A Review of Battery Materials as CDI Electrodes for Desalination
Abstract
:1. Introduction
2. Na-Ion Battery Materials
2.1. Sodium Transition-Metal Oxide and Transition-Metal Oxide
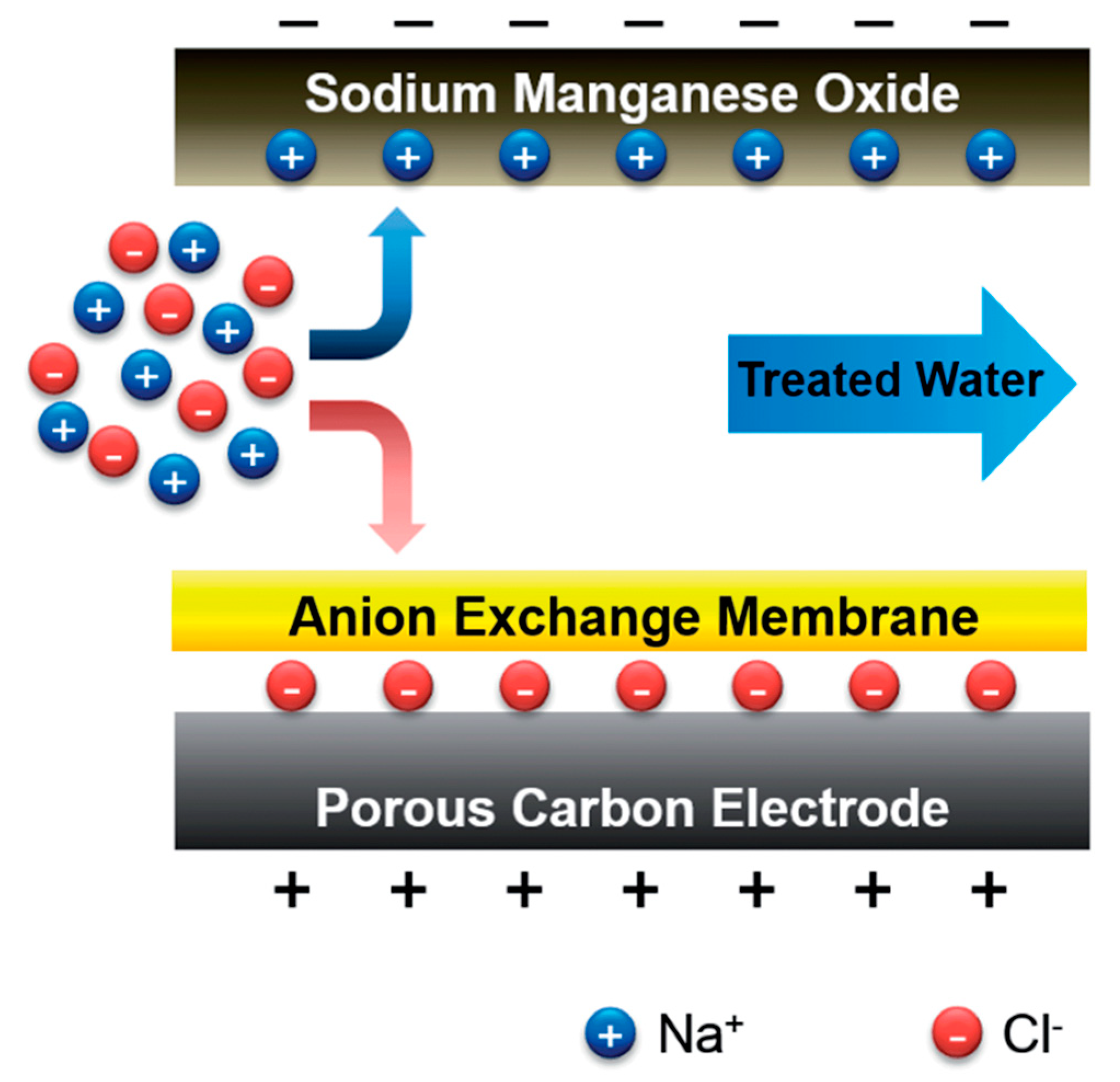
2.1.1. Sodium Manganese Oxide and Manganese Oxide
2.1.2. Sodium Cobalt Oxide and Cobalt Oxide
2.1.3. Sodium Titanium Oxide and Titanium Oxide
2.1.4. Tin Oxide
2.1.5. Zinc Oxide
2.1.6. Iron Oxide
2.1.7. Binary Transition Metal Oxide
2.2. NASICON Material
2.2.1. NaTi2(PO4)3
2.2.2. Na3V2(PO4)3
2.2.3. Na2FeP2O7
2.3. Prussian Blue Analogue
2.4. Iron Phosphate
2.5. Molybdenum Disulfide
2.6. Mxene
2.7. Ammonium Vanadate
3. Li-Ion Battery Materials
3.1. Lithium Titanium Oxide
3.2. Polyoxometalate
4. Cl-Ion Battery Materials
4.1. Bi-Based Material
4.2. Ag-Based Material
5. Conducting Polymers
5.1. Polypyrrole
5.2. Polyaniline
6. Radical Polymers
7. Flow Battery Electrode Materials
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.G.; Mooney, H.; Hull, V.; Davis, S.J.; Gaskell, J.; Hertel, T.; Lubchenco, J.; Seto, K.C.; Gleick, P.; Kremen, C.; et al. Systems integration for global sustainability. Science 2015, 347, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, S.; Balu, A.M.; van der Waal, J.C.; Luque, R. Biomass-Derived Porous Carbon Materials: Synthesis and Catalytic Applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. In Annual Review of Environment and Resources; Gadgil, A., Liverman, D.M., Eds.; Annual Reviews: Palo Alto, SF, USA, 2010; Volume 35, pp. 109–136. [Google Scholar]
- Oki, T.; Kanae, S. Global hydrological cycles and world water resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Svoboda, P.; Janska, A.; Spiwok, V.; Prasil, I.T.; Kosova, K.; Vitamvas, P.; Ovesna, J. Global Scale Transcriptional Profiling of Two Contrasting Barley Genotypes Exposed to Moderate Drought Conditions: Contribution of Leaves and Crowns to Water Shortage Coping Strategies. Front. Plant Sci. 2016, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M.A.; Liu, J.G.; Matthews, J.H.; Mumba, M.; D’Odorico, P. Water security: Gray or green? Science 2015, 349, 584–585. [Google Scholar] [CrossRef] [Green Version]
- Falkenmark, M. Growing water scarcity in agriculture: Future challenge to global water security. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2013, 371, 14. [Google Scholar] [CrossRef] [Green Version]
- Catley-Carlson, M. The Water Paradox: Overcoming the Global Crisis in Water Management. Nature 2019, 565, 426–427. [Google Scholar] [CrossRef]
- Hejazi, M.; Edmonds, J.; Chaturvedi, V.; Davies, E.; Eom, J. Scenarios of global municipal water-use demand projections over the 21st century. Hydrol. Sci. J. 2013, 58, 519–538. [Google Scholar] [CrossRef] [Green Version]
- De Graaf, I.E.M.; van Beek, L.P.H.; Wada, Y.; Bierkens, M.F.P. Dynamic attribution of global water demand to surface water and groundwater resources: Effects of abstractions and return flows on river discharges. Adv. Water Resour. 2014, 64, 21–33. [Google Scholar] [CrossRef]
- Parkinson, S.C.; Johnson, N.; Rao, N.D.; Jones, B.; Van Vliet, M.T.H.; Fricko, O.; Djilali, N.; Riahi, K.; Florke, M. Climate and human development impacts on municipal water demand: A spatially-explicit global modeling framework. Environ. Model. Softw. 2016, 85, 266–278. [Google Scholar] [CrossRef] [Green Version]
- Linares, R.V.; Li, Z.; Sarp, S.; Bucs, S.S.; Amy, G.; Vrouwenvelder, J.S. Forward osmosis niches in seawater desalination and wastewater reuse. Water Res. 2014, 66, 122–139. [Google Scholar] [CrossRef]
- Gao, M.; Connor, P.K.N.; Ho, G.W. Plasmonic photothermic directed broadband sunlight harnessing for seawater catalysis and desalination. Energy Environ. Sci. 2016, 9, 3151–3160. [Google Scholar] [CrossRef] [Green Version]
- Morillo, J.; Usero, J.; Rosado, D.; El Bakouri, H.; Riaza, A.; Bernaola, F.J. Comparative study of brine management technologies for desalination plants. Desalination 2014, 336, 32–49. [Google Scholar] [CrossRef]
- Catrini, P.; Cipollina, A.; Micale, G.; Piacentino, A.; Tamburini, A. Exergy analysis and thermoeconomic cost accounting of a Combined Heat and Power steam cycle integrated with a Multi Effect Distillation-Thermal Vapour Compression desalination plant. Energy Conv. Manag. 2017, 149, 950–965. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Wang, X.L.; Zhao, H.X. Design and numerical investigation of an adaptive nozzle exit position ejector in multi-effect distillation desalination system. Energy 2017, 140, 673–681. [Google Scholar] [CrossRef]
- Sharan, P.; Neises, T.; Turchi, C. Optimal feed flow sequence for multi-effect distillation system integrated with supercritical carbon dioxide Brayton cycle for seawater desalination. J. Clean. Prod. 2018, 196, 889–901. [Google Scholar] [CrossRef]
- El-Ghonemy, A.M.K. Performance test of a sea water multi-stage flash distillation plant: Case study. Alex. Eng. J. 2018, 57, 2401–2413. [Google Scholar] [CrossRef]
- Khoshrou, I.; Nasr, M.R.J.; Bakhtari, K. New opportunities in mass and energy consumption of the Multi-Stage Flash Distillation type of brackish water desalination process. Sol. Energy 2017, 153, 115–125. [Google Scholar] [CrossRef]
- Mahdi, T.; Ahmad, A.; Ripin, A.; Nasef, M.M. Separation of Azeotropic Mixture Using Multi-stage Ultrasound-Assisted Flash Distillation. Chem. Prod. Process Model. 2015, 10, 237–242. [Google Scholar] [CrossRef]
- Shen, J.B.; Xing, Z.W.; Wang, X.L.; He, Z.L. Analysis of a single-effect mechanical vapor compression desalination system using water injected twin screw compressors. Desalination 2014, 333, 146–153. [Google Scholar] [CrossRef]
- Shen, J.B.; Xing, Z.W.; Zhang, K.; He, Z.L.; Wang, X.L. Development of a water-injected twin-screw compressor for mechanical vapor compression desalination systems. Appl. Therm. Eng. 2016, 95, 125–135. [Google Scholar] [CrossRef]
- Askalany, A.A. Innovative mechanical vapor compression adsorption desalination (MVC-AD) system. Appl. Therm. Eng. 2016, 106, 286–292. [Google Scholar] [CrossRef]
- Choi, W.; Choi, J.; Bang, J.; Lee, J.H. Layer-by-Layer Assembly of Graphene Oxide Nanosheets on Polyamide Membranes for Durable Reverse-Osmosis Applications. ACS Appl. Mater. Interfaces 2013, 5, 12510–12519. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2 with improved desalination performance. J. Membr. Sci. 2015, 489, 43–54. [Google Scholar] [CrossRef]
