, 68–74. [Google Scholar]
- Belkina, N. Changes in the Processes of Redox Diagenesis of Bottom Sediments of Onega and Ladoga lakes under the Influence of Anthropogenic Factors. Ph.D. Thesis, Northern Water Problems Institute Karelian Research Centre Russian Academy of Science, Saint-Petersburg, Russia, 2003; 149p. (In Russian). [Google Scholar]
- Muller, B.; Maerki, M.; Schmid, M.; Vologina, E.G.; Wehrli, B.; Wüest, A.; Sturm, M. Internal carbon and nutrient cycling in Lake Baikal: Sedimentation, upwelling, and early diagenesis. Glob. Planet. Chang. 2005, 46, 101–124. [Google Scholar] [CrossRef]
- Gaskova, O.L.; Strakhovenko, V.D.; Ermolaeva, N.I.; Zarubina, E.Y.; Ovdina, E.A. A simple method to model the reduced environment of lake bottom sapropel formation. Chin. J. Ocean. Limnol. 2017, 35, 956–966. [Google Scholar] [CrossRef]
- Leonova, G.A.; Mal’tsev, A.E.; Melenevskii, V.N.; Miroshnichenko, L.V.; Kondrat’eva, L.M.; Bobrov, V.A. Geochemistry of Diagenesis of Organogenic Sediments: An Example of Small Lakes in Southern West Siberia and Western Baikal Area. Geochem. Int. 2018, 56, 344–361. [Google Scholar] [CrossRef]
- Strakhovenko, V.D.; Shcherbov, B.L.; Malikova, I.N.; Vosel’, Y. The regularities of distribution of radionuclides and rear-earth elements in bottom sediments of Siberian lakes. Russ. Geol. Geophys. 2010, 51, 1167–1178. [Google Scholar] [CrossRef]
- Zhdanova, A.N.; Solotchina, E.P.; Krivonogov, S.K.; Solotchin, P.A. Mineral Composition of the Sediments of Lake Malye Chany as an Indicator of Holocene Climate Changes (Southern West Siberia). Russ. Geol. Geophys. 2019, 60, 1163–1174. [Google Scholar]
- Borzenko, S.V.; Kolpakova, M.N.; Shvartsev, S.L.; Isupov, V.P. Biogeochemical conversion of sulfur species in saline lakes of Steppe Altai. J. Oceanol. Limnol. 2018, 36, 676–686. [Google Scholar] [CrossRef]
- Lebedeva Verba, M.P.; Lopukhina, O.V.; Kalinina, N.V. Specificity of the chemical and mineralogical composition of salts in solonchak playas and lakes of the Kulunda steppe. Eurasian Soil Sci. 2008, 41, 416–428. [Google Scholar] [CrossRef]
- Rudaya, N.; Nazarova, L.; Nourgaliev, D.; Palagushkina, O.; Papin, D.; Frolova, L. Mid-late Holocene environmental history of Kulunda, southern West Siberia: Vegetation, climate and humans. Quat. Sci. Rev. 2012, 48, 32–42. [Google Scholar] [CrossRef]
- Zarubina, E.Y.; Durnikin, D.A. Flora of saline lakes of Kulunda steppe (south of Western Siberia). Sib. Ecol. J. 2005, 2, 341–351. (In Russian) [Google Scholar]
- Kuznetsova, M.A.; Postnikova, O.V.; Sidorenkov, A.V. Hydrogeology of USSR; Nedra: Moscow, Russia, 1972; Volume 17, p. 344. (In Russian) [Google Scholar]
- Slyadnev, A. Climate of Altay Kray; Altai knizhnoye izdatel’stvo: Barnaul, Russia, 1958; 139p. (In Russian) [Google Scholar]
- Maximova, N.; Kantamaneni, K.; Morkovkin, G.; Arnaut, D.; Rice, L. The transformation of agro-climatic resources of the altai region under changing climate conditions. Agriculture 2019, 9, 68. [Google Scholar] [CrossRef]
- Roshydromet. A Report on Climate Features on the Territory of the Russian Federation in 2018. 2019. 79p. Available online: https://meteoinfo.ru/images/media/climate/rus-clim-annual-report.pdf (accessed on 22 September 2020).