- Duan, J.T.; Pan, Y.C.; Pacheco, F.; Litwiller, E.; Lai, Z.P.; Pinnau, I. High-performance polyamide thin-film-nanocomposite reverse osmosis membranes containing hydrophobic zeolitic imidazolate framework-8. J. Membr. Sci. 2015, 476, 303–310. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.H.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Coday, B.D.; Xu, P.; Beaudry, E.G.; Herron, J.; Lampi, K.; Hancock, N.T.; Cath, T.Y. The sweet spot of forward osmosis: Treatment of produced water, drilling wastewater, and other complex and difficult liquid streams. Desalination 2014, 333, 23–35. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A.F. A novel thin film composite forward osmosis membrane prepared from PSf-TiO2 nanocomposite substrate for water desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumee, L.; Zhang, J.H.; Li, J.D.; Duke, M.; Gomez, J.; Gray, S. Advances in Membrane Distillation for Water Desalination and Purification Applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef] [Green Version]
- Gahlot, S.; Sharma, P.P.; Gupta, H.; Kulshrestha, V.; Jha, P.K. Preparation of graphene oxide nano-composite ion-exchange membranes for desalination application. RSC Adv. 2014, 4, 24662–24670. [Google Scholar] [CrossRef]
- Hilal, N.; Kochkodan, V.; Al Abdulgader, H.; Johnson, D. A combined ion exchange-nanofiltration process for water desalination: II. Membrane selection. Desalination 2015, 363, 51–57. [Google Scholar] [CrossRef]
- Wang, T.; Yu, S.L.; Hou, L.A. Impacts of HPAM molecular weights on desalination performance of ion exchange membranes and fouling mechanism. Desalination 2017, 404, 50–58. [Google Scholar] [CrossRef]
- Zhang, C.Y.; He, D.; Ma, J.X.; Tang, W.W.; Waite, T.D. Faradaic reactions in capacitive deionization (CDI)—Problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar] [CrossRef]
- Huang, Z.H.; Yang, Z.Y.; Kang, F.Y.; Inagaki, M. Carbon electrodes for capacitive deionization. J. Mater. Chem. A 2017, 5, 470–496. [Google Scholar] [CrossRef]
- Khan, Z.U.; Yan, T.T.; Shi, L.Y.; Zhang, D.S. Improved capacitive deionization by using 3D intercalated graphene sheet-sphere nanocomposite architectures. Environ. Sci. Nano 2018, 5, 980–991. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Tewari, S. Capacitive deionization: Processes, materials and state of the technology. J. Electroanal. Chem. 2018, 813, 178–192. [Google Scholar] [CrossRef]
- Gao, X.; Omosebi, A.; Ma, Z.; Zhu, F.; Landon, J.; Ghorbanian, M.; Kernd, N.; Liu, K. Capacitive deionization using symmetric carbon electrode pairs. Environ. Sci. Water Res. Technol. 2019, 5, 660–671. [Google Scholar] [CrossRef]
- Porada, S.; Borchardt, L.; Oschatz, M.; Bryjak, M.; Atchison, J.S.; Keesman, K.J.; Kaskel, S.; Biesheuvel, P.M.; Presser, V. Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization. Energy Environ. Sci. 2013, 6, 3700–3712. [Google Scholar] [CrossRef] [Green Version]
- Porada, S.; Weinstein, L.; Dash, R.; Van der Wal, A.; Bryjak, M.; Gogotsi, Y.; Biesheuvel, P.M. Water Desalination Using Capacitive Deionization with Microporous Carbon Electrodes. Acs Appl. Mater. Interfaces 2012, 4, 1194–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Qi, J.W.; Li, J.S.; Shen, J.M.; Liu, Y.X.; Sun, X.Y.; Shen, J.Y.; Han, W.Q.; Wang, L.J. Nitrogen-Doped Hollow Mesoporous Carbon Spheres for Efficient Water Desalination by Capacitive Deionization. ACS Sustain. Chem. Eng. 2017, 5, 6635–6644. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, B.P. Toward anti-fouling capacitive deionization by using visible-light reduced TiO2/graphene nanocomposites. MRS Commun. 2015, 5, 613–617. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Zou, L.; Pan, L.; Sun, Z. Synthesis of TiO2-graphene composites via visible-light photocatalytic reduction of graphene oxide. J. Mater. Res. 2011, 26, 970–973. [Google Scholar] [CrossRef]
- Zhang, W.; Mossad, M.; Yazdi, J.S.; Zou, L.D. A statistical experimental investigation on arsenic removal using capacitive deionization. Desalin. Water Treat. 2016, 57, 3254–3260. [Google Scholar] [CrossRef]
- Wang, T.Y.; Liang, H.; Bai, L.M.; Zhu, X.W.; Gan, Z.D.; Xing, J.J.; Li, G.B.; Aminabhavi, T.M. Adsorption behavior of powdered activated carbon to control capacitive deionization fouling of organic matter. Chem. Eng. J. 2020, 384, 10. [Google Scholar] [CrossRef]
- Han, J.L.; Shi, L.Y.; Yan, T.T.; Zhang, J.P.; Zhang, D.S. Removal of ions from saline water using N, P co-doped 3D hierarchical carbon architectures via capacitive deionization. Environ.-Sci. Nano 2018, 5, 2337–2345. [Google Scholar] [CrossRef]
- Xu, X.T.; Tan, H.B.; Wang, Z.M.; Wang, C.; Pan, L.K.; Kaneti, Y.V.; Yang, T.; Yamauchi, Y. Extraordinary capacitive deionization performance of highly-ordered mesoporous carbon nano-polyhedra for brackish water desalination. Environ. Sci. Nano 2019, 6, 981–989. [Google Scholar] [CrossRef]
- Byles, B.W.; Cullen, D.A.; More, K.L.; Pomerantseva, E. Tunnel structured manganese oxide nanowires as redox active electrodes for hybrid capacitive deionization. Nano Energy 2018, 44, 476–488. [Google Scholar] [CrossRef]
- Wu, T.T.; Wang, G.; Wang, S.Y.; Zhan, F.; Fu, Y.; Qiao, H.Y.; Qiu, J.S. Highly Stable Hybrid Capacitive Deionization with a MnO2 Anode and a Positively Charged Cathode. Environ. Sci. Technol. Lett. 2018, 5, 98–102. [Google Scholar] [CrossRef]
- Divyapriya, G.; Vijayakumar, K.K.; Nambi, I. Development of a novel graphene/Co3O4 composite for hybrid capacitive deionization system. Desalination 2019, 451, 102–110. [Google Scholar] [CrossRef]
- Avraham, E.; Noked, M.; Bouhadana, Y.; Soffer, A.; Aurbach, D. Limitations of Charge Efficiency in Capacitive Deionization II. On the Behavior of CDI Cells Comprising Two Activated Carbon Electrodes. J. Electrochem. Soc. 2009, 156, P157–P162. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Heil, D.; Wang, G. Treatment of brackish produced water using carbon aerogel-based capacitive deionization technology. Water Res. 2008, 42, 2605–2617. [Google Scholar] [CrossRef]
- Sussz, M.E. Size-Based Ion Selectivity of Micropore Electric Double Layers in Capacitive Deionization Electrodes. J. Electrochem. Soc. 2017, 164, E270–E275. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.Y.; Wang, H.; Yan, T.T.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Grafting sulfonic and amine functional groups on 3D graphene for improved capacitive deionization. J. Mater. Chem. A 2016, 4, 5303–5313. [Google Scholar] [CrossRef]
- Wu, T.T.; Wang, G.; Dong, Q.; Qian, B.Q.; Meng, Y.L.; Qiu, J.S. Asymmetric capacitive deionization utilizing nitric acid treated activated carbon fiber as the cathode. Electrochim. Acta 2015, 176, 426–433. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, Y.; Dong, X.; Hou, M.; Wang, Y.; Xia, Y. Integrating Desalination and Energy Storage via Saltwater-based Hybrid Sodium-ion Supercapacitor. Chemsuschem 2018, 11, 1741–1745. [Google Scholar] [CrossRef]
- Ahirrao, D.J.; Tambat, S.; Pandit, A.B.; Jha, N. Sweet-Lime-Peels-Derived Activated-Carbon-Based Electrode for Highly Efficient Supercapacitor and Flow-Through Water Desalination. ChemistrySelect 2019, 4, 2610–2625. [Google Scholar] [CrossRef]
- Pasta, M.; Wessells, C.D.; Cui, Y.; La Mantia, F. A Desalination Battery. Nano Lett. 2012, 12, 839–843. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chen, J.F.; Lin, C.H.; Hou, C.H. Integrating a supercapacitor with capacitive deionization for direct energy recovery from the desalination of brackish water. Appl. Energy 2019, 252, 9. [Google Scholar] [CrossRef]
- Nam, D.H.; Lumley, M.A.; Choi, K.S. A Desalination Battery Combining Cu3[Fe(CN)(6)](2) as a Na-Storage Electrode and Bi as a Cl-Storage Electrode Enabling Membrane-Free Desalination. Chem. Mater. 2019, 31, 1460–1468. [Google Scholar] [CrossRef]
- Sarkar, A.; Sarkar, S.; Sarkar, T.; Kumar, P.; Bharadwaj, M.D.; Mitra, S. Rechargeable Sodium-Ion Battery: High-Capacity Ammonium Vanadate Cathode with Enhanced Stability at High Rate. ACS Appl. Mater. Interfaces 2015, 7, 17044–17053. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Manthiram, A. Progress in High-Voltage Cathode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 11. [Google Scholar] [CrossRef]
- Guo, L.; Mo, R.W.; Shi, W.H.; Huang, Y.X.; Leong, Z.Y.; Ding, M.; Chen, F.M.; Yang, H.Y. A Prussian blue anode for high performance electrochemical deionization promoted by the faradaic mechanism. Nanoscale 2017, 9, 13305–13312. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. Sodium-Ion Battery Materials and Electrochemical Properties Reviewed. Adv. Energy Mater. 2018, 8, 49. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Y. 2D material as anode for sodium ion batteries: Recent progress and perspectives. Energy Storage Mater. 2019, 16, 323–343. [Google Scholar] [CrossRef]
- Liu, W.L.; Zhi, H.Q.; Yu, X.B. Recent progress in phosphorus based anode materials for lithium/sodium ion batteries. Energy Storage Mater. 2019, 16, 290–322. [Google Scholar] [CrossRef]
- Li, X.N.; Zhu, X.B.; Liang, J.W.; Hou, Z.G.; Wang, Y.; Lin, N.; Zhu, Y.C.; Qian, Y.T. Graphene-Supported NaTi2(PO4)(3) as a High Rate Anode Material for Aqueous Sodium Ion Batteries. J. Electrochem. Soc. 2014, 161, A1181–A1187. [Google Scholar] [CrossRef]
- Zhang, L.D.; Huang, T.; Yu, A.S. Carbon-coated Na3V2(PO4)(3) nanocomposite as a novel high rate cathode material for aqueous sodium ion batteries. J. Alloy Compd. 2015, 646, 522–527. [Google Scholar] [CrossRef]
- Hung, T.F.; Lan, W.H.; Yeh, Y.W.; Chang, W.S.; Yang, C.C.; Lin, J.C. Hydrothermal Synthesis of Sodium Titanium Phosphate Nanoparticles as Efficient Anode Materials for Aqueous Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 7074–7079. [Google Scholar] [CrossRef]
- Song, J.; Gim, J.; Kim, S.; Kang, J.; Mathew, V.; Ahn, D.; Kim, J. A Sodium Manganese Oxide Cathode by Facile Reduction for Sodium Batteries. Chem. Asian J. 2014, 9, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.P.; Zhao, S.L.; Wu, F.G. Structure and electrochemical properties of layered sodium manganese oxide NaxMnO2. Mater. Res. Express 2019, 6, 6. [Google Scholar] [CrossRef]
- Bucher, N.; Hartung, S.; Gocheva, I.; Cheah, Y.L.; Srinivasan, M.; Hoster, H.E. Combustion-synthesized sodium manganese (cobalt) oxides as cathodes for sodium ion batteries. J. Solid State Electrochem. 2013, 17, 1923–1929. [Google Scholar] [CrossRef]
- Shi, W.J.; Zhang, D.; Meng, X.M.; Bao, C.X.; Xu, S.D.; Chen, L.; Wang, X.M.; Liu, S.B.; Wu, Y.C. Low-Strain Reticular Sodium Manganese Oxide as an Ultrastable Cathode for Sodium-Ion Batteries. Acs Appl. Mater. Interfaces 2020, 12, 14174–14184. [Google Scholar] [CrossRef]
- Guo, S.H.; Yu, H.J.; Jian, Z.L.; Liu, P.; Zhu, Y.B.; Guo, X.W.; Chen, M.W.; Ishida, M.; Zhou, H.S. A High-Capacity, Low-Cost Layered Sodium Manganese Oxide Material as Cathode for Sodium-Ion Batteries. Chemsuschem 2014, 7, 2115–2119. [Google Scholar] [CrossRef]
- Lu, D.; Yao, Z.J.; Li, Y.Q.; Zhong, Y.; Wang, X.L.; Xie, D.; Xia, X.H.; Gu, C.D.; Tu, J.P. Sodium-rich manganese oxide porous microcubes with polypyrrole coating as a superior cathode for sodium ion full batteries. J. Colloid Interface Sci. 2020, 565, 218–226. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, C.; Yoon, J. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. [Google Scholar] [CrossRef]
- Chen, F.; Huang, Y.X.; Guo, L.; Ding, M.; Yang, H.Y. A dual-ion electrochemistry deionization system based on AgCl-Na0.44MnO2 electrodes. Nanoscale 2017, 9, 10101–10108. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wu, J.S.; Xu, J.M.; Dravid, V.P. Synergistic sodiation of cobalt oxide nanoparticles and conductive carbon nanotubes (CNTs) for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 8669–8675. [Google Scholar] [CrossRef]
- Lin, S.C.; Lu, Y.T.; Chien, Y.A.; Wang, J.A.; Chen, P.Y.; Ma, C.C.M.; Hu, C.C. Asymmetric supercapacitors based on electrospun carbon nanofiber/sodium-pre-intercalated manganese oxide electrodes with high power and energy densities. J. Power Sources 2018, 393, 1–10. [Google Scholar] [CrossRef]
- Chen, B.W.; Wang, Y.F.; Chang, Z.; Wang, X.W.; Li, M.X.; Liu, X.; Zhang, L.X.; Wu, Y.P. Enhanced capacitive desalination of MnO2 by forming composite with multi-walled carbon nanotubes. RSC Adv. 2016, 6, 6730–6736. [Google Scholar] [CrossRef]
- Leong, Z.Y.; Yang, H.Y. A Study of MnO2 with Different Crystalline Forms for Pseudocapacitive Desalination. ACS Appl. Mater. Interfaces 2019, 11, 13176–13184. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, T.; Wang, C.-F.; Chen, C.-W.; Dong, C.-D.; Huang, C.P. The effect of crystal phase of manganese oxide on the capacitive deionization of simple electrolytes. Sci. Total Environ. 2019, 675, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.P.; Zhou, T.F.; Zhu, R.; Chen, X.Q.; Guo, Z.P.; Fan, J.W.; Liu, H.K.; Zhang, W.X. Highly Ordered Dual Porosity Mesoporous Cobalt Oxide for Sodium-Ion Batteries. Adv. Mater. Interfaces 2016, 3, 7. [Google Scholar] [CrossRef]
- Nagata, Y.; Nagao, K.; Deguchi, M.; Sakuda, A.; Hayashi, A.; Tsukasaki, H.; Mori, S.; Tatsumisago, M. Amorphization of Sodium Cobalt Oxide Active Materials for High-Capacity All-Solid-State Sodium Batteries. Chem. Mater. 2018, 30, 6998–7004. [Google Scholar] [CrossRef]
- Qi, S.H.; Wu, D.X.; Dong, Y.; Liao, J.Q.; Foster, C.W.; O’Dwyer, C.; Feng, Y.Z.; Liu, C.T.; Ma, J.M. Cobalt-based electrode materials for sodium-ion batteries. Chem. Eng. J. 2019, 370, 185–207. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Yue, Z.S.; Li, H.B. Na0.71CoO2 promoted sodium uptake via faradaic reaction for highly efficient capacitive deionization. Sep. Purif. Technol. 2020, 234, 10. [Google Scholar] [CrossRef]
- Zhou, R.J.; Guo, X.X.; Li, Z.H.; Luo, S.J.; Luo, M. More Ca2+, Less Na+: Increase the Desalination Capacity and Performance Stability of NaxCayCoO2. ACS Sustain. Chem. Eng. 2019, 7, 14561–14568. [Google Scholar] [CrossRef]