- Kolpakova, M.N.; Gaskova, O.L.; Naymushina, O.S.; Karpov, A.V.; Vladimirov, A.G.; Krivonogov, S.K. Saline lakes of Northern Kazakhstan: Geochemical correlations of elements and controls on their accumulation in water and bottom sediments. Appl. Geochem. 2019, 107, 8–18. [Google Scholar] [CrossRef]
- Rudaya, N.; Krivonogov, S.; Słowiński, M.; Cao, X.; Zhilich, S. Postglacial history of the Steppe Altai: Climate, fire and plant diversity. Quat. Sci. Rev. 2020. accepted. [Google Scholar]
- Dauvalter, V.A.; Rognerud, S. Heavy metal pollution in sediments of the Pasvik River drainage. Chemosphere 2001, 42, 9–18. [Google Scholar] [CrossRef]
- Tessier, A.; Cambell, P.G.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Bogush, A.A.; Lazareva, E.V. Behavior of heavy metals in sulfide mine tailings and bottom sediment (Salair, Kemerovo region, Russia). Environ. Earth Sci. 2011, 64, 1293–1302. [Google Scholar] [CrossRef]
- Duarte, C.M.; Prairie, Y.T.; Montes, C.; Cole, J.J.; Striegl, R.; Melack, J.; Downing, J.A. CO2 emissions from saline lakes: A global estimate of a surprisingly large flux. J. Geophys. Res. Biogeosci. 2008, 113. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. The principles of distribution of chemical elements in minerals and rocks. J. Chem. Soc. 1937, 74, 655–673. [Google Scholar] [CrossRef]
- Hardie, L.A.; Eugster, H.P. The Evolution of Closed-Basin Brines. Miner. Soc. Am. Spec. Publ. 1970, 3, 273–290. [Google Scholar]
- Babel, M.; Schreiber, B.C. Geochemistry of Evaporites and Evolution of Seawater. In Treatise on Geochemistry, 2nd ed.; Elsevier: San Diego, CA, USA, 2014; Volume 9. [Google Scholar]
- Kolpakova, M.N.; Gaskova, O.L. Major ions behaviour during evaporation of different saline type water of Western Mongolian lakes (geochemical modelling). Hydrol. Res. 2018, 49, 163–176. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A. Description of Input and Examples for PHREEQC Version 3—A Computer Program. for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Techniques and Methods: Denver, CO, USA, 2013; 497p.
- Gas’kova, O.L.; Strakhovenko, V.D.; Ovdina, E.A. Composition of brines and mineral zoning of the bottom sediments of soda lakes in the Kulunda steppe (West Siberia). Russ. Geol. Geophys. 2017, 58, 1207–1218. [Google Scholar] [CrossRef]
- Rudaya, N.; Zhilich, S. Changes in Annual Precipitation in the Younger Dryas and Holocene in Southwestern Siberia. Probl. Archaeol. Ethnogr. Anthropol. Sib. Neighboring Territ. 2019, 25, 211–217. [Google Scholar] [CrossRef]
- Chizhikova, N.P.; Khitrov, N.B. Diversity of clay minerals in soils of solonetzic complexes in the southeast of Western Siberia. Eurasian Soil Sci. 2016, 49, 1419–1431. [Google Scholar] [CrossRef]
- Raiswell, R. Pyrite texture, isotopic composition and the availability of iron. Am. J. Sci. 1982, 1, 1244–1263. [Google Scholar] [CrossRef]
- Picard, A.; Gartman, A.; Clarke, D.R.; Girguis, P.R. Sulfate-reducing bacteria influence the nucleation and growth of mackinawite and greigite. Geochim. Cosmochim. Acta 2018, 220, 367–384. [Google Scholar] [CrossRef]
- Popa, R.; Kinkle, B.K.; Badescu, A. Pyrite Framboids as Biomarkers for Iron-Sulfur Systems. Geomicrobiol. J. 2004, 21, 193–206. [Google Scholar] [CrossRef]
- Hass, A.; Fine, P. Sequential Selective Extraction Procedures for the Study of Heavy Metals in Soils, Sediments, and Waste Materials—A Critical Review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 365–399. [Google Scholar] [CrossRef]
- Lazareva, E. (IGM SB RAS, Novosibirsk, Russia). Personal communication, 2020.