- Kitta, M.; Kataoka, R.; Tanaka, S.; Takeichi, N.; Kohyama, M. Spinel-Type Sodium Titanium Oxide: A Promising Sodium-Insertion Material of Sodium-Ion Batteries. ACS Appl. Energ. Mater. 2019, 2, 4345–4353. [Google Scholar] [CrossRef]
- Xu, J.; Yang, D.Z.; Liao, X.Z.; He, Y.S.; Ma, Z.F. Electrochemical Performances of Reduced Graphene Oxide/Titanium Dioxide Composites for Sodium-Ion Batteries. Acta Phys. Chim. Sin. 2015, 31, 913–919. [Google Scholar]
- Kitta, M.; Kojima, T.; Kataoka, R.; Yazawa, K.; Tada, K. Realizing the Single-Phase Spinel-Type Sodium Titanium Oxide with the Li4Ti5O12-like Structure for Building Stable Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 9322–9331. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, H.M.; Obaid, M.; Nassar, M.M.; Abdelkareem, M.A.; Mahmoud, M.S. Titanium dioxide-decorated rGO as an effective electrode for ultrahigh-performance capacitive deionization. Sep. Purif. Technol. 2020, 235, 9. [Google Scholar] [CrossRef]
- Yue, Z.S.; Gao, T.; Li, H.B. Robust synthesis of carbon@Na4Ti9O20 core-shell nanotubes for hybrid capacitive deionization with enhanced performance. Desalination 2019, 449, 69–77. [Google Scholar] [CrossRef]
- Gu, M.; Kushima, A.; Shao, Y.Y.; Zhang, J.G.; Liu, J.; Browning, N.D.; Li, J.; Wang, C.M. Probing the Failure Mechanism of SnO2 Nanowires for Sodium-Ion Batteries. Nano Lett. 2013, 13, 5203–5211. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.D.; Hou, H.S.; Zou, G.Q.; Chen, J.; Zhang, Y.; Liao, H.X.; Li, S.M.; Ji, X.B. 3D Porous Carbon Encapsulated SnO2 Nanocomposite for Ultrastable Sodium Ion Batteries. Electrochim. Acta 2016, 214, 156–164. [Google Scholar] [CrossRef]
- Wang, Y.; Su, D.W.; Wang, C.Y.; Wang, G.X. SnO2@MWCNT nanocomposite as a high capacity anode material for sodium-ion batteries. Electrochem. Commun. 2013, 29, 8–11. [Google Scholar] [CrossRef]
- Li, F.; Luo, G.E.; Chen, W.Y.; Chen, Y.C.; Fang, Y.P.; Zheng, M.T.; Yu, X.Y. Rational Design and Controllable Synthesis of Multishelled Fe2O3@SnO2@C Nanotubes as Advanced Anode Material for Lithium-/Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 36949–36959. [Google Scholar] [CrossRef]
- El-Deen, A.G.; Barakat, N.A.M.; Khalil, K.A.; Motlak, M.; Kim, H.Y. Graphene/SnO2 nanocomposite as an effective electrode material for saline water desalination using capacitive deionization. Ceram. Int. 2014, 40, 14627–14634. [Google Scholar] [CrossRef]
- Yasin, A.S.; Jeong, J.; Mohamed, I.M.A.; Park, C.H.; Kim, C.S. Fabrication of N-doped & SnO2-incorporated activated carbon to enhance desalination and bio-decontamination performance for capacitive deionization. J. Alloy Compd. 2017, 729, 764–775. [Google Scholar]
- Xu, F.; Li, Z.R.; Wu, L.J.; Meng, Q.P.; Xin, H.L.L.; Sun, J.; Ge, B.H.; Sun, L.T.; Zhu, Y.M. In situ TEM probing of crystallization form-dependent sodiation behavior in ZnO nanowires for sodium-ion batteries. Nano Energy 2016, 30, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.Q.; Mo, M.S.; Li, Y.; Xue, J.L.; Zhao, H.L. Amorphous carbon-coated ZnO porous nanosheets: Facile fabrication and application in lithium- and sodium-ion batteries. J. Alloy Compd. 2018, 744, 712–720. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, Q.J.; Xue, W.D.; Jian, Z.; Zhao, R.; Wang, J.J. ZnO/rGO/C composites derived from metal-organic framework as advanced anode materials for Li-ion and Na-ion batteries. J. Mater. Sci. 2018, 53, 6785–6795. [Google Scholar] [CrossRef]
- Liu, J.Y.; Lu, M.; Yang, J.M.; Cheng, J.; Cai, W.S. Capacitive desalination of ZnO/activated carbon asymmetric capacitor and mechanism analysis. Electrochim. Acta 2015, 151, 312–318. [Google Scholar] [CrossRef]
- Arora, N.; Banat, F.; Bharath, G.; Alhseinat, E. Capacitive deionization of NaCl from saline solution using graphene/CNTs/ZnO NPs based electrodes. J. Phys. D Appl. Phys. 2019, 52, 12. [Google Scholar] [CrossRef]
- Qi, S.H.; Xu, B.L.; Tiong, V.T.; Hu, J.; Ma, J.M. Progress on iron oxides and chalcogenides as anodes for sodium-ion batteries. Chem. Eng. J. 2020, 379, 32. [Google Scholar] [CrossRef]
- Ni, J.F.; Sun, M.L.; Li, L. Highly Efficient Sodium Storage in Iron Oxide Nanotube Arrays Enabled by Built-In Electric Field. Adv. Mater. 2019, 31, 7. [Google Scholar] [CrossRef]
- Valvo, M.; Lindgren, F.; Lafont, U.; Bjorefors, F.; Edstrom, K. Towards more sustainable negative electrodes in Na-ion batteries via nanostructured iron oxide. J. Power Sources 2014, 245, 967–978. [Google Scholar] [CrossRef]
- Li, H.B.; Leong, Z.Y.; Shi, W.H.; Zhang, J.; Chen, T.P.; Yang, H.Y. Hydrothermally synthesized graphene and Fe3O4 nanocomposites for high performance capacitive deionization. RSC Adv. 2016, 6, 11967–11972. [Google Scholar] [CrossRef]
- Trinh, N.T.; Chung, S.; Lee, J.K.; Lee, J. Development of high quality Fe3O4/rGO composited electrode for low energy water treatment. J. Energy Chem. 2016, 25, 354–360. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Zhao, Y.C.; Wang, C.G.; Li, X.; Liu, J.D.; Yue, G.H.; Zhou, Z.D. Facile synthesis of hollow urchin-like NiCo2O4 microspheres for high-performance sodium-ion batteries. J. Mater. Sci. 2016, 51, 9296–9305. [Google Scholar] [CrossRef]
- Wang, X.H.; Fang, Y.; Shi, B.; Huang, F.F.; Rong, F.; Que, R.H. Three-dimensional NiCo2O4@NiCo2O4 core-shell nanocones arrays for high-performance supercapacitors. Chem. Eng. J. 2018, 344, 311–319. [Google Scholar] [CrossRef]
- Wu, Z.B.; Zhu, Y.R.; Ji, X.B. NiCo2O4-based materials for electrochemical supercapacitors. J. Mater. Chem. A 2014, 2, 14759–14772. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chien, H.C.; Lu, S.Y.; Hu, C.C. A Cost-Effective Supercapacitor Material of Ultrahigh Specific Capacitances: Spinel Nickel Cobaltite Aerogels from an Epoxide-Driven Sol-Gel Process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Z.; Xi, W.; Li, H.B. The feasibility of hollow echinus-like NiCo2O4 nanocrystals for hybrid capacitive deionization. Environ. Sci. Water Res. Technol. 2020, 6, 283–289. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zhang, N.; Yu, C.M.; Jiao, L.F.; Chen, J. MnFe2O4@C Nanofibers as High-Performance Anode for Sodium-Ion Batteries. Nano Lett. 2016, 16, 3321–3328. [Google Scholar] [CrossRef]
- Geng, L.; Yan, F.F.; Dong, C.H.; An, C.H. Design and Regulation of Novel MnFe2O4@C Nanowires as High Performance Electrode for Supercapacitor. Nanomaterials 2019, 9, 777. [Google Scholar] [CrossRef] [Green Version]
- Kogularasu, S.; Akilarasan, M.; Chen, S.M.; Elaiyappillai, E.; Johnson, P.M.; Chen, T.W.; Al-Hemaid, F.M.A.; Ali, M.A.; Elshikh, M.S. A comparative study on conventionally prepared MnFe2O4 nanospheres and template-synthesized novel MnFe2O4 nano-agglomerates as the electrodes for biosensing of mercury contaminations and supercapacitor applications. Electrochim. Acta 2018, 290, 533–543. [Google Scholar] [CrossRef]
- Kuo, S.L.; Lee, J.F.; Wu, N.L. Study on pseudocapacitance mechanism of aqueous MnFe2O4 supercapacitor. J. Electrochem. Soc. 2007, 154, A34–A38. [Google Scholar] [CrossRef]
- Younes, H.; Zou, L.D. Asymmetric configuration of pseudocapacitive composite and rGO electrodes for enhanced capacitive deionization. Environ. Sci. Water Res. Technol. 2020, 6, 392–403. [Google Scholar] [CrossRef]
- Anantharamulu, N.; Rao, K.K.; Rambabu, G.; Kumar, B.V.; Radha, V.; Vithal, M. A wide-ranging review on Nasicon type materials. J. Mater. Sci. 2011, 46, 2821–2837. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Guo, J.Z.; Yang, Y.; Zhao, X.X.; Yang, X.; Nie, X.J.; He, X.Y.; Wu, X.L. Research Progress on NASICON-Type Cathode Materials for Sodium Ion Batteries. Chin. J. Inorg. Chem. 2019, 35, 1535–1550. [Google Scholar]
- Zhang, Q.C.; Man, P.; He, B.; Li, C.W.; Li, Q.L.; Pan, Z.H.; Wang, Z.X.; Yang, J.; Wang, Z.; Zhou, Z.Y.; et al. Binder-free NaTi2(PO4)(3) anodes for high-performance coaxial-fiber aqueous rechargeable sodium-ion batteries. Nano Energy 2020, 67, 8. [Google Scholar] [CrossRef]
- Lei, P.; Li, S.J.; Luo, D.X.; Huang, Y.X.; Tian, G.R.; Xiang, X.D. Fabricating a carbon-encapsulated NaTi2(PO4)(3) framework as a robust anode material for aqueous sodium-ion batteries. J. Electroanal. Chem. 2019, 847, 8. [Google Scholar] [CrossRef]
- Shao, M.M.; Wang, B.; Liu, M.C.; Wu, C.; Ke, F.S.; Ai, X.P.; Yang, H.X.; Qian, J.F. A High-Voltage and Cycle Stable Aqueous Rechargeable Na-Ion Battery Based on Na2Zn3[Fe(CN)(6)](2)-NaTi2(PO4)(3) Intercalation Chemistry. ACS Appl. Energy Mater. 2019, 2, 5809–5815. [Google Scholar] [CrossRef]
- Liu, Z.X.; Pang, G.; Dong, S.Y.; Zhang, Y.D.; Mi, C.H.; Zhang, X.G. An aqueous rechargeable sodium-magnesium mixed ion battery based on NaTi2(PO4)(3)-MnO2 system. Electrochim. Acta 2019, 311, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.X.; Chen, F.M.; Guo, L.; Yang, H.Y. Ultrahigh performance of a novel electrochemical deionization system based on a NaTi2(PO4)(3)/rGO nanocomposite. J. Mater. Chem. A 2017, 5, 18157–18165. [Google Scholar] [CrossRef]
- Pei, J.; Geng, H.B.; Ang, H.X.; Zhang, L.L.; Wei, H.X.; Cao, X.Q.; Zheng, J.W.; Gu, H.W. Three-dimensional nitrogen and sulfur co-doped holey-reduced graphene oxide frameworks anchored with MoO2 nanodots for advanced rechargeable lithium-ion batteries. Nanotechnology 2018, 29, 11. [Google Scholar] [CrossRef]
- Yang, Z.X.; Qian, K.; Lv, J.; Yan, W.H.; Liu, J.H.; Ai, J.W.; Zhang, Y.X.; Guo, T.L.; Zhou, X.T.; Xu, S.; et al. Encapsulation of Fe3O4 Nanoparticles into N, S co-Doped Graphene Sheets with Greatly Enhanced Electrochemical Performance. Sci. Rep. 2016, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.J.; Han, C.L.; Meng, Q.H.; Tian, G.Y. Nitrogen and sulfur co-doped NaTi2(PO4)(3)/hole graphene composite as high-performance electrosorption electrodes for hybrid capacitive deionization. J. Mater. Sci. 2020, 55, 6017–6029. [Google Scholar] [CrossRef]
- Huang, Y.X.; Chen, F.M.; Guo, L.; Zhang, J.; Chen, T.P.; Yang, H.Y. Low energy consumption dual-ion electrochemical deionization system using NaTi2(PO4)(3)-AgNPs electrodes. Desalination 2019, 451, 241–247. [Google Scholar] [CrossRef]
- Wang, L.B.; Mu, C.N.; Li, H.X.; Li, F.J. A dual-function battery for desalination and energy storage. Inorg. Chem. Front. 2018, 5, 2522–2526. [Google Scholar] [CrossRef]
- Zhang, X.X.; Ma, J.; Hu, P.; Chen, B.B.; Lu, C.L.; Zhou, X.H.; Han, P.X.; Chen, L.H.; Cui, G.L. An insight into failure mechanism of NASICON-structured Na3V2(PO4)(3) in hybrid aqueous rechargeable battery. J. Energy Chem. 2019, 32, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Alfaruqi, M.H.; Putro, D.Y.; Mathew, V.; Kim, S.; Jo, J.; Kim, S.; Sun, Y.K.; Kim, K.; Kim, J. Pyrosynthesis of Na3V2(PO4)(3)@C Cathodes for Safe and Low-Cost Aqueous Hybrid Batteries. Chemsuschem 2018, 11, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Yang, Z.; Jiang, Y.; Zhang, W.X.; Huang, Y.H. Hybrid aqueous battery based on Na3V2(PO4)(3)/C cathode and zinc anode for potential large-scale energy storage. J. Power Sources 2016, 308, 52–57. [Google Scholar] [CrossRef]
- Cao, J.L.; Wang, Y.; Wang, L.; Yu, F.; Ma, J. Na3V2(PO4)(3)@C as Faradaic Electrodes in Capacitive Deionization for High-Performance Desalination. Nano Lett. 2019, 19, 823–828. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.T.; Chen, C.J.; Li, Z.; Huang, Y.H.; Hu, X.L. Flexible and Binder-Free Electrodes of Sb/rGO and Na3V2(PO4)(3)/rGO Nanocomposites for Sodium-Ion Batteries. Small 2015, 11, 3822–3829. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Y.; Ding, M.; Guo, L.; Yang, H.Y. Dual-Ion Electrochemical Deionization System with Binder-Free Aerogel Electrodes. Small 2019, 15, 8. [Google Scholar] [CrossRef]
- Jung, Y.H.; Lim, C.H.; Kim, J.H.; Kim, D.K. Na2FeP2O7 as a positive electrode material for rechargeable aqueous sodium-ion batteries. RSC Adv. 2014, 4, 9799–9802. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Kim, C.; Yoon, J. Na2FeP2O7 as a Novel Material for Hybrid Capacitive Deionization. Electrochim. Acta 2016, 203, 265–271. [Google Scholar] [CrossRef]
- Matos-Peralta, Y.; Antuch, M. Review-Prussian Blue and Its Analogs as Appealing Materials for Electrochemical Sensing and Biosensing. J. Electrochem. Soc. 2019, 167, 9. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Chikyow, T. Recent advances in Prussian blue and Prussian blue analogues: Synthesis and thermal treatments. Coord. Chem. Rev. 2017, 352, 328–345. [Google Scholar] [CrossRef]
- Liu, Q.N.; Hu, Z.; Chen, M.Z.; Zou, C.; Jin, H.L.; Wang, S.; Chou, S.L.; Liu, Y.; Dou, S.X. The Cathode Choice for Commercialization of Sodium-Ion Batteries: Layered Transition Metal Oxides versus Prussian Blue Analogs. Adv. Funct. Mater. 2020, 30, 15. [Google Scholar] [CrossRef]
- Yin, J.W.; Shen, Y.; Li, C.; Fan, C.Y.; Sun, S.X.; Liu, Y.; Peng, J.; Qing, L.; Han, J.T. In Situ Self-Assembly of Core-Shell Multimetal Prussian Blue Analogues for High-Performance Sodium-Ion Batteries. Chemsuschem 2019, 12, 4786–4790. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Yoon, J. Rocking Chair Desalination Battery Based on Prussian Blue Electrodes. ACS Omega 2017, 2, 1653–1659. [Google Scholar] [CrossRef]