- Rozanov, A.G. Redox system of the bottom sediments of the western Kara Sea. Geochem. Int. 2015, 53, 987–1001. [Google Scholar] [CrossRef]
- Manning-Berg, A.R.; Kah, L.C. Proterozoic microbial mats and their constraints on environments of silicification. Geobiology 2017, 15, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Wooyong, U.; Serne, R.J.; Brown, C.F.; Rod, K.A. Uranium(VI) sorption on iron oxides in Hanford Site sediment: Application of a surface complexation model. Appl. Geochem. 2008, 23, 2649–2657. [Google Scholar] [CrossRef]
- Fontes, J.C.; Matray, J.M. Geochemistry and origin of formation brines from the Paris Basin, France. 1. Brines associated with Triassic salts. Chem. Geol. 1993, 109, 149–175. [Google Scholar] [CrossRef]
- Issar, A. Climate Changes during the Holocene and Their Impact on Hydrological Systems (International Hydrology Series); Cambridge University Press: Cambridge, UK, 2003. [Google Scholar] [CrossRef]
- Hardie, L.A. Anhydrite and gypsum. In Sedimentology. Encyclopedia of Earth Science; Springer: Berlin/Heidelberg, Germany, 1978. [Google Scholar]
- Yechieli, Y.; Wood, W.W. Hydrogeologic processes in saline systems: Playas, sabkhas, and saline lakes. Earth Sci. Rev. 2002, 58, 343–365. [Google Scholar] [CrossRef]
- De Lange, G.J.; Krijgsman, W. Primary Messinian Salinity Crisis shallow gypsum vs. deep dolomite formation: A unifying biogeochemical mechanism. Mar. Geol. 2010, 275, 273–277. [Google Scholar] [CrossRef]
- Komlev, A.E. Anionic composition of groundwaters of Altai Krai. Izv. Altai Univ. 2010, 3–2, 99–103. (In Russian) [Google Scholar]
- Paramonov, E.; Rybkina, I.; Gubarev, M. Agrarian and Forest Landscapes in Steppe: Prevention of Soil Deflation during Climate Warming. Eur. Geogr. Stud. 2019, 6, 39–49. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolpakova, M.; Gaskova, O.; Borzenko, S.; Krivonogov, S.; Naymushina, O.; Rudaya, N. Distribution Profile of Chemical Elements during the Last 13 Thousand Years from the Sediments of Maloye Yarovoe Lake (Western Siberia, Russia). Water 2020, 12, 3001. https://doi.org/10.3390/w12113001
Kolpakova M, Gaskova O, Borzenko S, Krivonogov S, Naymushina O, Rudaya N. Distribution Profile of Chemical Elements during the Last 13 Thousand Years from the Sediments of Maloye Yarovoe Lake (Western Siberia, Russia). Water. 2020; 12(11):3001. https://doi.org/10.3390/w12113001
Chicago/Turabian StyleKolpakova, Marina, Olga Gaskova, Svetlana Borzenko, Sergey Krivonogov, Olga Naymushina, and Natalia Rudaya. 2020. "Distribution Profile of Chemical Elements during the Last 13 Thousand Years from the Sediments of Maloye Yarovoe Lake (Western Siberia, Russia)" Water 12, no. 11: 3001. https://doi.org/10.3390/w12113001
APA StyleKolpakova, M., Gaskova, O., Borzenko, S., Krivonogov, S., Naymushina, O., & Rudaya, N. (2020). Distribution Profile of Chemical Elements during the Last 13 Thousand Years from the Sediments of Maloye Yarovoe Lake (Western Siberia, Russia). Water, 12(11), 3001. https://doi.org/10.3390/w12113001