- Porada, S.; Shrivastava, A.; Bukowska, P.; Biesheuvel, P.M.; Smith, K.C. Nickel Hexacyanoferrate Electrodes for Continuous Cation Intercalation Desalination of Brackish Water. Electrochim. Acta 2017, 255, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.S.; Zhang, D.P.; Niu, F.; Li, X.T.; Wang, C.S.; Yang, J. FeFe(CN)(6) Nanocubes as a Bipolar Electrode Material in Aqueous Symmetric Sodium-Ion Batteries. ChemPlusChem 2017, 82, 1170–1173. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Lu, Y.C. Facile Construction of High-Performance Amorphous FePO4/Carbon Nanomaterials as Cathodes of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 13225–13233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Huang, C.; Min, H.; Shu, H.B.; Gao, P.; Liang, Q.Q.; Yang, X.K.; Liu, L.; Wang, X.Y. Bowl-like double carbon layer architecture of hollow carbon@FePO4@reduced graphene oxide composite as high-performance cathodes for sodium and lithium ion batteries. J. Alloy Compd. 2019, 795, 34–44. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Z.; Laul, D.; Zhu, W.; Provencher, M.; Trudeau, M.L.; Guerfi, A.; Zaghib, K. Ultra-low cost and highly stable hydrated FePO4 anodes for aqueous sodium-ion battery. J. Power Sources 2018, 374, 211–216. [Google Scholar] [CrossRef]
- Yang, G.L.; Ding, B.; Wang, J.; Nie, P.; Dou, H.; Zhang, X.G. Excellent cycling stability and superior rate capability of a graphene-amorphous FePO4 porous nanowire hybrid as a cathode material for sodium ion batteries. Nanoscale 2016, 8, 8495–8499. [Google Scholar] [CrossRef]
- Guo, L.; Huang, Y.X.; Ding, M.; Leong, Z.Y.; Vafakhah, S.; Yang, H.Y. A high performance electrochemical deionization method to desalinate brackish water with an FePO4/RGO nanocomposite. J. Mater. Chem. A 2018, 6, 8901–8908. [Google Scholar] [CrossRef]
- Ma, J.; Wang, L.; Yu, F.; Dai, X.H. Mesoporous amorphous FePO4 nanosphere@Graphene as a faradic electrode in capacitive deionization for high-capacity and fast removal of NaCl from water. Chem. Eng. J. 2019, 370, 938–943. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Zhang, D.; Wang, Y.; Luo, X.; Liu, X.; Kim, J.-K.; Luo, Y. Inter-overlapped MoS2/C composites with large-interlayer-spacing for high-performance sodium-ion batteries. Nanoscale horizons 2020, 5, 1127–1135. [Google Scholar] [CrossRef]
- Zhang, X.D.; Liu, K.L.; Zhang, S.J.; Miao, F.J.; Xiao, W.D.; Shen, Y.L.; Zhang, P.; Wang, Z.; Shao, G.S. Enabling remarkable cycling performance of high-loading MoS2@Graphene anode for sodium ion batteries with tunable cut-off voltage. J. Power Sources 2020, 458, 10. [Google Scholar] [CrossRef]
- Srimuk, P.; Lee, J.; Fleischmann, S.; Choudhury, S.; Presser, V. Faradaic deionization of brackish and sea water via pseudocapacitive cation and anion intercalation into few layered molybdenum disulfide. J. Mater. Chem. A 2017, 5, 15640–15649. [Google Scholar] [CrossRef]
- Feifei, J.; Kaige, S.; Bingqiao, Y.; Xian, Z.; Qingmiao, W.; Shaoxian, S. Defect-rich molybdenum disulfide as electrode for enhanced capacitive deionization from water. Desalination 2018, 446, 21–30. [Google Scholar]
- Wang, Q.; Jia, F.; Song, S.; Li, Y. Hydrophilic MoS2/Polydopamine (PDA) Nanocomposites as The Electrode for Enhanced Capacitive Deionization. Sep. Purif. Technol. 2019, 236, 116298. [Google Scholar] [CrossRef]
- Jiang, Q.; Lei, Y.J.; Liang, H.F.; Xi, K.; Xia, C.; Alshareef, H.N. Review of MXene electrochemical microsupercapacitors. Energy Storage Mater. 2020, 27, 78–95. [Google Scholar] [CrossRef]
- Du, L.; Duan, H.H.; Xia, Q.; Jiang, C.; Yan, Y.R.; Wu, S.P. Hybrid Charge-Storage Route to Nb2CTx MXene as Anode for Sodium-Ion Batteries. ChemistrySelect 2020, 5, 1186–1192. [Google Scholar] [CrossRef]
- Meng, J.N.; Zhang, F.F.; Zhang, L.; Liu, L.Y.; Chen, J.T.; Yang, B.J.; Yan, X.B. Rolling up MXene sheets into scrolls to promote their anode performance in lithium-ion batteries. J. Energy Chem. 2020, 46, 256–263. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L.; Leong, Z.Y.; Mo, R.; Sun, L.; Yang, H.Y. Ar plasma modification of 2D MXene Ti3C2Tx nanosheets for efficient capacitive desalination. Flatchem 2018, 8, 17–24. [Google Scholar]
- Bao, W.Z.; Tang, X.; Guo, X.; Choi, S.; Wang, C.Y.; Gogotsi, Y.; Wang, G.X. Porous Cryo-Dried MXene for Efficient Capacitive Deionization. Joule 2018, 2, 778–787. [Google Scholar] [CrossRef] [Green Version]
- Li, C.X.; Wang, S.Y.; Wang, G.; Wang, S.F.; Che, X.P.; Li, D.Z.; Qiu, J.S. NH4V4O10/rGO Composite as a high-performance electrode material for hybrid capacitive deionization. Environ. Sci. Water Res. Technol. 2020, 6, 303–311. [Google Scholar] [CrossRef]
- Bubulinca, C.; Sapurina, I.; Kazantseva, N.E.; Vilcakova, J.; Cheng, Q.; Saha, P. Fabrication of a flexible binder-free lithium manganese oxide cathode for secondary Li—Ion batteries. J. Phys. Chem. Solids 2020, 137, 9. [Google Scholar] [CrossRef]
- Berhe, G.B.; Su, W.N.; Huang, C.J.; Hagos, T.M.; Hagos, T.T.; Bezabh, H.K.; Weret, M.A.; Abrha, L.H.; Yang, Y.W.; Hwang, B.J. A new class of lithium-ion battery using sulfurized carbon anode from polyacrylonitrile and lithium manganese oxide cathode. J. Power Sources 2019, 434, 9. [Google Scholar] [CrossRef]
- Wang, K.; Wan, J.J.; Xiang, Y.X.; Zhu, J.P.; Leng, Q.Y.; Wang, M.; Xu, L.M.; Yang, Y. Recent advances and historical developments of high voltage lithium cobalt oxide materials for rechargeable Li-ion batteries. J. Power Sources 2020, 460, 16. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Zhang, W.X.; Li, G.M.; Zhu, H.C.; Huang, J.W.; He, W.Z. Ultrasonic renovating and coating modifying spent lithium cobalt oxide from the cathode for the recovery and sustainable utilization of lithium-ion battery. J. Clean. Prod. 2020, 257, 11. [Google Scholar] [CrossRef]
- Joo, S.H.; Shin, D.J.; Oh, C.; Wang, J.P.; Senanayake, G.; Shin, S.M. Selective extraction and separation of nickel from cobalt, manganese and lithium in pre-treated leach liquors of ternary cathode material of spent lithium-ion batteries using synergism caused by Versatic 10 acid and LIX 84-I. Hydrometallurgy 2016, 159, 65–74. [Google Scholar] [CrossRef]
- Song, B.F.; Dhanabalan, A.; Biswal, S.L. Evaluating the capacity ratio and prelithiation strategies for extending cyclability in porous silicon composite anodes and lithium iron phosphate cathodes for high capacity lithium-ion batteries. J. Energy Storage 2020, 28, 8. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Liu, H.; An, L.W.; Zhao, X.X.; Liang, G.C. Blended spherical lithium iron phosphate cathodes for high energy density lithium-ion batteries. Ionics 2019, 25, 61–69. [Google Scholar] [CrossRef]
- Guo, L.; Kong, D.Z.; Pam, M.E.; Huang, S.Z.; Ding, M.; Shang, Y.; Gu, C.D.; Huang, Y.X.; Yang, H.Y. The efficient faradaic Li4Ti5O12@C electrode exceeds the membrane capacitive desalination performance. J. Mater. Chem. A 2019, 7, 8912–8921. [Google Scholar] [CrossRef]
- Genovese, M.; Lian, K. Polyoxometalate modified inorganic-organic nanocomposite materials for energy storage applications: A review. Curr. Opin. Solid State Mater Sci. 2015, 19, 126–137. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, Z.J.; Hou, W.; Wang, Q.; Wang, J. Polyoxometalate-based phase transfer catalysis for liquid-solid organic reactions: A review. Catal. Sci. Technol. 2015, 5, 4324–4335. [Google Scholar] [CrossRef]
- Jia, X.Y.; Wang, J.X.; Hu, H.B.; Song, Y.F. Three-Dimensional Carbon Framework Anchored Polyoxometalate as a High-Performance Anode for Lithium-Ion Batteries. Chem. Eur. J. 2020, 26, 5257–5263. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, P.P.; Ma, X.L.; Li, W.J.; Tan, Z.L.; Sha, J.Q. Graphite-like polyoxometalate-based metal-organic framework as an efficient anode for lithium ion batteries. Crystengcomm 2020, 22, 1340–1345. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Xu, X.X.; Wang, Q. A Polyoxometalate-Based Binder-Free Capacitive Deionization Electrode for Highly Efficient Sea Water Desalination. Chem. Eur. J. 2020, 26, 4403–4409. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Y.; Xu, X.; Wang, Y. A binder free hierarchical mixed capacitive deionization electrode based on a polyoxometalate and polypyrrole for brackish water desalination. Dalton Trans. 2020, 49, 6321–6327. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Shen, K.X.; Zhou, Y.; Hou, X.H.; Ru, Q.; He, Q.Y.; Su, C.Y.; Sun, L.F.; Aung, S.H.; Chen, F.M. The composite electrode of Bi@carbon-texture derived from metal-organic frameworks for aqueous chloride ion battery. Ionics 2020, 26, 2395–2403. [Google Scholar] [CrossRef]
- Yin, Q.; Luo, J.N.; Zhang, J.; Zhang, S.X.; Han, J.B.; Lin, Y.J.; Zhou, J.S.; Zheng, L.R.; Wei, M. Ultralong-Life Chloride Ion Batteries Achieved by the Synergistic Contribution of Intralayer Metals in Layered Double Hydroxides. Adv. Funct. Mater. 2020, 30, 8. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Ren, S.H.; Bruns, M.; Fichtner, M. Chloride ion battery: A new member in the rechargeable battery family. J. Power Sources 2014, 245, 706–711. [Google Scholar] [CrossRef]
- Chen, F.M.; Leong, Z.Y.; Yang, H.Y. An aqueous rechargeable chloride ion battery. Energy Storage Mater. 2017, 7, 189–194. [Google Scholar] [CrossRef]
- Chang, J.J.; Duan, F.; Su, C.L.; Li, Y.P.; Cao, H.B. Removal of chloride ions using a bismuth electrode in capacitive deionization (CDI). Environ. Sci. Water Res. Technol. 2020, 6, 373–382. [Google Scholar] [CrossRef]
- Nam, D.H.; Choi, K.S. Electrochemical Desalination Using Bi/BiOCl Electrodialysis Cells. ACS Sustain. Chem. Eng. 2018, 6, 15455–15462. [Google Scholar] [CrossRef]
- Chen, F.M.; Huang, Y.X.; Guo, L.; Sun, L.F.; Wang, Y.; Yang, H.Y. Dual-ions electrochemical deionization: A desalination generator. Energy Environ. Sci. 2017, 10, 2081–2089. [Google Scholar] [CrossRef]
- Kim, K.; Hwang, S.M.; Park, J.S.; Han, J.; Kim, J.; Kim, Y. Highly improved voltage efficiency of seawater battery by use of chloride ion capturing electrode. J. Power Sources 2016, 313, 46–50. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Doong, R.A. Hierarchically ordered mesoporous carbons and silver nanoparticles as asymmetric electrodes for highly efficient capacitive deionization. Desalination 2016, 398, 171–179. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.X.; Shen, J.M.; Li, Y.; Yan, X.; Qi, J.W.; Sun, X.Y.; Shen, J.Y.; Han, W.Q.; Wang, L.J.; et al. Ag-doped hollow ZIFs-derived nanoporous carbon for efficient hybrid capacitive deionization. Desalination 2020, 473, 9. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, J.; Kim, S.; Kim, C.; Lee, J.; Biesheuvel, P.M.; Yoon, J. High performance electrochemical saline water desalination using silver and silver-chloride electrodes. Desalination 2020, 476, 7. [Google Scholar] [CrossRef]
- Srimuk, P.; Husmann, S.; Presser, V. Low voltage operation of a silver/silver chloride battery with high desalination capacity in seawater. RSC Adv. 2019, 9, 14849–14858. [Google Scholar] [CrossRef] [Green Version]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Abdelhamid, M.E.; O’Mullane, A.P.; Snook, G.A. Storing energy in plastics: A review on conducting polymers & their role in electrochemical energy storage. RSC Adv. 2015, 5, 11611–11626. [Google Scholar]
- Oka, K.; Strietzel, C.; Emanuelsson, R.; Nishide, H.; Oyaizu, K.; Stromme, M.; Sjodin, M. Conducting Redox Polymer as a Robust Organic Electrode-Active Material in Acidic Aqueous Electrolyte towards Polymer-Air Secondary Batteries. Chemsuschem 2020, 13, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Hudak, N.S. Chloroaluminate-Doped Conducting Polymers as Positive Electrodes in Rechargeable Aluminum Batteries. J. Phys. Chem. C 2014, 118, 5203–5215. [Google Scholar] [CrossRef]
- Wang, Z.M.; Xu, X.T.; Kim, J.; Malgras, V.; Mo, R.; Li, C.L.; Lin, Y.Z.; Tan, H.B.; Tang, J.; Pan, L.K.; et al. Nanoarchitectured metal-organic framework/polypyrrole hybrids for brackish water desalination using capacitive deionization. Mater. Horizons 2019, 6, 1433–1437. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, Y.; Xu, S.C.; Wang, J.X.; Wang, Z.; Wang, S.C. Polypyrrole nanowire modified graphite (PPy/G) electrode used in capacitive deionization. Synth. Met. 2010, 160, 1392–1396. [Google Scholar] [CrossRef]
- Feng, J.T.; Zhang, Q.; Wang, J.J.; Yang, H.H.; Xu, H.; Yan, W. Application of chemically synthesized polypyrrole with hydro-sponge characteristic as electrode in water desalination. RSC Adv. 2015, 5, 71593–71600. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.Y.; Wang, R.G.; Xu, S.C.; Wang, J.X. Preparation optimization on the coating-type polypyrrole/carbon nanotube composite electrode for capacitive deionization. Electrochim. Acta 2015, 182, 81–88. [Google Scholar] [CrossRef]
- Haq, O.U.; Choi, J.-H.; Lee, Y.-S. Synthesis of Ion-Exchange Polypyrrole/Activated Carbon Composites and Their Characterization as Electrodes for Capacitive Deionization. Macromol. Res. 2020, 28, 877–880. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Y.H.; An, Z.H.; Xu, G.R.; Gadgil, A.J.; Ruan, G.L. The polymeric conformational effect on capacitive deionization performance of graphene oxide/polypyrrole composite electrode. Desalination 2020, 486, 9. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Li, L.; Wang, Y.F.; Zhang, C.; Liu, T.X. Polyaniline/graphene nanocomposites towards high-performance supercapacitors: A review. Compos. Commun. 2018, 8, 83–91. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, S.; Shimpi, N.G. Synthesis and sensing applications of polyaniline nanocomposites: A review. RSC Adv. 2016, 6, 42196–42222. [Google Scholar] [CrossRef]
- Yan, C.; Kanaththage, Y.W.; Short, R.; Gibson, C.T.; Zou, L.D. Graphene/Polyaniline nanocomposite as electrode material for membrane capacitive deionization. Desalination 2014, 344, 274–279. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, R.G.; Xu, S.C.; Liu, Q.; Wang, J.X. Polypyrrole/polyaniline composites with enhanced performance for capacitive deionization. Desalin. Water Treat. 2015, 54, 3248–3256. [Google Scholar] [CrossRef]
- Tian, S.C.; Zhang, Z.H.; Zhang, X.H.; Ostrikov, K. Capacitative deionization using commercial activated carbon fiber decorated with polyaniline. J. Colloid Interface Sci. 2019, 537, 247–255. [Google Scholar] [CrossRef]
- ul Haq, O.; Choi, J.H.; Lee, Y.S. Synthesis of ion-exchange polyaniline-carbon composite electrodes for capacitive deionization. Desalination 2020, 479, 9. [Google Scholar]
- Janoschka, T.; Hager, M.D.; Schubert, U.S. Powering up the Future: Radical Polymers for Battery Applications. Adv. Mater. 2012, 24, 6397–6409. [Google Scholar] [CrossRef]
- Zhang, K.; Hu, Y.X.; Wang, L.Z.; Monteiro, M.J.; Jia, Z.F. Pyrene-Functionalized PTMA by NRC for Greater pi-pi Stacking with rGO and Enhanced Electrochemical Properties. ACS Appl. Mater. Interfaces 2017, 9, 34900–34908. [Google Scholar] [CrossRef]
- Lopez-Pena, H.A.; Hernandez-Munoz, L.S.; Cardoso, J.; Gonzalez, F.J.; Gonzalez, I.; Frontana, C. Electrochemical and spectroelectrochemical properties of nitroxyl radical species in PTMA, an organic radical polymer. Influence of the microstructure. Electrochem. Commun. 2009, 11, 1369–1372. [Google Scholar] [CrossRef]
- Li, Y.Q.; Ding, Z.B.; Li, J.B.; Wang, K.; Lu, T.; Pan, L.K. Novel membrane-free hybrid capacitive deionization with a radical polymer anode for stable desalination. Desalination 2020, 481, 9. [Google Scholar] [CrossRef]
- Hou, X.H.; Liang, Q.; Hu, X.Q.; Zhou, Y.; Ru, Q.; Chen, F.M.; Hu, S.J. Coupling desalination and energy storage with redox flow electrodes. Nanoscale 2018, 10, 12308–12314. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Beh, E.S.; Sahu, S.; Vedharathinam, V.; van Overmeere, Q.; de Lannoy, C.F.; Jose, A.P.; Volkel, A.R.; Rivest, J.B. Electrochemical Desalination of Seawater and Hypersaline Brines with Coupled Electricity Storage. ACS Energy Lett. 2018, 3, 375–379. [Google Scholar] [CrossRef]









| NO. | Materials of the Electrode Pair | Initial NaCl Concentration | CC (Constant Current)/CV (Constant Voltage) | Current Density /Voltage | Desalination Capacity | Ref. |
|---|---|---|---|---|---|---|
| 1 | Na4Mn9O18||AC | 50 mM | CV | 1.2 V | 31.2 mg·g−1 | [79] |
| 2 | Na0.44MnO2||AgCl | 890 mg·g−1 | CC | 0.1A·g−1 | 57.4 mg·g−1 | [80] |
| 3 | MnO2/CNT||AC | 30 mg·g−1 | CV | 1.8 V | 6.65 mg·g−1 | [83] |
| 4 | α-MnO2||AC δ-MnO2||AC | 5 mM | CV | 1 V | 9.93 mg·g−1 9.35 mg·g−1 | [84] |
| 5 | β-MnO2||carbon | 1 mM | CV | 1.8 V | about 18.4 mg·g−1 | [85] |
| 6 | Na0.71CoO2||Ag/rGO | 500 mg·g−1 | CV | 1.4 V | 31 mg·g−1 | [89] |
| 7 | Na0.27Ca0.03CoO2||AC | 529 mg·g−1 | CV | 1.4 V | 83.5 ± 2.4 mg·g−1 | [90] |
| 8 | rGO/TiO2||rGO/TiO2 | 150 μS·cm−1 | CV | 1.2 V | 24.576 mg·g−1 | [94] |
| 9 | Na4Ti9O20/C||AC | 500 μS·cm−1 | CV | 1.4 V | 80.56 mg·g−1 | [95] |
| 10 | graphene/SnO2||graphene/SnO2 | 61 μS·cm−1 | CV | 1.4 V | 1.49 mg·g−1 | [100] |
| 11 | N-AC/SnO2||N-AC/SnO2 | 50 mg·L−1 | CV | 1.2 V | 3.42 mg·g−1 | [101] |
| 12 | AC||ZnO/AC | 500 mg·L−1 | CV | 1.2 V | 9.4 mg·g−1 | [105] |
| 13 | graphene/CNT/ZnO||graphene/CNT/ZnO | 600 mg·L−1 | CV | 1.2 V | 28.62 mg·g−1 | [106] |
| 14 | Fe3O4/rGO||Fe3O4/rGO | 110 μS·cm−1 | CV | 1.5 V | 4.3 mg·g−1 | [111] |
| 15 | NiCo2O4||AC | 1000 μS·cm−1 | CV | 1.2 V | 44.3 mg·g−1 | [116] |
| 16 | MnFe2O4/rGO||MnO2/rGO | 50 mg·L−1 | CV | 1.6 V | 38.28 mg·g−1 | [121] |
| 17 | AC||NaTi2(PO4)3/rGO | 1000 mg·L−1 | CC | 0.1 A·g−1 | 140 mg·g−1 | [128] |
| 18 | N,S-NaTi2(PO4)3/hole rGO||AC | 800 mg·L−1 | CV | 1.4 V | 36.87 mg·g−1 | [131] |
| 19 | Ag/rGO||NaTi2(PO4)3/rGO | 2500 mg·L−1 | CC | 0.1 A·g−1 | 105 mg·g−1 | [132] |
| 20 | Ag||NaTi2(PO4)3 | 1 M | —— | —— | 151.5 mg·g−1 | [133] |
| 21 | Na3V2(PO4)3/C||AC | 100 mM | CV | 1 V | 137.2 mg·g−1 | [137] |
| 22 | Na3V2(PO4)3/rGO||AgCl/rGO | 1000 mg·L−1 | CC | 0.1 A·g−1 | 107.5 mg·g−1 | [139] |
| 23 | Na2FeP2O7/C||AC | 100 mM | CV | 1.2 V | 32.6 mg·g−1 | [141] |
| 24 | NaNiHCF||NaFeHCF | 0.5 M | CC | 0.5 mA·cm−2 | 39.9 mg·g−1 | [146] |
| 25 | NaNiFe(CN)6||Na2NiFe(CN)6 | 20 mM | CC | 0.28 mA·cm−2 | 34 mg·g−1 | [147] |
| 26 | FeFe(CN)6/rGO||AC | 1937 μS·cm−1 | CC | 0.125 A·g−1 | 120 mg·g−1 | [66] |
| 27 | FePO4/rGO||AC | 2500 mg·L−1 | CC | 0.1 A·g−1 | 100 mg·g−1 | [153] |
| 28 | FePO4/rGO||rGO | 40 mM | CV | 1.8 V | 85.94 mg·g−1 | [154] |
| 29 | MoS2/CNT||MoS2/CNT | 500 mM | CV | 0.8 V | 25 mg·g−1 | [157] |
| 30 | Defect-rich MoS2||Defect-rich MoS2 | 254.35 mg·L−1 | CV | 0.8 V | 24.6 mg·g−1 | [158] |
| 31 | AC||MoS2/PDA | 200 mg·L−1 | CV | 1.2 V | 14.8 mg·g−1 | [159] |
| 32 | Ar-modified Ti3C2Tx||AC | 500 mg·L−1 | CV | 1.2 V | 26.8 mg·g−1 | [163] |
| 33 | Porous Ti3C2Tx||Porous Ti3C2Tx | 10,000 mg·L−1 | CV | 1.2 V | 45 mg·g−1 | [164] |
| 34 | NH4V4O10/rGO||AC | 500 mg·L−1 | CV | 1.2 V | 20.1 mg·g−1 | [165] |
| 35 | Li4Ti5O12/C||carbon cloth | 2500 mg·L−1 | CC | 0.16 mA·cm−2 | 25 mg·g−1 | [173] |
| 36 | SiW12/PANI/graphite | 500 mg·L−1 | CV | 1.2 V | 23.1 mg·g−1 | [178] |
| 37 | P2Mo18/PPy/graphite | 600 mg·L−1 | CV | 1.2 V | 17.8 mg·g−1 | [179] |
| 38 | AC||Bi | 500 mg·L−1 | CV | 1.2 V | 55.52 mg·g−1 | [184] |
| 39 | Na0.44MnO2||BiOCl | 760 mg·L−1 | CC | 0.1A/g | 68.5 mg·g−1 | [186] |
| 40 | mesoporous carbon||Ag | 1 mM | CV | 1.2 V | 20.82 mg·g−1 | [188] |
| 41 | hollow carbon||Ag/C | 500 mg·L−1 | CV | 1.2 V | 29.18 mg·g−1 | [189] |
| 42 | AgCl||Ag | 500 mM | CC | 1 mA·cm−2 | 85 mg·g−1 | [190] |
| 43 | AgCl||Ag | 600 mM | CC | 0.1 A·g−1 | 115 mg·g−1 | [191] |
| 44 | hydro-PPy||graphite | 88,930.43 mg·g−1 | CV | 2 V | 2917.66 mg·g−1 | [198] |
| 45 | graphite||PPy/CNT | 1000 μS·cm−1 | CV | 1.4 V | 93.68 mg·g−1 | [199] |
| 46 | NH2-PPy/AC||HSO3-PPy/AC | 8.55 mM | CV | 1 V | 18.4 mg·g−1 | [200] |
| 47 | GO/PPy||GO/PPy | 200 mg·L−1 | CV | 1.2 V | 88.43 mg·g−1 | [201] |
| 48 | CNT||PPy/PANI | 500 mg·L−1 | CV | 1.4 V | 197.8 mg·g−1 | [205] |
| 49 | AC/PANI||AC/PANI | 200 mg·L−1 | CV | 2 V | 19.9 mg·g−1 | [206] |
| 50 | NH2-PANI/AC||HSO3-PANI/AC | 8.55 mM | CV | 1 V | 17.7 mg·g−1 | [207] |
| 51 | AC||PTMA | 250 mg·L−1 | CV | 1.2 V | 20.9 mg·g−1 | [211] |
| 52 | NaI||VCl3/VCl2 | 19,000 mg·L−1 | CC | 0.22 mA·cm−2 | 82.6 mg·g−1 | [212] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Alhassan, S.I.; Wei, D.; Wang, H. A Review of Battery Materials as CDI Electrodes for Desalination. Water 2020, 12, 3030. https://doi.org/10.3390/w12113030
Jiang Y, Alhassan SI, Wei D, Wang H. A Review of Battery Materials as CDI Electrodes for Desalination. Water. 2020; 12(11):3030. https://doi.org/10.3390/w12113030
Chicago/Turabian StyleJiang, Yuxin, Sikpaam Issaka Alhassan, Dun Wei, and Haiying Wang. 2020. "A Review of Battery Materials as CDI Electrodes for Desalination" Water 12, no. 11: 3030. https://doi.org/10.3390/w12113030
APA StyleJiang, Y., Alhassan, S. I., Wei, D., & Wang, H. (2020). A Review of Battery Materials as CDI Electrodes for Desalination. Water, 12(11), 3030. https://doi.org/10.3390/w12113030




